Abstract
Purpose
Understanding how differentiation, microenvironment, and hormonal milieu influence human breast cell susceptibility to malignant transformation will require the use of physiologically relevant in vitro systems. We sought to develop a 3D culture model that enables the propagation of normal estrogen receptor alpha (ER) + cells.
Methods
We tested soluble factors and protocols for the ability to maintain progenitor and ER + cells in cultures established from primary cells. Optimized conditions were then used to profile estrogen-induced gene expression changes in cultures from three pathology-free individuals.
Results
Long-term representation of ER + cells was optimal in medium that included three different TGFβ/activin receptor-like kinase inhibitors. We found that omitting the BMP signaling antagonist, Noggin, enhanced the responsiveness of the PGR gene to estradiol exposure without altering the proportions of ER + cells in the cultures. Profiling of estradiol-exposed cultures showed that while all the cultures showed immediate and robust induction of PGR, LRP2, and IGFB4, other responses varied qualitatively and quantitatively across specimens.
Conclusions
We successfully identified conditions for the maintenance and propagation of functional ER + cells from normal human breast tissues. We propose that these 3D cultures will overcome limitations of conventional 2D cultures of partially or fully transformed cell lines by sustaining normal endocrine function and growth regulation of the cell populations that comprise intact breasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While good progress has been made in understanding a number of fundamental mechanisms of cancer progression, a comprehensive pathologically relevant model for the oncogenic transformation of normal human breast cells is still lacking. Rodent models have been used productively to study effects of pregnancy, carcinogens, and radiation on breast cancer development; however establishing causality at the molecular level is often complicated by difficulties involved in manipulation and observation of individual cells in such models. Moreover, evidence indicates that there are fundamental differences in both normal hormonal regulation and tumorigenesis in rodents and humans [1, 2]. For these reasons, various tractable human cell culture systems have been employed. Cultured human cells have been used historically to: a) study cellular responses to specific changes under conditions where other potential variables are controlled, b) distinguish local from systemic effects on cellular physiology, and c) identify phenotypes that may be uniquely human and thus not amenable to study using animal models. Although such culture systems lack the spatial and systemic influences of intact tissue, they have proven useful in modeling certain aspects of tumor initiation and progression.
Optimally, understanding the roles that differentiation, microenvironment, and hormonal milieu play in determining the relative susceptibility of human breast cells to malignant transformation, and determining the favored routes of in situ transformation, require the use of more sophisticated, physiologically relevant in vitro systems. Bissell, Brugge, and others pioneered and popularized the use of reconstituted laminin-rich extracellular matrix (lrECM) preparations as one means of better recapitulating the normal microenvironment of human breast cells [3, 4]. The ability of non-malignant human mammary epithelial cells (HMECs) suspended in lrECM to form growth-arrested polarized structures readily distinguished them from malignant cells that lost this ability. Nevertheless, incubation of established HMEC lines in lrECM did not result in the expression of estrogen receptor alpha (ER) by these cells. Since estrogen and ER have been implicated in the majority of human breast cancers, the inability to maintain or propagate normal ER + cells has been a major deficiency of past models.
In 2009, Clarke and co-workers [5] reported that, when embedded in lrECM prior to adherent culture, primary normal human breast tissue fragments (organoids) maintained the morphological characteristics, cell lineages, and hormone responsiveness observed in vivo. In fact, gene expression profiling revealed no significant differences in transcript abundance between uncultured breast epithelial tissue fragments and cultured organoids maintained in lrECM for 12–14 days, other than increases in transcripts involved in cell proliferation and normal morphogenesis. As in breast tissue, ER and progesterone receptors (PGR) were frequently co-localized in luminal epithelial cells. Notably, PGR levels were increased by estrogen pretreatment, confirming the functionality of ER in the lrECM cultures. While this and other recent work [6] provided small-scale organotypic cultures for studies of human mammary gland development and hormone biology, maintenance of ER and PGR required passaging of intact organoids—heterogenous in size and composition, complicating efforts to generate replicate cultures for quantitative analyses.
In 2015, Ronnov-Jessen and co-workers [7] showed for the first time that small percentages of normal ER/PGR + human breast cells could be successfully propagated in monolayer cultures in the presence of two small molecule inhibitors of TGFβ signaling. The ER + human breast cells showed small but significant growth stimulation as well as elevated PGR and GREB1 expression in the presence of estrogen. Curiously however, immortalized cells derived from these cultures lost ER expression after formation of acinus-like structures in lrECM [8]. Similarly, in recently published work, Jin et al. [9] were able to demonstrate retention of some ER + cells in short-term 2D cultures containing growth-arrested murine fibroblasts as well as 5% fetal bovine serum. However, the ER signal decreased markedly in subsequent passages.
To design a robust 3D culture system for use in uncovering events involved in the pre-malignant transformation of ER + human breast cells, as well as for screening chemicals for roles in promoting or inhibiting these events, we have used information gleaned from the aforementioned studies. Our culture system offers the following advantages over pre-existing systems: 1) it allows colonies containing epithelial cells with both basal and luminal markers to be generated from single cells or small cell clusters in repeated passages, 2) the medium does not contain serum, and with the exception of lrECM, is completely defined, 3) functional ER expression is maintained in physiologically relevant proportions among the cultured cells.
Methods
Dissociation of breast tissues
De-identified histologically normal breast tissues obtained with informed consent from four women (N88—Black, 34 years old; N129—Caucasian, 26 years old; N182—Caucasian, 33 years old; N191—Asian, 43 years old) undergoing reduction mammoplasties were purchased from the Cooperative Human Tissue Network (CHTN). Tissue samples were minced and enzymatically dissociated using 0.1% w/v collagenase I in Dulbecco’s Modified Eagle Medium at 37 °C for 12–18 h. Small tissue fragments (organoids) remaining after digestion were collected by centrifugation at 100 ×g for 2 min. These primary organoids were suspended in cryogenic medium and immediately frozen in 1 ml aliquots.
Cell culture
The wells of a 24-well cell culture plate were uniformly coated with 50 µl lrECM (BME, Matrigel Basement Membrane Matrix Growth Factor Reduced, Corning)/well and allowed to polymerize at 37 °C for 20 min. Organoid aliquots were thawed, diluted in baseline medium: Advanced DMEM/F12 (ThermoFisher) containing 30 mM HEPES (Sigma), 1 ×GlutaMAX (ThermoFisher), 140 nM hydrocortisone (Sigma), 10 ng/ml EGF (Sigma), 1% PenStrep (ThermoFisher), and centrifuged twice before resuspension in 600 µl lrECM. 50 µl portions were plated at the centers of the pre-coated wells. Following 1 h polymerization, 500 µl of pre-warmed culture medium was added to each well and organoids were incubated for 2 days at 37 °C, 5% CO2 in a humidified incubator. Organoids were retrieved from lrECM by removing medium and adding 500 µl of Cell Harvesting Buffer/Dispase (Corning) to each well and incubating for 1 h at 37 °C. Organoids were then collected and mixed with an equal volume of 0.5 mM EDTA in phosphate buffered saline (PBS), and incubated for an additional 15 min at room temperature before centrifugation. Organoids were then dissociated to single cells using 1 ml of TryPLE (ThermoFisher) for 10 min at 37 °C, while shaking. Following dissociation, the suspension was filtered through 35 µm nylon mesh to obtain single cells. The cells were then washed in baseline medium, resuspended in lrECM and replated in pre-coated 24-well plates at a density of 25,000 cells/well. Following lrECM polymerization for 1 h, 500 µl medium was added per well, with indicated additional factors including EGF (50 ng/ml, Sigma), Noggin (100 ng/ml, PeproTech), R-Spondin-1 (500 ng/ml, PeproTech), Neuregulin1 (100 ng/ml, PeproTech), Jagged-1 (1 µM, AnaSpec), A 83-01 (500 nM, Tocris), SB 431542 (10 µM, Sigma), RepSox (25 µM, Sigma). ROCK inhibitor Y-27632 (10 µM, Tocris) was added to all the cultures during dissociation and for the first 2–3 days of culture. Cells were incubated for 8–14 days (until the mean diameter of colonies was > 100 µm) and medium was changed every other day. The procedure was repeated for subsequent passages. Growth and morphology were documented using bright field microscopy. For estrogen exposures, a 1 mM stock of 17β-Estradiol (Sigma) was dissolved in ethanol and used at a final concentration of 10 nM.
Immunofluorescent imaging
Colonies or individual cells were retrieved from lrECM as described above. Intact colonies were fixed in 4% paraformaldehyde in PBS for 5 min, centrifuged and resuspended in 4% paraformaldehyde and 0.5% Triton X-100 in PBS for another 10 min, washed in blocking buffer (PBS containing 0.1% bovine serum albumin, 0.3% Triton X-100, 0.3% Tween, 10% goat serum), then incubated with primary antibodies in blocking buffer at 4˚ overnight. The colonies were then washed 5 × before incubation with secondary antibodies at 4 °C overnight. The colonies were then washed 2 × and cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 20 ng/ml) for 20 min at room temperature before mounting on glass slides using Fluoromount-G mounting medium (Southern Biotechnology). Dissociated cells were fixed in ice-cold ethanol at − 80 °C, washed in staining buffer (PBS containing 1% glucose, 10 mM EDTA, 1% goat serum, and 0.3 M glycine), and incubated with primary antibodies overnight. Cells were then washed 3 × with staining buffer and incubated with secondary antibodies for 1 h at room temperature, washed again, and counterstained with DAPI before mounting. Confocal microscopy images were acquired using a Zeiss LSM 710 microscope and analyzed using Zen software. Antibodies used included K14 (RB-9020, Thermo Fisher), K19 (MA5-12663, Thermo Fisher), ER (ab16660, AbCam), goat anti-rabbit Alexa Fluor 488 conjugate (A-11034, Thermo Fisher), and goat anti-mouse Alexa Fluor 594 conjugate (A-11032, Thermo Fisher). MCF10A and MCF7 cells cultured under standard 2D culture conditions were used as positive and negative controls, as appropriate.
RNA isolation and qRT-PCR
RNA was isolated directly from intact cultures using RNA STAT-60 (Amsbio) following the manufacturer’s instructions, and quantitated using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher). cDNA was synthesized using the high capacity cDNA Reverse Transcription Kit (Thermo Fisher). qRT-PCR was carried out in a LightCycler 480 Real-Time qPCR instrument (Roche) using SYBR™ Select Master Mix (Applied Biosystems) and primers (Thermo Fisher). Relative PGR mRNA expression levels were calculated using for normalization the geometric mean of the average values of three gene transcripts—Ubiquitin C (UBC), Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ), and Hypoxanthine Phosphoribosyltransferase 1 (HPRT1), with relatively invariant expression in cells under a variety of conditions [10].
RNA-Seq
For transcriptome analysis, triplicate cultures of each specimen incubated with or without 10 nM estradiol for 6 or 24 h were run in triplicate using RNA-seq technology. RNA-seq libraries were prepared using the NEBNext Ultra II RNA Library Prep Kit for Illumina (New England Biolabs) from 1 µg of total RNA input per sample, following the manufacturer’s instructions. Sequencing and initial bioinformatics analyses were conducted at the University of Florida, ICBR facility. Libraries were multiplexed with Illumina barcodes, and 39 samples were sequenced (13 per lane) using an Illumina HiSeq 3000 instrument. Paired end reads were generated with an average read-length of 101-bp. A minimum of 7.8 M reads was produced for each library with 26 M on average (only 2 of 39 samples had less than 10 M reads). QC checks for reads were performed using FASTQC/MULTIQC software [11, 12]. We applied the HISAT2/htseq-count pipeline [13, 14] with default alignment options and chose union mode for mapping reads against the human genome (hg38 gtf file downloaded from UCSC [15]). R environment [16] and tools from Bioconductor [17] packages DESeq 2/edgeR [18,19,20] were applied to generate summaries for raw counts and check for artifacts and outliers. Specifically, we used the tools to generate: 1) sums of raw counts per sample (per replicate), 2) sums of raw counts per gene through all the samples, 3) multi-dimensional scaling (MDS) plots to check for clusters in samples (conditions), 4) Cook’s distances to detect/discard outliers in counts. DESeq 2 uses a built-in model-based normalization procedure by calculating and applying sample-specific scaling factors. To quantify candidate genes, an R wrapper was used for the DESeq 2 package [20] creating annotated consolidated summary tables for results. We applied default options for the DESeq 2 data analysis with cutoffs for non-specific filtering and p-values = 0.1.
Results
Optimization of conditions favoring growth of colonies containing cells with progenitor features and ER expression
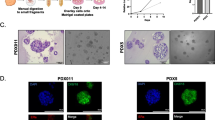
Historically, in normal breast tissues of non-pregnant, non-lactating adult human females, only 7–15% of epithelial cells were reported to express immunologically detectable ER at a given time [21, 22]. Moreover, ER and proliferation-associated markers, such as the nuclear protein Ki67, were rarely expressed together in individual cells in normal premenopausal human breasts [23]. These observations led to speculation that ER + cells are normally post-mitotic, converting estrogen-induced signals to localized paracrine or juxtacrine signals that promote growth in surrounding ER-cells [22, 24], or that ER + cells comprise a stem/progenitor cell population that responds to estrogen by down-regulating ER prior to proliferation and differentiation [25]. We reasoned that in either case, propagation of ER + cells would necessitate finding conditions for the maintenance of stem/progenitor cells. In our initial approach, we attempted to leverage information generated in studies of organotypic cultures initiated from a variety of adult human tissue sources in which investigators had identified factors crucial for maintenance of stem/progenitor cell features [26,27,28,29]. In the absence of a definitive marker of stemness in the adult human mammary gland, we selected dual positivity for keratins 14 and 19 as a marker for multipotent progenitor cells in our cultures [30]. We tested soluble factors and protocols for the ability to maintain and/or enhance the growth of dual keratin positive, as well as ER + cells in 3D lrECM cultures established from single cells and small (< 40 µM in diameter) cell clusters isolated from primary organoids. During the course of this work, Jarde et al. [31] showed that manipulation of Wnt signaling using the protein R-spondin (R-spo1) and addition of the protein Neuregulin1 (Nrg1) could extend the lifespan of mouse mammary organoids in 3D cultures. We performed optimization experiments in which we varied these parameters as well as EGF (Table 1), and found that we could achieve good growth rate and greater than ten population doublings (Fig. 1a) while increasing representation of dual keratin positive cells in cultures (Fig. 1b). In the medium designated LM-2, representation of keratin 14 + cells initially decreased, keratin 19 + cells remained roughly constant, and dual keratin 14/19 + cells increased, before stabilizing with increasing passage. Colonies were mostly round, often contained mixtures of cells expressing all three keratin phenotypes (Fig. 1c), and were devoid of polarized structural organization. In later passages, many colonies contained hollow areas (Fig. 1d); however the cells did not organize into distinct layers in colonies with or without hollow areas, indicating lack of mature lumen formation.
Cultures maintained in Luminal Medium-2 (LM-2) exhibited good growth and high percentages of dual keratin positive cells. a Growth curves of cells from specimens N88 and N129. Colonies were dissociated to single cells and passaged when the mean diameters of colonies were > 100 µm (see Materials & Methods). b Keratins 14 and 19 were identified in dissociated single cells from specimens N88 and N191 at indicated passages through immunofluorescent staining, and quantified using CellProfiler cell image analysis software [38]. c A representative immunofluorescent image of a colony exhibiting a mixture of K14 + , K19 + , and dual keratin positive cells. d Bright field images of colonies from specimens N88 and N191 showing increasing incidence of colonies with vesicular spaces with passage
While our initial medium formulation included one small molecule inhibitor of TGFβ signaling, A83-01, use of this inhibitor alone was insufficient to maintain robust expression of ER in cells derived from primary organoids. In further optimization experiments prompted by work [7] showing that non-malignant ER + human breast cells could be successfully propagated in monolayer cultures in the presence of two different inhibitors of TGFβ signaling (SB431542 and RepSox), we examined the effects of individual and combined inhibitors of TGFβ signaling. Surprisingly, while A83-01, SB431542, and RepSox have been reported to have overlapping effects on TGFβ/activin receptor-like kinase (ALK 5) and related kinases in cell-free assays, we observed significant long-term improvement in the representation of ER + cells in our cultures when we included all three inhibitors in the LM-2 medium (Fig. 2a). RepSox, in particular, proved especially important in maintaining ER expression. In complete LM-2 medium, as in situ, the intensity of ER staining within individual nuclei varied. Without a distinct bimodal distribution, as seen for keratin immunofluorescence, it was not possible to set a threshold, and therefore quantitative evaluation of ER positivity in individual cells was not possible. However, the cultures containing strongly ER + nuclei corresponded qualitatively to those expressing the highest percentages of dual keratin + cells (Fig. 2b).
Long-term representation of ER + and dual keratin + cells was optimal in LM-2 medium that included three different TGFβ/activin receptor-like kinase inhibitors. a Bright green nuclear immunofluorescent staining of ER was apparent in colonies from specimen N88 at passage four grown in LM-2 medium in which all three inhibitors (A83-01, RepSox, SB431542) were included, but not in LM-2 medium in which RepSox (ΔRepSox) or SB431542 (ΔSB431542) were left out. b Quantification of keratin staining in dissociated single cells indicated that the highest levels of cells containing both keratins 14 and 19 (yellow; dual positive) were found in N88 cultures grown in LM-2 medium that included all three inhibitors. In contrast, most cells grown in LM-2 medium lacking RepSox contained only keratin 19 (red) and most cells grown in LM-2 medium lacking SB431542 contained only keratin 14 (green)
Factors influencing ER transcriptional activation
We performed expression analysis using qRT-PCR to determine whether the ER expressed in the 3D cultures were functional. We exposed the cultures to supraphysiological (10 nM) levels of estradiol for brief periods of time to ensure saturation of available ER and to measure direct rather than indirect effects of exposure to the ligand. In complete LM-2 medium, relative progesterone receptor (PGR) mRNA expression was only marginally increased 6 h after stimulation (Fig. 3a). While some researchers have reported that phenol red or a contaminant in commercial phenol red preparations can bind to estrogen receptors and affect ER dependent signal transduction, other researchers have not observed such effects [32]. In control experiments using ER + breast cancer cell line MCF7, we found no improvements in estrogen induction of PGR mRNA when phenol red was omitted from the medium (data not shown). We therefore reasoned that although colonies in complete LM-2 medium expressed ER, the medium lacked necessary component(s) and/or contained inhibitor(s) of ER function. We examined the effects of addition or subtraction of individual factors on baseline and estrogen-induced levels of PGR mRNA. In doing so, we noted that Noggin—a factor included in our original medium formulation due to its requirement for passaging intestinal crypts in culture [27], is an antagonist of BMP action, and BMPs have been reported to be necessary for normal ductal elongation and lumen formation in murine mammary glands [33]. We found that omitting Noggin from LM-2 medium significantly increased estrogen induction of PGR expression in cultures established from three different specimens (Fig. 3b), although it did not significantly affect ER expression or dual keratin + cells (Fig. 3c, d).
Leaving Noggin out of LM-2 medium enhanced the responsiveness of the PGR gene to E2 exposure without altering the proportions of ER + or dual keratin + cells. a Quantitative RT-PCR was performed using RNA preparations from replicate cultures established from specimen N182 incubated for five passages in the presence of Wnt3a (100 ng/ml), Wnt4 (100 ng/ml), Wnt9a (100 ng/ml), or FGF-7 (5 ng/ml)/FGF-10 (20 ng/ml), or absence of A83-01, Noggin, or Jagged-1, followed by treatment with 10 nM 17β-estradiol (E2, red bars) or ethanol (control, blue bars) for 6 h on day 11. After normalization, relative PGR transcript levels were divided by those in control cultures in complete LM-2 medium treated with vehicle alone. Bars indicating standard deviations are included. b Quantitative RT-PCR was performed using RNA preparations from replicate cultures established from specimens N88, N129, and N182 incubated in LM-2 medium without Noggin treated with ethanol or E2 for 6 or 24 h. Normalized PGR transcript levels for individual wells for each of the three specimens are indicated. c Immunofluorescent imaging of ER expression in N129 cultures incubated in media with or without Noggin showed no qualitative differences. d Quantification of keratin expression in N129 cultures incubated in media with or without Noggin showed no quantitative differences in the percentages of dual positive cells
Estrogen regulation of gene expression in 3D cultures of normal breast cells versus tissues and breast cancer cells
We performed RNA-seq on RNA prepared from replicate 3D cultures from three specimens grown in LM-2 medium without Noggin, exposed to 10 nM estradiol or vehicle alone for 6 or 24 h. The sets of genes identified as differentially expressed in response to estradiol showed specimen-dependent differences. For example, 94 genes were identified with a logFC of > 0.5 and FDR < 0.1 in N129 cultures exposed to estradiol for 6 h. Of these, 80 were also identified in the 24 h time point. Using the same criteria, 21 genes were identified in N182 as responsive to estrogen after 6 h and 13 of these were also identified at 24 h. N88 cultures showed the least consistency with only eight genes identified as differentially expressed at 6 h and of these, four also showed differential expression at 24 h. 24 genes were identified as differentially expressed in at least one time point in two or more specimens (Table 2). Of these, 21 showed increased transcript levels relative to corresponding untreated cultures while three showed decreased transcript levels. Three genes—PGR, LRP2, and IGFB4, were identified as estrogen responsive in all specimens at both 6 and 24 h. No significant changes were noted in the levels of transcripts encoding paracrine mediators RANKL (TNFS11), WNT4, or AREG.
The set of genes identified as differentially expressed in this study showed limited overlap with the set of genes identified in a study of MCF7 breast cancer cell monocultures exposed to 10 nM estradiol for 24 h [34]. Our reanalysis of the RNA-seq data from Liu et al. identified 1595 genes with logFC of > 0.5 and FDR < 0.1 and 1397 genes with LogFC < − 0.5 and FDR < 0.1. Of the 24 genes differentially expressed in two or more normal specimens exposed to estradiol, nine (PGR, CISH, NPY1R, IGFBP4, MREG, LOXL4, MYBL1, PTGS2, and DRAM1) showed a similar pattern of expression in MCF7 cells exposed for 24 h. Several genes—LRP2, SEMA3G, SLC40A1, and ZFANDS, showed significant differential expression in the normal specimens and in MCF7, but in the opposite direction (e.g., increased in normal specimens versus decreased in MCF7). While AREG was induced in MCF7 cells exposed to estradiol, both WNT4 and TNFSF11 transcripts were down regulated.
Discussion
Even though estrogen regulates normal breast development, cumulative exposure to cycling levels of ovarian hormones throughout women’s reproductive lives increases the risk of breast tumorigenesis [35]. While progress has been made in understanding fundamental mechanisms of breast cancer progression, a relevant in vitro model that supports and maintains the cell types and signaling pathways observed in normal breast tissues, and which can be used to study carcinogenic disruption of communication among normal human breast cells, is still lacking. The development of an in vitro system in which normal levels of hormone receptors and responses are maintained provides a truly representative model of the human breast with which to perform “proof-of-principle” studies of genetic determinants as well as to perform toxicological studies on environmental factors. We have developed a novel three-dimensional human breast cell culture model that enables the propagation of cells that express functional ER. Our culture system offers a number of advantages over pre-existing systems, while still being amenable to standardization and scale-up required for medium to high throughput applications. We propose that these 3D cultures will overcome limitations of conventional 2D cultures of partially or fully transformed cell lines by sustaining normal endocrine function and growth regulation of the various breast cell populations that comprise the intact organ.
A significant finding is that while the presence of Noggin did not interfere with ER expression by the normal cell cultures, it did interfere with at least some ER-dependent transcriptional responses. Noggin’s canonical role is that of an inhibitor of signaling by members of the BMP family of morphogens. Past studies have suggested that BMP 2 and 4 play roles in mammary gland development, including cell proliferation and lumen formation [36, 37]. However to our knowledge, a specific interaction between BMP signaling and normal ER function has not been demonstrated. It is possible that BMP activity is necessary for the synthesis or function of transcriptional co-factor(s) that interact with ER at specific sites within chromatin. The influence of secreted BMPs on such co-factors is likely to be context dependent. Other molecular interactions and networks influence elements of involved signal transduction pathways, such as Smads. Notably, we assessed ER functionality in 3D colonies that were still growing and which had not been induced to exhibit polarization or form lumens. Additional studies will be needed to determine how different stages of growth and differentiation of ER expressing normal cells modulate ER-dependent transcriptional responses.
Importantly, we demonstrated that the optimized culture system would support the propagation of functional ER expressing colonies from several specimens. While the specimens were all from non-pregnant, non-lactating women, they were derived from patients of diverse racial backgrounds, ranging from 26 to 43 years of age. No attempt was made to ascertain menstrual cycle status or gross anatomical location at the time of tissue collection. Given this initial heterogeneity, it is perhaps not surprising that the actual percentages of ER + cells and transcriptional responses to estradiol exposure varied considerably across specimens. While all the cultures tested showed immediate and robust induction of PGR, as well as LRP2 and IGFB4, other responses varied qualitatively and quantitatively across specimens. Due to the dilution effect of ER- cells in the cultures, the values for genes that did show significant changes in expression were necessarily underestimates of differential gene expression in the ER + cell populations. Similarly, this dilution effect may have obscured more subtle expression changes in ER + cells responding to estradiol. While the quantitative variation might be attributable to differences in the proportions of ER + cells in the individual cultures, this cannot entirely explain the qualitative heterogeneity, the lack of direct proportionality in gene expression signal strength, among the specimens. One possibility is that epigenetic differences present in the tissues may be maintained in the cultures during the extended period of passaging prior to hormone exposure.
There was relatively limited overlap in the estradiol-induced gene expression patterns of the normal cultures and that of MCF7, a commonly used ER + breast cancer cell line. Potential contributors to the differences include genetic and epigenetic alterations accompanying immortal and malignant transformation, as well as in vitro growth conditions (e.g., 2D vs. 3D, serum vs. no serum, etc.). In addition, the homogeneity of ER expression in the MCF7 cultures would presumably allow identification of more subtle changes in gene expression than in the heterogeneously ER-expressing normal cultures. Most perplexing are the differentially expressed genes that showed opposite responses to estradiol. These results indicate significant differences in signaling in the two systems, and suggest caution in the interpretation of MCF7 responses as being representative of ER + cells in the normal breast.
Data availability
RNA-seq data that support the findings of this study have been deposited in NCBI Gene Expression Omnibus (GEO) with the accession code GSE115112. All other remaining data are available within the Article and Supplementary Files, or available from the authors upon request.
References
Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, Gold LI (2005) The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation 73(6):313–322
Rangarajan A, Weinberg RA (2003) Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer 3(12):952–959
Petersen O, Ronnov-Jessen L, Howlett A, Bissell M (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation patterns of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA 89:9064–9068
Debnath J, Brugge JS (2005) Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer 5(9):675–688. https://doi.org/10.1038/nrc1695
Graham JD, Mote PA, Salagame U, Balleine RL, Huschtscha LI, Clarke CL (2009) Hormone-responsive model of primary human breast epithelium. J Mammary Gland Biol Neoplasia 14(4):367–379. https://doi.org/10.1007/s10911-009-9160-6
Sokol ES, Miller DH, Breggia A, Spencer KC, Arendt LM, Gupta PB (2016) Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res 18(1):19. https://doi.org/10.1186/s13058-016-0677-5
Fridriksdottir AJ, Kim J, Villadsen R, Klitgaard MC, Hopkinson BM, Petersen OW, Ronnov-Jessen L (2015) Propagation of oestrogen receptor-positive and oestrogen-responsive normal human breast cells in culture. Nat Commun 6:8786. https://doi.org/10.1038/ncomms9786
Hopkinson BM, Klitgaard MC, Petersen OW, Villadsen R, Rønnov-Jessen L, Kim J (2017) Establishment of a normal-derived estrogen receptor-positive cell line comparable to the prevailing human breast cancer subtype. Oncotarget 8:10580–10593
Jin L, Qu Y, Gomez LJ, Chung S, Han B, Gao B, Yue Y, Gong Y, Liu X, Amersi F, Dang C, Giuliano AE, Cui X (2018) Characterization of primary human mammary epithelial cells isolated and propagated by conditional reprogrammed cell culture. Oncotarget 9(14):11503–11514. https://doi.org/10.18632/oncotarget.23817
Vandesompele J, DePreter K, Pattyn F, Poppe B, VanRoy N, DePaepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):RESEARCH0034
Andrews S FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Ewels P, Magnusson M, Lundin S, Kaller M (2016) MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32(19):3047–3048. https://doi.org/10.1093/bioinformatics/btw354
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12(4):357–360. https://doi.org/10.1038/nmeth.3317
Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31(2):166–169. https://doi.org/10.1093/bioinformatics/btu638
UCSC Genome browser. https://genome.ucsc.edu/cgi-bin/hgTables?GALAXY_URL=https%3A//usegalaxy.org/tool_runner&tool_id=ucsc_table_direct1&hgta_compressType=none&sendToGalaxy=1&hgta_outputType=bed
The R Project for Statistical Computing. https://www.r-project.org/
Bioconductor. http://www.bioconductor.org/
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. https://doi.org/10.1093/bioinformatics/btp616
McCarthy DJ, Chen Y, Smyth GK (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40(10):4288–4297. https://doi.org/10.1093/nar/gks042
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550. https://doi.org/10.1186/s13059-014-0550-8
Petersen OW, Hoyer PE, van Deurs B (1987) Frequency and distribution of estrogen receptor-positive cells in normal, non-lactating human breast tissue. Cancer Res 47:5748–5751
Clarke RB, Howell A, Potten CS, Anderson E (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57(22):4987–4991
Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, Sibson DR, Sloane JP (1999) Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol 155(6):1811–1815
Mallepell S, Krust A, Chambon P, Brisken C (2006) Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA 103(7):2196–2201
Ronnov-Jessen L, Petersen OW, Bissell MJ (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76(1):69–125
Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM (2014) Niche-independent high-purity cultures of Lgr5 + intestinal stem cells and their progeny. Nat Methods 11(1):106–112. https://doi.org/10.1038/nmeth.2737
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244):262–265. https://doi.org/10.1038/nature07935
Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160(1–2):299–312. https://doi.org/10.1016/j.cell.2014.11.050
Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel H, Sachs N, Vries RG, Cuppen E, Chen Y, Sawyers CL, Clevers HC (2014) Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159(1):163–175. https://doi.org/10.1016/j.cell.2014.08.017
Petersen OW, Polyak K (2010) Stem cells in the human breast. Cold Spring Harb Perspect Biol 2(5):a003160
Jarde T, Lloyd-Lewis B, Thomas M, Kendrick H, Melchor L, Bougaret L, Watson PD, Ewan K, Smalley MJ, Dale TC (2016) Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids. Nat Commun 7:13207. https://doi.org/10.1038/ncomms13207
Moreno-Cuevas JE, Sirbasku DA (2000) Estrogen mitorgenic action. III. Is phenol red a “red herring”. In Vitro 36:447–464
Forsman CL, Ng BC, Heinze RK, Kuo C, Sergi C, Gopalakrishnan R, Yee D, Graf D, Schwertfeger KL, Petryk A (2013) BMP-binding protein twisted gastrulation is required in mammary gland epithelium for normal ductal elongation and myoepithelial compartmentalization. Dev Biol 373(1):95–106. https://doi.org/10.1016/j.ydbio.2012.10.007
Liu Y, Zhou J, White KP (2014) RNA-seq differential expression studies: more sequence or more replication? Bioinformatics 30(3):301–304. https://doi.org/10.1093/bioinformatics/btt688
Anderson E (2002) The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4(5):197–201
Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, Woo I, Roberts-Clark D, Francis-West PH, Liu YH, Maxson R, Hill RE, Dale TC (1996) Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development 122(9):2729–2737
Montesano R (2007) Bone morphogenetic protein-4 abrogates lumen formation by mammary epithelial cells and promotes invasive growth. Biochem Biophys Res Commun 353(3):817–822. https://doi.org/10.1016/j.bbrc.2006.12.109
Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM (2006) Cell Profiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7(10):R100. https://doi.org/10.1186/gb-2006-7-10-r100
Acknowledgements
This work was supported by grants from the California Breast Cancer Research Program (21UB-8012, P.Y.; 17UB-8708, C.V.) and the Avon Research Foundation (L1779, C.V.), and a fellowship (to M.V.) from the Tegger Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
De-identified histologically normal breast tissues obtained with informed consent from women undergoing reduction mammoplasties were purchased from the Cooperative Human Tissue Network (CHTN). CHTN operating policies and procedures protect subjects from whom specimens are obtained. These policies and procedures are consistent with current regulations and guidance for repositories from the United States Office of Human Research Protections (OHRP, DHHS). All divisions of the CHTN operate with the review and approval of their local Institutional Review Board (IRB). In addition, the Lawrence Berkeley National Laboratory maintains its own IRB that confirmed that all methods and experimental protocols were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meng, P., Vaapil, M., Tagmount, A. et al. Propagation of functional estrogen receptor positive normal human breast cells in 3D cultures. Breast Cancer Res Treat 176, 131–140 (2019). https://doi.org/10.1007/s10549-019-05229-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05229-5