Abstract
Background
Over the last 20 years, aromatase inhibitors (AI) have been tested in clinical trials as first-line therapy for hormone receptor-positive (HR-positive) advanced breast cancer (ABC), firstly as experimental arms, when they proved to be effective, and recently as control arms. This analysis aims to evaluate trends in progression-free survival (PFS) and time to progression (TTP) over time.
Patients and methods
A literature review was conducted using the MEDLINE database to identify randomized controlled phase II or III trials which reported PFS or TTP of at least one arm using first-line AI HR-positive ABC patients. A linear correlation was used to access the association between the year of the first patient enrolled and the observed PFS/TTP.
Results
The search retrieved 19 trials, accounting for 4552 postmenopausal patients divided into 21 separate AI treatment arms. The PFS/TTP increased from 6 to 9 months in the earlier trials to 13–16 months in the current era, representing an absolute gain of approximately 7 months, without the addition of any other drug. Our analysis showed a positive correlation between the year of the first patient enrolled in these trials and median PFS/TTP reported (R 2 = 0.34; p < 0.01). No correlation was found between the year of the first patient included in these trials and other potential prognostic factors such as visceral metastasis at baseline (R 2 = 0.26; p = 0.20) or exposure to adjuvant therapy (R 2 = 0.05; p = 0.18).
Conclusion
Patients treated with first-line AIs in the more recently conducted trials have longer PFS/TTP when compared to their counterparts treated with the same drugs in older studies. These findings have important implications for the estimation of sample size and follow-up periods for the planning of future trials as well as in the translation of the results into clinical practice decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent type of malignant neoplasm worldwide, and hormone receptor (HR)-positive breast cancer is the most common phenotype. Since their introduction in the 90s, aromatase inhibitors (AIs)—anastrozole, letrozole, and exemestane—became the standard of care for postmenopausal HR-positive breast cancer both in early-stage and advanced disease settings. For patients with HR-positive advanced breast cancer (ABC), clinical guidelines recommend sequential treatment with endocrine therapy, unless they are experiencing visceral crises and/or endocrine resistance is known or suspected [1, 2].
Progression-free survival (PFS) and time to progression (TTP) have been the most frequently used endpoints in ABC. In recent studies, both PFS and TTP (PFS/TTP) have been considered as surrogate endpoints for overall survival (OS), for the purpose of drug approval regulatory process. PFS/TTP are attractive endpoints because they are available earlier than OS, are less likely than OS to be influenced by competing causes of death, and are not affected by the treatments administered after progression. On the other hand, these endpoints are subject to measurement errors and bias [3].
Recently published randomized clinical trials evaluating new therapeutic approaches used AI monotherapy arms as the control group. Better than expected survival outcomes in patients treated with single-agent AI have been observed. This finding has potential implications for therapeutics decisions regarding the sequencing of endocrine agents as well as for the design and conduct of ongoing and future clinical trials.
This study aims to evaluate the evolution over time of the PFS/TTP obtained in trials testing first-line AI monotherapy in patients with HR-positive ABC.
Methods
A literature review was conducted using the MEDLINE database to identify randomized, controlled phase II or III trials which included at least one arm of first-line AI to treat HR-positive ABC patients using the following strategy: (((((breast cancer[Title]) AND aromatase inhibitor[Title/Abstract])) OR ((breast cancer[Title]) AND anastrozole[Title/Abstract])) OR ((breast cancer[Title]) AND letrozole[Title/Abstract])) OR ((breast cancer[Title]) AND exemestane[Title/Abstract]). For this analysis, first-line treatment was defined as the first endocrine therapy used for ABC irrespective of prior (neo) adjuvant endocrine therapies for early-stage disease. The search strategy included terms applicable to the patient population (postmenopausal women with locally advanced or metastatic breast cancer). Publications reporting PFS or TTP data in patients treated with first-line AI monotherapy (anastrozole, letrozole, or exemestane) were considered eligible for further assessment. However, there are some subtle differences between these outcomes that must be pointed out. While PFS is defined as the time elapsed between treatment initiation and tumor progression or death from any cause; TTP considers only disease progression as event, censoring patients who die from any cause before progression. Language was restricted to English, but there was no date of publication restriction. Data were evaluated from publications reporting either the primary or follow-up analyses. Studies designed for HER-2-positive breast cancer were excluded.
The following data were extracted from each relevant publication identified: phase, sample size, year of publication, the period of patients` accrual, estimated median PFS/TTP used for sample size calculation and median observed PFS/TTP. We also evaluated baseline patients’ characteristics such as age, the proportion of patients with visceral disease, previous endocrine therapy exposure, the proportion of patients with confirmed HR positivity and the proportion of patients with confirmed HER2 negativity.
A linear correlation was used to access the association between the year of the first patient enrolled and the PFS/TTP reported with AI as first-line treatment for HR-positive ABC in each study. We hypothesized that the reported PFS/TTP times would be longer as the year of the first patient enrolled increases. p values lower than 5% were deemed to be significant.
Results
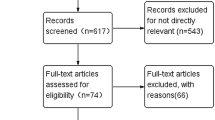
The literature search retrieved 568 publications, of which 19 met the inclusion criteria for this review, accounting for 4552 postmenopausal patients divided into 21 separate treatment arms with an AI as first-line monotherapy treatment for HR-positive ABC. Figure 1 summarizes the flowchart for study selection. The studies included in this review are described in Table 1.
The overall median PFS/TTP ranged from 6.1 to 15.6 months among trials. Nine studies reported median PFS/TTP for anastrozole ranging from 8.2 to 15 months [4,5,6,7,8,9,10,11,12,13,14], while seven studies reported PFS/TTP between 9 and 15.6 months with letrozole [15,16,17,18,19,20,21,22] and 6.1 and 13.8 months with exemestane in three studies [7, 14, 23] Our analysis showed a positive correlation between the year of the first patient enrolled in these trials and median PFS/TTP reported (R 2 = 0.34; p < 0.01), meaning that patients treated with first-line AIs in the more recently conducted trials have longer PFS/TTP than their counterparts treated with the same drugs but in older studies (see Fig. 2). In univariate analyses, no correlation was found between the year of the first patient included in these trials and other potential prognostic factors such as the proportion of patients with visceral metastasis at baseline (R 2 = 0.26; p = 0.20), median age (R 2 = 0.10; p = 0.15), and percentage of patients who had never been exposed to adjuvant therapy (R 2 = 0.05; p = 0.18). Multivariate analysis was not carried out because of the reduced number of studies available to be included in this exploratory estimation. No other correlations with potential prognostic factors were sought. The number of patients accrued in each individual study is a factor that could influence the precision of the median PFS/TTP times reported by the studies included in this analysis. Univariate linear regression did not show significant variation in the sample size magnitude along time (R 2 < 0.01; p = 0.68). As described in Table 1, 10 studies reported the estimated 95% confidence interval for their median PFS/TTP, while nine did not. Therefore, this variable was not included in the estimations presented and represents a limitation of this analysis.
Table 2 describes the nine trials in which the estimated PFS/TTP in the AI monotherapy group used for sample size calculations were reported. In all but one trial, the expected PFS/TTP was lower than the observed PFS/TTP.
Discussion
Our analysis suggests that the PFS/TTP achieved with aromatase inhibitors therapy as first-line treatment for advanced breast cancer have shown progressive improvement. The PFS/TTP increased from 6 to 9 months in the earlier trials to 13–16 months in the current era, representing an absolute gain of approximately 7 months, without the addition of any other drug. These advances raise challenges on how to incorporate these findings into the interpretation of available clinical trial data and the design of future research. Immediate implications are related to sample size and follow-up time estimation for ET trials design.
Most likely, a combination of improved medical care and patient selection factors justifies most of this progressive improvement in outcomes obtained with the same therapeutic strategy. While clinical trial entry criteria have not changed significantly over time, more recent studies are probably including a different population of patients. With the widespread use of modern radiology tests, the population included in the latest trials may include a higher proportion of patients with a lower burden of metastatic disease. Exclusion of HER2 patients is routinely done in recently published endocrine therapy trials; however, HER2 status was not available in earlier studies. At the same time, older trials included a minority of patients with unknown HR status. Therefore, a small fraction of patients with HER2-positive or HR-negative breast cancer was included, and this could lead to inferior PFS/TTP in older studies.
Better outcomes may also be a result of improved loco-regional treatments and increasing experience with managing the expected adverse effects. Additionally, improved general medical care and the use of adjuvant therapies (i.e., radiotherapy, bisphosphonates, and RANKL inhibitors) is another potential explanation for the observed improvement in PFS outcomes using the same therapy on similar population, even though these factors are hard to measure and probably of small importance.
An alternative explanation that may contribute to a trend for better survival outcomes in more recent trials is a change in tumor biologic features, with a hypothetical shift towards a higher proportion of endocrine-sensitive tumors. Some studies have reported a difference in the epidemiology of breast cancer over time, with more ER-positive tumors being observed than in the past [24,25,26]. Whether these changes occur in the metastatic setting or are simply related to improvements in screening strategies of early-stage indolent tumors has not been adequately studied.
The inclusion of a population with characteristics associated with hormone sensitivity and/or less aggressive disease, such as a higher proportion of endocrine therapy-naive patients or patients with non-visceral metastases, could lead to longer PFS. However, our analysis shows that there were no significant differences over time in the proportion of endocrine therapy-naïve patients neither on the proportion of patients with non-visceral disease.
Estimating the event rate to proceed with sample size calculation in clinical trials is challenging in this scenario. For instance, PFS/TTP results for the AI monotherapy arms in the trials included in this review have consistently surpassed the expectations used for calculations of HR and sample size (see Table 2). With the exception of one study, in all studies, the observed rates surpassed the estimated PFS/TTP in trial design. The CALGB 40503 trial provides an extreme example. This phase III study was designed to investigate whether bevacizumab prolongs PFS when added to first-line letrozole as treatment of HR-positive advanced breast cancer [21]. This trial had 90% power to detect a 50% improvement in median PFS from 6 to 9 months based on literature available when the study was designed. Surprisingly, the observed PFS for the letrozole monotherapy arm was 15.6 months.
We expect that ongoing trials will face similar challenges. The VICTORIANE trial is an ongoing randomized phase 3 study assessing the addition of oral vinorelbine to aromatase inhibitors for the treatment of patients with endocrine therapy-naive HR-positive ABC. The sample size for this trial was calculated based on an expected PFS for the AI monotherapy arm of only 9 months [27]. The MONARCH 3 is a randomized phase III study of AI plus abemaciclib, a CDK4/6 inhibitor, or placebo in first-line treatment of women with ER+ advanced breast cancer [28]. Similarly, the estimated PFS in the AI monotherapy arm considered for study planning was 10 months. These trials are examples where there is a high probability that the PFS in the AI monotherapy control arm will surpass the estimative. Calculating sample sizes considering a PFS/TTP lower than the actual one and an excessively optimistic hazard ratio may result in underpowered clinical trials with critical issues impacting sample size and follow-up time.
Interestingly, similar improvements in survival outcomes have also been identified in studies of early-stage breast cancer with AI monotherapy as the control group. Because an annual risk of relapse persists over decades, the development of new adjuvant ET approaches requires protracted follow-up and large sample sizes. In the FACE trial [29], comparing anastrozole versus letrozole in the adjuvant setting, investigators were challenged with a lower-than-expected event rate and decided to report results before reaching the expected number of events. Similarly, in the SOFT [30] and TEXT [31] trials, that evaluated the role of ovarian function suppression and exemestane in premenopausal patients with early-stage HR-positive breast cancer, the expected survival outcomes used for sample size calculation were overly pessimistic. Consequently, the event rate was over-estimated and the duration of follow-up time to reach the targeted number of events for final analysis was under-estimated [32].
Other than the impact on methodological issues for future research, these findings also impact clinical practice. Given continued efforts to develop therapies that delay the emergence of endocrine resistance, the results of this analysis demonstrating the favorable outcome for these endocrine-sensitive subgroups has immediate relevance. With added clinical and financial toxicities, it is important to define HR-positive populations that are more likely to benefit from combination therapy and to identify those patients who may do well with endocrine therapy alone. [33]. Future research on prognostic and predictive biomarkers might assist in estimating the pattern of endocrine sensitivity or resistance and guide therapeutic decisions on ET sequencing strategies.
Conclusion
While acknowledging the inherent cross-trial analyses limitations, our work suggests a progressive improvement in PFS/TTP of first-line endocrine therapy with aromatase Inhibitors containing trials over time. Our scrutiny of the data does not reveal any definitive reason that fully explains this phenomenon. Nevertheless, these findings have important implications for the planning and sample calculation of currently ongoing and future randomized clinical trials.
References
Cardoso F, Costa A, Senkus E et al (2017) 3rd ESO–ESMO International consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol. https://doi.org/10.1093/annonc/mdx036
Rugo HS, Rumble RB, Macrae E et al (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 34(25):3069–3103
Saad ED (2011) Endpoints in advanced breast cancer: methodological aspects & clinical implications. Indian J Med Res 134:413–418
Bergh J, Jonsson PE, Lidbrink EK et al (2012) FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30:1919–1925
Bonneterre J, Thurlimann B, Robertson J et al (2000) Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Eficacy and Tolerability study. J Clin Oncol 18:3748–3757
Baum M, Buzdar AU, Cuzick J et al (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet 359(9324):2131–2139
Iwata H, Masuda N, Ohno S et al (2013) A randomized, double-blind, controlled study of exemestane versus anastrozole for the first-line treatment of postmenopausal Japanese women with hormone-receptor-positive advanced breast cancer. Breast Cancer Res Treat 139:441–451
Johnston S, Basik M, Hegg R et al (2016) Inhibition of EGFR, HER2, and HER3 signaling with AZD8931 in combination with anastrozole as an anticancer approach: phase II randomized study in women with endocrine-therapy-naı¨ve advanced breast cancer. Breast Cancer Res Treat 160(1):91–99
Mehta RS, Barlow W, Albain KS et al (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367:435–444
Nabholtz JM, Buzdar A, Pollak K et al (2000) Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women; results of a North American multicenter randomized trial. J Clin Oncol 18(22):3758–3767
Robertson J, Bondarenko I, Trishkina E et al (2016) Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388(10063):2997–3005
Robertson J, Llombart-Cussac A, Rolski J et al (2009) Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol 27:4530–4535
Cristofanilli M, Valero V, Mangalik A et al (2010) Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res 16:1904–1914
Llombart-Cussac A, Ruiz A, Anton A et al (2012) Exemestane versus anastrozole as front-line endocrine therapy in postmenopausal patients with hormone receptor-positive. Advanced breast cancer. Cancer 118:241–247
Finn R, Martin M, Rugo H et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1926–1936
Finn RS, Crown J, Lang I et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as fi rst-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 stud. Lancet Oncol 16:25–35
Johnston S, Pippen J, Pivot X et al (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor -positive metastatic breast cancer. J Clin Oncol 27:5538–5546
Martín M, Loibl S, von Minckwitz G et al (2015) Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. J Clin Oncol 33:1045–1052
Mouridsen H, Gershanovich M, Sun Y et al (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19(10):2596–2606
Wolff AC, Lazzar AA, Bondarenko I et al (2013) Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 31:195–202
Dickler MN, Barry W, Cirrincione CT et al (2015) Phase III trial evaluating the addition of bevacizumab to letrozole as first-line endocrine therapy for treatment of hormone-receptor positive advanced breast cancer: CALGB 40503 (Alliance). J Clin Oncol 33:501
Hortobagyi G, Stemmer S, Burris H et al (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375(18):1738–1748
Paridaens RJ, Dirix L, Beex LV et al (2008) Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastastic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Breast Cancer Cooperative Group. J Clin Oncol 26:4883–4890
Brown S, Mallon E, Edwards J et al (2009) Is the biology of breast cancer changing? a study of hormonereceptor status 1984–1986 and 1996–1997. Br J Cancer 100:807–810
Pujol P, Chamness GC, Elledge RM (1994) Rising levels of estrogen receptor in breast cancer over 2 decades. Cancer 74:1601–1606
Anderson WF, Rosenberg PS (2011) Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst 103:1397–1402
Villanueva C, Bazan F, Barthelemy P et al. VICTORIANE: Open Label, Randomised Multi-center Phase III Study to Assess the Efficacy and Tolerability of metronomic oral OT 1-04-07 vinorelbine in combination with aromatase inhibitors for the treatment of patients with advanced breast cancer. (NCT02730091). Cancer Res 2016
Goetz M, Toi M, Klise S et al (2015) MONARCH 3: a randomized phase III study of anastrozole or letrozole plus abemaciclib, a CDK4/6 inhibitor, or placebo in firstline treatment of women with HR + , HER2locoregionally recurrent or metastatic breast cancer (MBC). J Clin Oncol 33(15):tps624
Smith I, Yardley D, Burris H et al (2017) Comparative efficacy and safety of adjuvant letrozole versus anstrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: final results of the randomized phase III Femara Versus Anastrozole Clinical Evaluation (FACE) trial. J Clin Oncol 35(10):1041–1048
Francis P, Regan M, Fleming G et al (2015) Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 372:436–446
Pagani O, Regan M, Walley B et al (2014) Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371:107–118
Regan M, Pagani O, Fleming G et al (2013) Adjuvant treatment of premenopausal women with endocrine responsive early breast cancer: design of the TEXT and SOFT trials. Breast 22:1094–1100
Turner N, Neven P, Loibl S, Andre F (2016) Advances in the treatment of estrogen receptor-positive advanced breast cancer. Lancet. https://doi.org/10.1016/S0140-6736(16)32419-9
Llombart-Cussac A, Guerrero A, Galan A et al (2012) Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol 14:125–131
Robertson J, Llombart-Cussac A, Felti D, et al. Fulvestrant 500 mg versus anastrozole as first-line treatment for advanced breast cancer: Overall survival from the phase II ‘FIRST’ study. San Antonio Breast Cancer Symposium Abstract S6-04 2014
Finn R, Martin M, Rugo H et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925–1936
Disclosures
TR—Speaker honoraria (AstraZeneca and Novartis). Research funding (AstraZeneca), MD—No disclosures. JB—Travel expenses (AstraZeneca). CHB—Clinical Research and consultant/advisory services (AstraZeneca, Novartis, Pfizer, and Roche).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reinert, T., Debiasi, M., Bines, J. et al. Trends in progression-free survival (PFS) and time to progression (TTP) over time within first-line aromatase inhibitors trials in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat 168, 457–465 (2018). https://doi.org/10.1007/s10549-017-4593-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4593-x