Abstract
Purpose
Prostate-specific membrane antigen (PSMA), a protein product of the folate hydrolase 1 (FOLH1) gene, is gaining increasing acceptance as a target for positron emission tomography/computer tomography (PET/CT) imaging in patients with several cancer types, including breast cancer. So far, PSMA expression in breast cancer endothelia has not been sufficiently characterized.
Methods
This study comprised 315 cases of invasive carcinoma of no special type (NST) and lobular breast cancer (median follow-up time 9.0 years). PSMA expression on tumor endothelia was detected by immunohistochemistry. Further, vascular mRNA expression of the FOLH1 gene (PSMA) was investigated in a cohort of patients with invasive breast cancer provided by The Cancer Genome Atlas (TCGA).
Results
Sixty percent of breast cancer cases exhibited PSMA-positive endothelia with higher expression rates in tumors of higher grade, NST subtype with Her2-positivity, and lack of hormone receptors. These findings were confirmed on mRNA expression levels. The highest PSMA rates were observed in triple-negative carcinomas (4.5 × higher than in other tumors). Further, a case of a patient with metastatic breast cancer showing PSMA expression in PET/CT imaging and undergoing PSMA radionuclide therapy is discussed in detail.
Conclusions
This study provides a rationale for the further development of PSMA-targeted imaging in breast cancer, especially in triple-negative tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate-specific membrane antigen (PSMA), a protein product of the folate hydrolase 1 (FOLH1) gene, is increasingly used as a molecular target for positron emission tomography/computer tomography (PET/CT) imaging in patients with several cancer types, besides prostate cancer [1,2,3]. Despite its misleading name, PSMA is not at all prostate specific but widely expressed on the neovasculature of many tumors, including renal cell and squamous cell carcinoma, gastric cancer, and colorectal cancer [1, 4, 5]. Also in breast cancer, several smaller studies reported PSMA-positive tumor vessels [6,7,8]. Recently, the positive experience with PSMA-PET/CT imaging in patients with metastatic renal cell carcinoma, thyroid cancer, and also breast cancer was reported, indicating that vascular PSMA expression might be a promising tracer for PET imaging [2, 9, 10].
This study aimed to characterize PSMA protein expression in a large well-characterized cohort of breast cancer cases and to correlate with clinicopathological parameters including survival times. Additionally, we validated our findings using a The Cancer Genome Atlas (TCGA) breast cancer cohort at mRNA expression level and present a first case of PSMA-based therapy in a patient with metastatic breast cancer.
Materials and methods
Patient cohort (immunohistochemistry)
Three hundred fifteen treatment-naïve female patients from one institution (gynecological department of the University Hospital Bonn) were included in this study (Table 1). All patients underwent surgery for breast cancer between 2002 and 2012. Second malignancy, neoadjuvant therapy, histological types other than invasive carcinoma of no special type (NST) and invasive lobular cancer as well as metastases at the time of surgery were regarded as exclusion criteria. During follow-up, 38 patients (12.1%) developed metastases after median 38 months (range 10–165 months). Sixteen patients (5.1%) experienced locoregional disease progression after median 44 months (range 9–165 months) and 47 patients (14.9%) died after median 49 months (range 1–176 months).
Materials
Construction of tissue microarray (TMA)
Formalin-fixed paraffin-embedded archival specimens were used for TMA construction using three tumor cores per patient (1 mm in diameter). An experienced pathologist supervised the tissue content of the resultant TMA.
Immunohistochemistry protocol
TMA blocks were freshly cut (3 µm) and mounted on superfrost slides (Menzel Gläser, Brunswick, Germany). After deparaffinization with xylene and gradual rehydration, antigen retrieval was achieved by pressure cooking in 0.01 mol/L citrate buffer for 5 min. Slides were incubated with primary antibody (mouse monoclonal antibody, Dako/Agilent, Clone 3E6; dilution 1:100), counterstained with hematoxylin, and aqueously mounted.
Immunohistochemistry evaluation
The slides were evaluated by two experienced pathologists, quantifying staining intensity on tumor endothelia using a 4-tiered scoring system (0: negative; 1: weakly positive; 2: moderately positive; 3: strongly positive). Also, the percentage of positive vessels was recorded.
Case report of PSMA-based radionuclide therapy
A first treatment attempt was performed to a 38-year-old woman with triple-negative breast cancer first diagnosed 2 years before. The patient was initially treated with 4 cycles epirubicin, cyclophosphamide, and bevacizumab followed by docetaxel and bevacizumab as neoadjuvant regimen, followed by extensive surgical resection. After resection a wide field radiation treatment was performed. Besides this intensive therapy local recurrence was diagnosed 5 months after the radiation treatment was finished. Additional surgery was performed followed by a second chemotherapy with carboplatin. Tumor progress in the thorax wall was already diagnosed at the end of this adjuvant treatment. Another extensive surgical resection was performed including the left thorax wall. Due to the fast progress after a wide range of systemic therapy in the interdisciplinary tumor conference, a treatment with [177Lu]Lu-PSMA was decided as individual treatment attempt and after PSMA-receptor status was proven in [68 Ga]Ga-PSMA-PET/CT (Fig. 3). The patient obtained two times 7.5 GBq [177Lu]Lu-PSMA with an interval of 4 weeks.
Ethical issues
This project was approved by the ethical committee of the University Hospital Bonn (Number 169/16). The patient (case report) gave written and informed consents for the treatment and the scientific use of the data.
Analysis of the TCGA data
Clinical data and normalized mRNA expression data of 975 breast cancer cases were downloaded from the TCGA dataset. As described before, we performed a normalization of FOLH1 (PSMA) data using mRNA expression of a vascular marker (CD34), which is not expressed by tumor cells, by calculating a ratio [1].
Statistical analysis
Statistical analysis was carried out using R (R Foundation for Statistical Computing; version 3.4.1).
Results
Correlation between PSMA vascular expression and clinicopathological parameters
Immunohistochemistry cohort
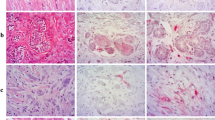
As expected, PSMA displayed a diffuse cytoplasmic staining pattern. Sixty percent of patients in our study showed a PSMA positivity in tumor vessels: weak PSMA expression was seen in 27.6% (n = 87) of cases, moderate expression in 24.4% (n = 77), and strong expression in 8% (n = 25) (Fig. 1).
Immunohistochemical staining patterns of the prostate-specific membrane antigen (PSMA) in NST and invasive lobular breast carcinoma. a—Negative staining of the neovasculature (NST carcinoma). b—Weak staining of the neovasculature (NST carcinoma). c—Moderate staining of the neovasculature (NST carcinoma). d—Strong staining of the neovasculature (NST carcinoma). e—Moderate staining of the neovasculature (invasive lobular carcinoma). f—Occasional staining of the tumor cells (NST carcinoma)
The percentage of positive vessels correlated highly with the intensity of vascular staining (Pearson’s r = 0.87, p < 2.2e−16). Higher vascular PSMA protein expression was observed in higher grade, NST subtype, hormone receptor-negative, Her2-positive, and triple-negative tumors (Table 2). A relatively strong negative correlation was present between PSMA vascular expression and hormone receptor expression of the tumor (Pearson’s r): estrogen receptor − 0.25 with p = 1.1e−05, and progesterone receptor − 0.28 with p = 4.8e−07. There was no association between pN-stage, locoregional progression status, development of distant metastases, and the patients’ overall survival status and PSMA protein expression (all p > 0.2). There was also no correlation with tumor size or multifocality of disease (all p > 0.4).
Very few cases (3%, 10 cases) showed an additional weak expression of PSMA in epithelial tumor cells (Fig. 1f).
TCGA cohort
On mRNA expression levels the findings were similar to the results of the immunohistochemistry studies with higher grade cancer, NST subtype, hormone receptor-negative, Her2-positive, and triple-negative tumors expressing significantly more FOLH1 (PSMA) mRNA, even when normalized using a vascular marker (Table 3). Similarly, high levels of negative correlation were observed for estrogen and progesterone receptor status and FOLH1 mRNA expression (Pearson’s r): estrogen receptor − 0.28 with p < 2.2e−16, and progesterone receptor − 0.27 with p < 2.2e-−16. Importantly, normalized vascular expression of FOLH1 was 4.5 times higher in triple- and hormone receptor-negative tumors (Table 3).
Survival analysis
Immunohistochemistry cohort
Univariate survival analyses (Kaplan–Meier and Cox) failed to demonstrate significant associations between PSMA protein expression and metastasis-free survival or overall survival (Fig. 2a, b).
Kaplan–Meier estimates for metastasis-free and overall survival with log-rank test. a Immunohistochemistry cohort: all patients, metastasis-free survival, PSMA protein expression on the vessels (Staining intensity 0, 1, 2, or 3). b Immunohistochemistry cohort: all patients, overall survival, PSMA protein expression on the vessels (Staining intensity 0, 1, 2, or 3). c TCGA cohort: all patients, overall survival, cut-off for normalized vascular expression of FOLH1 (FOLH1/CD34) = 0.0355. d TCGA cohort: patients with UICC-stage > 2, overall survival, cut-off for normalized vascular expression of FOLH1 (FOLH1/CD34) = 0.0355
TCGA cohort
Only overall survival was available in TCGA cohort as endpoint for survival analyses. For the whole cohort of patients, there was only a trend towards longer survival times in patients with lower normalized vascular expression of FOLH1 mRNA (Fig. 2c, Table 4). However, the analysis of subgroups of patients with positive lymph nodes (log-rank p = 0.019), pN-stage ≥ pN2 (p = 0.001), pT-stage ≥ pT2 (p = 0.015) and ≥ pT3 (p = 0.002), and UICC-Stage ≥ 2 (p = 0.028) and ≥ 3 (p = 0.001) showed a statistically significant worse survival of cases with higher normalized vascular expression of FOLH1 mRNA (using optimized cut-off of 0.0355 in all analyses) (Fig. 2d, Suppl. Data).
PSMA-PET/CT imaging and therapy in a female patient with metastatic breast cancer
The treatment described before was tolerated well by the patient, no side effects were observed. [177Lu]Lu-PSMA accumulated well as shown by the post-therapeutic scintigraphy (Fig. 3). However clinical follow-up showed severe progress four weeks after the second cycle of treatment with [177Lu]Lu-PSMA, so no further cycles were applied.
Discussion
Even though PSMA protein was originally considered to be prostate specific, we now know that this is a misnomer, as it is expressed on the vessels of many tumor types, including breast cancer [6]. Also normal breast epithelia may express PSMA [11], which we confirm in our study. This study demonstrates endothelial PSMA positivity in up to 60% of breast carcinomas. An earlier study demonstrated a slightly higher rate of 74% and also reported clinicopathological correlations of PSMA expression [6]. Strengths of our study were: (a) a larger well-characterized cohort with a wide spectrum of clinicopathological characteristics and available follow-up, (b) several survival endpoints (n = 315), and (c) a validation of our data using a normalized vascular mRNA expression of the FOLH1 gene (PSMA) in the TCGA breast cancer cohort with a vascular normalization methodology we have developed earlier [1]. Another important point is the optimized dilution of the antibody used in our study. Primarily, PSMA positivity of the tumor is considered with regard to the potential application of PSMA-PET/CT in patients, for example for the diagnosis of metastases. In other tumor types, besides prostate cancer, immunohistochemistry against PSMA in the tumor tissue could represent a test, positive results of which could serve as an indication for PSMA-PET/CT in a metastatic setting [1]. Therefore the dilution of the antibody should be precisely adjusted to guarantee further applicability of PSMA-PET/CT. In our study, we used the same antibody as Wernicke et al. [6] but at a lower concentration (1:100 in the present study vs 1:20 in [6]). Therefore, we think that our results might be more informative with regard to a potential diagnostic application of vascular PSMA expression (PSMA-PET/CT).
Importantly, vascular PSMA expression is pronounced in higher grade, NST subtype, Her2-positive, and hormone receptor-negative tumors, as described before [6]. All these associations (except for histological tumor grade, which was not available for the TCGA cohort) were present at both the protein and mRNA level. Interestingly, there was no association of PSMA expression with nodal status. The highest PSMA levels were observed in hormone receptor-negative and triple-negative carcinomas. Normalized vascular mRNA expression was 4.5 times higher in these tumors compared to all other carcinomas under investigation, reaching the quantitative expression levels of renal cell carcinomas, for which PSMA-PET/CT shows promising results for the visualization of metastases [1, 9]. Therefore, patients with this carcinoma subtype may be the best candidates for PSMA-based radionuclide imaging. Given the paucity of therapeutic options for these patients, PSMA-based therapy could also represent a novel therapeutic modality, which warrants further studies. There also is supporting experimental evidence [12, 13] for this concept showing that endothelial cells expressing PSMA could internalize the antibody and nanoparticles carrying a PSMA-binding ligand/inhibitor in a similar way as prostate cancer cells, which is a necessary prerequisite for the application of radiopharmaceuticals with therapeutic intent.
Importantly, PSMA expression in prostate cancer was shown to be similar to breast cancer in that it negatively correlated to androgen receptor activation and was very responsive to changes in AR pathway activity, which led to the experimental use of antiandrogen blockade as a sensitizer prior to PSMA-PET/CT with a positive effect in several small studies [14, 15]. Whether this might be the case for estrogen-antagonizing drugs in breast cancer remains unclear.
Several case reports have suggested a PSMA-PET/CT as a diagnostic tool in patients with metastatic breast cancer [10, 16]. In the current study, we add a case to the already published evidence and report the first case of PSMA-based radionuclide therapy in a female patient with triple-negative breast cancer. In this young patient the two cycles with 7.5 GBq [177Lu]Lu-PSMA were tolerated very well. No side effects were reported by the patient, also no bone marrow or kidney toxicity was observed in the follow-up after the treatment. Unfortunately the tumor did not respond to the internal radiation treatment and progressed under therapy. This may be due to the very high proliferation index of 90% found in the last surgical specimens in this patient. No response to the previously applied chemotherapies was observed as well. Even though this treatment failed oncologically in this case, it also demonstrated that [177Lu]Lu-PSMA has a rather convenient toxicity profile and that further attempts are warranted to clarify the therapeutic efficacy of this approach.
Although many tumors were PSMA-positive in our study, we should state one limitation for using PSMA immunohistochemistry as indication for PSMA-PET/CT use, namely, heterogeneity of PSMA expression in tumor tissue. In our study, we used three tumor tissue cores (each 1 mm in diameter) and derived a maximal staining from all cores for further analyses. In clinical practice, the investigation of conventional tumor slides may be more representative before considering PET/CT. One other limitation of our study is that in both cohorts (immunohistochemistry and TCGA) only primary tumors were available for analysis and not the metastatic foci that can also affect the productivity of PSMA-PET/CT imaging. However, some small case series have shown that PSMA expression could be higher in metastases than in the primary tumors with supporting evidence from the largest case series of PET/CT imaging in patients with metastatic breast cancer [6, 10]. One other important issue to consider is that commercially available antibodies for immunohistochemistry and also the ligands used for the diagnostic setting target a number of different PSMA epitopes [17, 18] that could affect the diagnostic and therapeutic properties of the concrete products. In light of the growing popularity of PSMA as pan-cancer imaging agent in nuclear medicine, we have to bear in mind PSMA is not only expressed by a variety of tumors, but also by benign conditions, including those affecting the breast [19,20,21,22].
Conclusions
This study provides a detailed examination of PSMA expression in breast cancer on protein and mRNA level. One of the main findings is a high-expression rate of PSMA on tumor endothelia in patients with hormone-negative and triple-negative tumors, who may be the best candidates for PSMA-PET/CT imaging in the metastatic setting. We also report on a first case of PSMA radionuclide therapy in a female patient with metastatic breast cancer, which unfortunately progressed under therapy, but the low toxicity suggests further studies to clarify the efficacy of PSMA-directed therapy especially in triple-negative breast cancer cases.
References
Spatz S, Tolkach Y, Jung K et al (2017) Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: implications for imaging studies and prognostic role. J Urol. https://doi.org/10.1016/j.juro.2017.08.079
Lütje S, Gomez B, Cohnen J et al (2017) Imaging of prostate-specific membrane antigen expression in metastatic differentiated thyroid cancer using 68 Ga-HBED-CC-PSMA PET/CT. Clin Nucl Med 42:20–25. https://doi.org/10.1097/RLU.0000000000001454
Hangaard L, Jochumsen MR, Vendelbo MH, Bouchelouche K (2017) Metastases from colorectal cancer avid on 68 Ga-PSMA PET/CT. Clin Nucl Med 42:532–533. https://doi.org/10.1097/RLU.0000000000001700
Haffner MC, Laimer J, Chaux A et al (2012) High expression of prostate-specific membrane antigen in the tumor-associated neo-vasculature is associated with worse prognosis in squamous cell carcinoma of the oral cavity. Mod Pathol 25:1079–1085. https://doi.org/10.1038/modpathol.2012.66
Haffner MC, Kronberger IE, Ross JS et al (2009) Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol 40:1754–1761. https://doi.org/10.1016/j.humpath.2009.06.003
Wernicke AG, Varma S, Greenwood EA et al (2014) Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS 122:482–489. https://doi.org/10.1111/apm.12195
Nomura N, Pastorino S, Jiang P et al (2014) Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int 14:26. https://doi.org/10.1186/1475-2867-14-26
Chang SS, Reuter VE, Heston WD et al (1999) Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res 59:3192–3198
Sawicki LM, Buchbender C, Boos J et al (2017) Diagnostic potential of PET/CT using a 68 Ga-labelled prostate-specific membrane antigen ligand in whole-body staging of renal cell carcinoma: initial experience. Eur J Nucl Med Mol Imaging 44:102–107. https://doi.org/10.1007/s00259-016-3360-2
Sathekge M, Lengana T, Modiselle M et al (2017) 68 Ga-PSMA-HBED-CC PET imaging in breast carcinoma patients. Eur J Nucl Med Mol Imaging 44:689–694. https://doi.org/10.1007/s00259-016-3563-6
Kinoshita Y, Kuratsukuri K, Landas S et al (2006) Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J Surg 30:628–636. https://doi.org/10.1007/s00268-005-0544-5
Nguyen DP, Xiong PL, Liu H et al (2016) Induction of PSMA and internalization of an anti-PSMA mAb in the vascular compartment. Mol Cancer Res 14:1045–1053. https://doi.org/10.1158/1541-7786.MCR-16-0193
Milowsky MI, Nanus DM, Kostakoglu L et al (2007) Vascular targeted therapy with anti–prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J Clin Oncol 25:540–547. https://doi.org/10.1200/JCO.2006.07.8097
Meller B, Bremmer F, Sahlmann CO et al (2015) Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res 5:66. https://doi.org/10.1186/s13550-015-0145-8
Murga JD, Moorji SM, Han AQ et al (2015) Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate 75:242–254. https://doi.org/10.1002/pros.22910
Sathekge M, Modiselle M, Vorster M et al (2015) 68Ga-PSMA imaging of metastatic breast cancer. Eur J Nucl Med Mol Imaging 42:1482–1483. https://doi.org/10.1007/s00259-015-3066-x
Bühler P, Wolf P, Elsässer-Beile U (2009) Targeting the prostate-specific membrane antigen for prostate cancer therapy. Immunotherapy 1:471–481. https://doi.org/10.2217/imt.09.17
Tykvart J, Navrátil V, Sedlák F et al (2014) Comparative analysis of monoclonal antibodies against prostate-specific membrane antigen (PSMA). Prostate 74:1674–1690. https://doi.org/10.1002/pros.22887
Sasikumar A, Joy A, Nair BP et al (2017) False positive uptake in bilateral gynecomastia on 68 Ga-PSMA PET/CT Scan. Clin Nucl Med 42:e412–e414. https://doi.org/10.1097/RLU.0000000000001742
Malik D, Basher RK, Mittal BR et al (2017) 68 Ga-PSMA expression in pseudoangiomatous stromal hyperplasia of the breast. Clin Nucl Med 42:58–60. https://doi.org/10.1097/RLU.0000000000001445
Gordon IO, Tretiakova MS, Noffsinger AE et al (2008) Prostate-specific membrane antigen expression in regeneration and repair. Mod Pathol 21:1421–1427. https://doi.org/10.1038/modpathol.2008.143
Ardies PJ, Gykiere P, Goethals L et al (2017) PSMA uptake in mediastinal sarcoidosis. Clin Nucl Med 42:303–305. https://doi.org/10.1097/RLU.0000000000001543
Acknowledgements
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Markus Essler, Walther Kuhn and Glen Kristiansen—shared senior authors.
Rights and permissions
About this article
Cite this article
Tolkach, Y., Gevensleben, H., Bundschuh, R. et al. Prostate-specific membrane antigen in breast cancer: a comprehensive evaluation of expression and a case report of radionuclide therapy. Breast Cancer Res Treat 169, 447–455 (2018). https://doi.org/10.1007/s10549-018-4717-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4717-y