Abstract
Purpose
To outline the demographics, clinical presentation, imaging features, and treatment modalities observed among a series of patients diagnosed with biopsy-proven granulomatous mastitis (GM).
Method
Following approval by institutional review board, retrospective chart review was performed on patients with biopsy-proven granulomatous mastitis at our institution in the period from January 2013 until October 2017.
Results
A total of 90 patients were identified: 87 women and 3 men. The mean age was 35 years, mostly women in their reproductive age. In our study, patients with GM were more likely to be Hispanic compared to the general population. Sixty-three percent of patients were within 5 years of previous pregnancy. Painful palpable mass-like lesion was the most common physical finding. Breast ultrasound (US) was performed in all patients, and most commonly showed a hypoechoic irregular-shaped mass. Mammography (MG) showed asymmetry or irregular mass as the main finding. Definitive diagnosis was obtained by imaging-guided core needle biopsies in 94.4%. Conservative management was preferred, and only one patient underwent surgery.
Conclusion
Although clinical and radiological findings of patients with GM may mimic those of breast carcinoma, our study showed that women of childbearing age, especially among Hispanic ethnicity with a recent history of pregnancy or high prolactin level and newly tender mass-like lesion, in addition to new focal asymmetry on mammogram and heterogeneous hypoechoic irregular-shaped mass on ultrasound exam, should raise concern for GM. Non-invasive approach and clinical follow-up were the preferred treatment method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Granulomatous mastitis (GM) is a rare, chronic benign inflammatory entity of the breast, with a remarkable variable etiology, including infectious and non-infectious causes. The importance of recognizing this disease is because its presentation can clinically and radiographically mimic breast cancer [1], leading to a diagnostic challenge, as well as anxiety during the evaluation.
A non-specific type of inflammatory response, “granuloma” is defined as an organized collection of mature mononuclear phagocytes that may or may not be associated with necrosis or the infiltration of other inflammatory leukocytes [2]. It is thought to be caused by either infectious agents or foreign material, which triggers the immune response system, leading to granuloma formation [3]. Some of the known inflammatory etiologies of GM are tuberculosis (most common, caused by Mycobacterium tuberculosis), sarcoidosis, fungal infection, and autoimmune disease, such as Granulomatosis with Polyangiitis and giant cell arteritis. These entities are clinically, pathologically, and radiographically indistinguishable from idiopathic granulomatous mastitis (IGM) [3].
First described in 1972 by Kessler and Wolloch [1], idiopathic granulomatous mastitis is a rare benign chronic inflammatory disease of the breast of unclear etiology. Many articles [1,2,3,4,5,6,7] describe several associations, such as elevated hormonal states (e.g., pregnancy, lactation, or oral contraceptive use), autoimmune diseases, diabetes, and genetic factors. Young women of childbearing age who develop GM often have the afore-mentioned associations. GM is a diagnosis of exclusion, so carcinoma, chronic inflammatory conditions, and acute/chronic infections and autoimmune entities are in the differential diagnosis. The definitive diagnosis of GM can only be confirmed by histopathology. GM is characterized by non-caseating granulomas around the lobules and ducts in the breast without specific infectious agents, trauma, or foreign bodies. Associated microabscess formation is variable [2]. Not all cases have characteristic granulomas, but all cases have epithelioid histiocytes [8].
The heterogeneous clinical and radiological presentation of GM can be similar to breast inflammation and breast cancer, frequently presenting as a palpable lump. The lump can have inflammatory features (e.g., pain, tenderness, erythema, skin thickening, and sinus formation). Nipple retraction, nipple discharge, and axillary adenopathy can also be associated with GM [9, 10]. Due to overlapping symptoms with breast cancer and mastitis, delay of diagnosis is very common. Radiological findings of GM depend upon the clinical duration of the disease [4, 9, 11, 12].
Treatment of granulomatous mastitis is still a challenge, and an optimal treatment has not been established. Some authors have suggested conservative management as the appropriate treatment method [5, 10, 13, 14]. These authors have recommended antibiotics, anti-inflammatory drugs, and topical or systemic corticosteroids. Other authors have advocated surgical intervention as the primary curative therapy, such as wide surgical resection and mastectomy [4, 15,16,17].
Our goal is to review a large series of patients diagnosed with GM and find the common associations with clinical, radiological, and demographic features to decrease the time between diagnosis and treatment in an efficient way.
Materials and methods
This is a retrospective HIPAA-compliant study of patients in 2 breast-imaging clinics (one privately insured and one safety-net hospital) between January 1, 2013, and October 31, 2017. A chart review was performed on patients who had undergone breast biopsy and were found to have a pathologic diagnosis of granulomatous mastitis. The patient’s clinical, pathological, and radiological records were collected via electronic medical records. The records were reviewed for demographic data (age, race/ethnicity, menopause and pregnancy status, hormonal contraceptive use, history of breast cancer and trauma, smoking, and BMI), and comorbid diseases such as tuberculosis, sarcoidosis, and autoimmune disease. Clinical findings and treatment methods (at presentation and after histopathologic report) were reviewed. Breast imaging studies (mammography, ultrasound, and MRI) were reviewed, and findings were recorded using the terminology described in American College of Radiology Breast Imaging report and Data System (BI-RADS 5th) lexicon. Mammography, ultrasound, and MRI examinations were interpreted by 1 of the 7 fellowship-trained breast imaging radiologists, with 2–19 years of dedicated to breast imaging. Patients with a pathologic finding of foreign body and granulomatous lymphadenitis (with no breast findings) were excluded. After histopathologic granulomatous mastitis diagnosis was made, all patients were tested for tuberculosis, either with the skin test and/or QuantiFERON. If a positive result was found, patients were screened for active tuberculosis with chest X-ray.
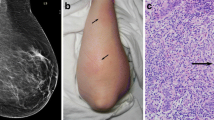
The core biopsy specimen was fixed in 10% neutral-buffered formalin and processed to yield 5 micrometer sections (Fig. 1). These were stained using Hematoxylin and Eosin (H&E) staining system. Histopathological examination of the core reveals loose collections of macrophages surrounded by lymphoplasmacytic infiltrate (granulomata) in a background of acute and predominantly chronic inflammation.
Core biopsy specimen of a granulomatous mastitis demonstrating. a Medium power H&E, 100x magnification showing a closer look at the granuloma formation (arrow) with a collection of paler staining macrophages surrounded by a lymphoplasmacytic infiltrate. b Medium power, 100x magnification of the GMS stain showing no fungal organisms. c Medium power, 100x magnification of the AFB stain showing no acid-fast bacilli
Special stains were performed for acid-fast bacilli (AFB stain) and fungal organisms (Grocott-Gomori methenamine silver—GMS stain) to exclude the presence of any micro-organisms.
Statistical analysis
Patient characteristics and descriptors of imaging features were analyzed. Differences in distribution of variables in the subgroups were analyzed by means of Fisher’s exact test. All comparisons were done with missing data excluded. A p value less than 0.05 was considered statistically significant. All analyses were performed in SAS version 9.4 statistical software (SAS Institute Inc.)
Results
Demographics and risk factors
From January 2013 to October 2017, a total of 90 patients with diagnosed biopsy-proven granulomatous mastitis were identified. Of the patients in the cohort, 87 were women (96.6%) and 3 (3.4%) were male. One man was HIV positive which demonstrated Mycobacterium tuberculosis complex DNA at breast biopsy. The second was a male-to-female transgender patient with a history of hyperthyroidism on estrogen replacement and anti-androgen therapy for 8 years [6]. The third patient was a 21-year-old young man with a history of cryptorchidism corrected with surgery when he was 10 years of age.
The baseline characteristics and risk factors of these patients were analyzed and are summarized in Table 1. The mean age of the patients at presentation was 35 ± 9 years (range: 18–62), with the majority of women at reproductive age (n = 83 [92.2%]), and only 4 (4.4%) were postmenopausal. Eighty patients were Hispanic (88.9%), 7 African-American (7.8%), and 3 were other ethnicities (3.3%). Hispanic ethnicity was more likely to have GM than any other ethnicity in our data (p < 0.0001). Sixty-three percent of patients were within 5 years of pregnancy; of these, 8 women (9.5%) were pregnant at the time of presentation. Five of these pregnant women (63%) also experienced a GM recurrence after the pregnancy. Fifteen patients (17%) were using a hormonal contraceptive at time of diagnosis. One (1.1%) of the patients had a lifetime personal history of breast cancer (triple-negative invasive ductal carcinoma, near the site of GM), whereas 5 patients (5.6%) had a family history of breast cancer (first-degree relatives). One patient described an episode of breast trauma 1 week prior to the beginning of the symptoms. Eight patients (8.9%) had a smoking history within the previous 5 years. Fifty-four percent of the patients were obese (BMI > 30 kg/m2). Positive tuberculosis test (PPD or QuantiFERON) was found in 14 (15.5%) patients. Seven patients (7.7%) had diabetes mellitus, and elevated prolactin level was found in five non-pregnant or lactating patients. Pulmonary sarcoidosis was found in one patient, and another patient had skin sarcoidosis; however, neither had a histopathological finding of sarcoid at breast biopsy.
Clinical presentation
The most common symptom was a palpable mass (n = 60 [66.7%]). Pain/tenderness was the second most common complaint, (n = 25 [27.8%]) (Table 2). Fifty patients (55.6%) had GM in the right breast only, and 8 cases (8.9%) had bilateral involvement. On physical exam, breast mass (n = 75 [83.3%]), pain/tenderness (n = 75 [83.3%]), and erythema (n = 36 [40.0%]) were the most common findings, with the average size of the mass being 4.0 cm. Nipple discharge was seen in thirty-one percent of the patients. The primary clinical diagnosis, usually at the emergency department, was an inflammatory/ infectious process in 44.4% of cases, and breast cancer was suspected in 3.3%. Definitive diagnosis was obtained by core needle biopsies in 94.4% (n = 85), by fine needle aspiration in four cases (4.5%) and excisional biopsy in one case. At the time of presentation, 39 (43.3%) patients were initially treated with a course of systemic antibiotics, and 42 (46.7%) had imaging and/or clinical follow-up. After a GM histopathologic diagnosis was made, 32 (35.5%) patients received a course of oral steroid therapy, either alone or in combination with oral antibiotics (n = 22 [24.4%]) or methotrexate (n = 2 [2.2%]).
Radiologic findings
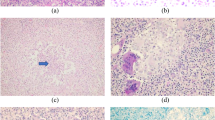
Mammography was done in 50 patients, and the most frequent mammographic finding was an asymmetry (n = 21 [42.0%]). Of these patients, “focal asymmetry” was described in 13 patients (61.9%), “asymmetry” in 6 patients (28.9%), and “global asymmetry” in 2 patients (9.5%) (Fig. 2). The second most common finding in mammogram was a mass (n = 15 [30.0%]) (Fig. 3). Masses with irregular shape were seen in 11 patients (73.3%) and oval shape were identified in 4 patients (26.7%). One patient presented with skin thickening/nipple retraction as the only imaging findings. Architectural distortion was described in 2 (4.0%) cases. Eleven (22.0%) patients showed no findings on mammography. Skin thickening was seen in 8 (16.0%) cases. Calcifications were not present in any of the cases. The mean size of the lesions on mammography was 3.9 cm.
A 39-year-old woman with a 3-week history of a slightly tender palpable right breast mass, bloody nipple discharge, and inverted nipple. a Right breast craniocaudal 2 D mammogram view (left) with spot compression (right) shows a focal asymmetry in the retroareolar (arrow) associated with nipple retraction and shin thickening (arrowhead). b Target right breast ultrasound shows an irregular hypoechoic mass with angular margins (caliper) and small internal anechoic area (arrow) at retroareolar region. Ultrasound findings are highly suggestive of direct invasion of the nipple-areolar complex (arrowhead)
A 35-year-old woman complained of a new tender palpable mass on left breast for 2 weeks, with skin redness and spontaneous drainage through adjacent skin at periareolar region, and nipple inversion. a Left breast mediolateral oblique 2 D mammogram view shows an irregular mass in the superior aspect of the breast anterior depth (arrow), associated with skin thickening and nipple retraction underlying a triangular marker denoting the palpable mass. b Targeted left breast ultrasound shows an irregular hypoechoic mass (arrows) with increased peripheral vascularity, and a tract extension to an open skin ulceration at periareolar region (arrowhead)
Breast ultrasonography was performed in all 90 patients. A mass was described in 83 (92.2%) cases; of these patients, 23 (27.8%) had 2 or more masses, and 60 (72.2%) had a single mass (Fig. 2). The average size of the masses was 3.9 cm. Thirty-six masses (43.3%) had an irregular shape, 50 masses (60.2%) were hypoechoic, and 18 masses (21.6%) were heterogeneous. The most common sonographic finding was an irregularly shaped hypoechoic mass (n = 28 [33.7%]) (Fig. 2). Five patients presented with inflammatory changes (skin thickening/increased vascularity) and no masses were seen. No lesions were sonographically found in 2 patients. Associated enlarged ipsilateral axillary adenopathy were present in 24 cases (26.6%).
MRI of the breast was performed in 2 patients. One patient had a long-term personal history of GM with multiple recurrences and poor response to clinical treatment. The second patient had no findings seen on the mammogram, a breast MRI was indicated for breast pain. In both cases, an enhancing irregular mass was characterized and a second-look ultrasound was performed. The radiologic findings are summarized in Table 3.
Discussion
This is one of the largest studies of women and men who have been diagnosed with granulomatous mastitis. In this study, GM cases were diagnosed mostly in women of reproductive age, in line with previously published reports [4, 7, 8, 18, 19]. The vast majority of our patients were Hispanic (88.9%). The incidence of GM among Hispanic ethnicity was significantly higher when compared to other ethnicities (p < 0.001), also reported in other published papers [13, 19]. The literature shows a prevalence of GM being 12 times more frequent in Hispanic women in the United States (2.4 per 100,000 women aged 20–40 years); however, the cause for this predilection remains uncertain. In a study by Sheybani et al. [7] and Altintoprak et al. [20], a population from the Mediterranean area and within developing regions from Asia, raised the question about HLA association, which could be associated with some unknown mechanism to increase the chances of developing GM.
High levels of estrogen and/or progesterone (either through pregnancy or exogenous use) and elevated prolactin have been postulated in the pathogenesis of GM, as some studies describe a statistically significant association between history of pregnancy and breastfeeding with a recurrence of GM [21,22,23,24,25]. Fifty-nine percent of our patients had been pregnant within the last 5 years which is consistent with other reports. Interestingly, of these, 8 (8.9%) were pregnant at the time of presentation, with recurrence described in five of them. Five patients from our study had elevated prolactin levels; however, none of them were pregnant, breastfeeding, or taking an antipsychotic drug at the time of the diagnosis of GM. Another interesting finding from our data is among the 3 male patients with GM; one of them was a male-to-female transgender with a long history of estrogen replacement and anti-androgen therapy [6], and another patient was a young adult man with a history of cryptorchidism corrected with surgery only when he was 10 years old. These two patients suggest the possibility of a hormonal influence as a possible trigger in the pathophysiology of the disease. In contrast to a study by Uysal et al, which described a statistically significant association of smoking with GM pathogenesis, fewer than 9% of our patients had a history of smoking. Diabetes mellitus type 2 has been associated with a few cases of GM. In our series, 7.7% of patients had diabetes mellitus type 2, likely related to the fact that 54% of patients were obese (BMI > 30 kg/m2). The obesity may be related to increased estrogen level due to biosynthesis from the adipose tissue and, again, suggests the possibility of high estrogen levels as a trigger for the disease.
In agreement with previous studies [2, 9, 10], the vast majority of our patients (83.3%) presented with a painful mass-like lesion as the main physical finding, with erythema seen in about 40% of cases. The mean size of the lesions at the time of presentation on physical examination was 4.0 ± 2.2 cm, which is in accordance with some studies [13, 26]. Due to the similar clinical finding and rapid growth of the mass, the primary clinical diagnosis, usually in the emergency setting, was a breast inflammatory process (e.g., mastitis, abscess, or infectious cyst), leading to an initial course of oral antibiotics. More than half of our patients sought medical attention more than 2 weeks after the onset of symptoms. This delay in seeking medical care might be attributed to financial issues since most of the affected patients are of low-income and/or underinsured population.
Although there are not specific radiologic features for GM, this entity should be included in the differential diagnosis by an experienced breast radiologist in the correct clinical scenario. With the exception of one patient (Fig. 2), our radiologists did not classify any of our cases as BI-RADS 5 on mammography or ultrasonography, and they were mostly classified as BI-RADS 4a-4c. All of these patients had a biopsy, the most reliable method for definitive diagnosis of GM [2, 27, 28]. Once a diagnosis of GM is suspected, the work-up should begin with conventional imaging studies; a diagnostic mammogram if the patient is above 30 years of age and an ultrasound if the patient is under 30 years of age. Mammographic findings of GM are in several studies and in our large study as an asymmetry (focal or regional) with increased density and ill-defined borders, or as an irregular mass (single or multiple) with non-circumscribed margins. Associated findings are skin thickening and axillary adenopathy [4, 9, 11, 29]. Twenty-two percent of the patients in our study had no mammographic findings, possibly due to breast density, since the majority of patients were at reproductive age with heterogeneous dense breast tissue on mammograms. Calcifications were not associated with GM on mammography. Mammographic findings of GM cannot be precisely distinguishable from other pathologies, therefore breast ultrasound is the recommended next step in the work-up of a patient with suspected GM.
In our study, ultrasound was performed in all 90 cases, and the most common sonographic findings were a heterogeneously echogenic irregular-shaped mass with indistinct margins and areas of tubular echogenicity extending to the superficial breast with or without overlying skin thickening. Although these all together may be suggestive of GM lesion, neither mammography nor sonography is highly specific. A few studies have described the role of MRI in the work-up of diagnosis of GM, and some advocate that MRI can help discriminate benign from malignant lesions but MRI lacks adequate specificity in differentiating tumor from an inflammatory process [30, 31]. MRI was performed in only two patients in our study; one of them due to multiple recurrences and poor response to treatment, and the other one as a problem-solving MRI due to lack of findings on mammography and ultrasound, regardless of intense breast pain. The MRI was unable to distinguish a specific cause for an enhancing irregular mass and did not avoid the need for biopsy.
The treatment of idiopathic granulomatous mastitis is still controversial, probably due to its low incidence and lack of understanding its pathophysiology and its prevalence in impoverished patients. Treatment approaches include observation, oral antibiotics, oral corticosteroids, limited or wide surgical excision, and mastectomies [1, 9, 10, 13,14,15,16, 18, 32,33,34,35,36]. In our study, non-surgical management was preferred; 41% of cases received only oral antibiotic therapy (often due to the initial clinical diagnosis of mastitis). Some may argue that antibiotics should not to have a significant role in the management of GM because of no associated bacterial etiology [4, 9]. Steroids are the first-line treatment in some studies, either as a single drug or prior to surgical excision, since it allows multiple and complicated lesions to decrease in size [5, 18, 28, 37]. Other authors advocate it is recommended only for resistant and recurrent cases [33, 38, 39]. In our series of patients, 35.5% received a course of systemic steroid therapy (after histopathology was confirmed by biopsy), either alone or in combination with antibiotic or methotrexate. Only one patient underwent surgery because she had a refractory response to conservative therapy, which is consistent with management of some protocols [5, 37, 40].
Our study has several limitations, including its retrospective and descriptive nature. We could not estimate the chance of relapse/recurrence with each treatment approach, mostly due to a short duration of patient follow-up. In addition, the interval between breastfeeding and onset of GM symptoms could not be evaluated.
Conclusion
Although granulomatous mastitis is a rare benign inflammatory disease of the breast, its morbidity can be debilitating. Women of childbearing age, especially of Hispanic ethnicity with a recent history of pregnancy and/or elevated prolactin levels, obesity, complaining of a new tender mass-like lesion should raise a clinical suspicion for GM. A focal asymmetry on mammogram corresponding to heterogeneous hypoechoic irregular-shaped mass on ultrasound exam are the most common imaging findings. Clinical suspicion associated with demographic characteristics, followed by dedicated breast imaging work-up and image-guided needle breast biopsy minimizes the delay between diagnosis and appropriate treatment, decreasing the chances of recurrence and resistance to treatment.
References
Kessler E, Wolloch Y (1972) Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol 58(6):642–646
Lacambra M et al (2011) Granulomatous mastitis: the histological differentials. J Clin Pathol 64(5):405–411
Bakaris S et al (2006) Granulomatous mastitis including breast tuberculosis and idiopathic lobular granulomatous mastitis. Can J Surg 49(6):427–430
Aghajanzadeh M et al (2015) Granulomatous mastitis: presentations, diagnosis, treatment and outcome in 206 patients from the north of Iran. Breast 24(4):456–460
Freeman CM et al (2017) Idiopathic granulomatous mastitis: a diagnostic and therapeutic challenge. Am J Surg 214(4):701–706
Sam KQ et al (2017) Granulomatous mastitis in a transgender patient. J Radiol Case Rep 11(2):16–22
Sheybani F et al (2016) Idiopathic granulomatous mastitis: long-discussed but yet-to-be-known. Autoimmunity 49(4):236–239
Tse GM et al (2004) Granulomatous mastitis: a clinicopathological review of 26 cases. Pathology 36(3):254–257
Hovanessian Larsen LJ et al (2009) Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol 193(2):574–581
Kiyak G et al (2014) Management of idiopathic granulomatous mastitis: dilemmas in diagnosis and treatment. BMC Surg 14:66
Fazzio RT et al (2016) Idiopathic granulomatous mastitis: imaging update and review. Insights Imaging 7(4):531–539
Memis A et al (2002) Granulomatous mastitis: imaging findings with histopathologic correlation. Clin Radiol 57(11):1001–1006
Joseph KA, Luu X, Mor A (2014) Granulomatous mastitis: a New York public hospital experience. Ann Surg Oncol 21(13):4159–4163
Oran E et al (2013) Management of idiopathic granulomatous mastitis diagnosed by core biopsy: a retrospective multicenter study. Breast J 19(4):411–418
Yabanoğlu H et al (2015) A comparative study of conservative versus surgical treatment protocols for 77 patients with idiopathic granulomatous mastitis. Breast J 21(4):363–369
Atak T et al (2015) Strategies to treat idiopathic granulomatous mastitis: retrospective analysis of 40 patients. Breast Dis 35(1):19–24
Moris D et al (2017) Is idiopathic granulomatous mastitis a surgical disease? The jury is still out. Ann Transl Med 5(15):309
Al-Khaffaf B, Knox F, Bundred NJ (2008) Idiopathic granulomatous mastitis: a 25-year experience. J Am Coll Surg 206(2):269–273
(CDC) (2009) C.f.D.C.a.P., Idiopathic granulomatous mastitis in Hispanic women - Indiana, 2006–2008. MMWR Morb Mortal Wkly Rep 58(47):1317–1321
Altintoprak F, Kivilcim T, Ozkan OV (2014) Aetiology of idiopathic granulomatous mastitis. World J Clin Cases 2(12):852–858
Destek S et al. (2017) Pituitary Adenoma and Hyperprolactinemia Accompanied by Idiopathic Granulomatous Mastitis. Case Rep Endocrinol 3974291
Goldberg J et al (2000) Granulomatous mastitis in pregnancy. Obstet Gynecol 96(5 Pt 2):813–815
Laghzaoui Boukaidi M et al (2000) [Granulomatous recurrent mastitis during pregnancy]. J Gynecol Obstet Biol Reprod (Paris) 29(1):102–104
Uysal E et al (2017) Factors related to recurrence of idiopathic granulomatous mastitis: what do we learn from a multicentre study? ANZ J Surg 88(6):635–639
Omranipour R, Mohammadi SF, Samimi P (2013) Idiopathic granulomatous lobular mastitis—report of 43 cases from iran; introducing a preliminary clinical practice guideline. Breast Care (Basel) 8(6):439–443
Yilmaz E et al (2001) Mammographic and sonographic findings in the diagnosis of idiopathic granulomatous mastitis. Eur Radiol 11(11):2236–2240
Tewari M, Shukla HS (2005) Breast tuberculosis: diagnosis, clinical features & management. Indian J Med Res 122(2):103–110
Seo HR et al (2012) Differential diagnosis in idiopathic granulomatous mastitis and tuberculous mastitis. J Breast Cancer 15(1):111–118
Longman CF et al (2017) Imaging features and diagnosis of tuberculosis of the breast. Clin Radiol 72(3):217–222
Yilmaz R et al (2016) Magnetic resonance imaging features of idiopathic granulomatous mastitis: is there any contribution of diffusion-weighted imaging in the differential diagnosis? Radiol Med 121(11):857–866
Dursun M et al (2012) Multimodality imaging features of idiopathic granulomatous mastitis: outcome of 12 years of experience. Radiol Med 117(4):529–538
Gunduz Y et al (2014) Effect of topical steroid treatment on idiopathic granulomatous mastitis: clinical and radiologic evaluation. Breast J 20(6):586–591
Akcan A et al (2014) Idiopathic granulomatous mastitis: comparison of wide local excision with or without corticosteroid therapy. Breast Care (Basel) 9(2):111–115
Gautier N et al (2013) Chronic granulomatous mastitis: Imaging, pathology and management. Eur J Radiol 82(4):e165–e175
Giovane R et al (2015) Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol 126(2):449–450
Wilson JP et al (2007) Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg 73(8):798–802
Lai EC et al (2005) The role of conservative treatment in idiopathic granulomatous mastitis. Breast J 11(6):454–456
Kayahan M, Kadioglu H, Muslumanoglu M (2012) Management of patients with granulomatous mastitis: analysis of 31 cases. Breast Care (Basel) 7(3):226–230
Asoglu O et al (2005) Feasibility of surgical management in patients with granulomatous mastitis. Breast J 11(2):108–114
Bani-Hani KE et al (2004) Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J 10(4):318–322
Funding
The authors did not receive funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals. The study was approved by the institutional review board for a retrospective chart review.
Rights and permissions
About this article
Cite this article
Barreto, D.S., Sedgwick, E.L., Nagi, C.S. et al. Granulomatous mastitis: etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res Treat 171, 527–534 (2018). https://doi.org/10.1007/s10549-018-4870-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4870-3