Abstract
Purpose
Prospective information regarding the tolerability and efficacy of endocrine therapy (ET) alone and in combination with targeted agents in older patients in the metastatic setting is limited. This review summarizes available trial data in this population.
Methods
We searched PubMed for Phase 2 or 3 trials with age-stratified patient cohorts (≥ 65 vs. < 65 years in most studies) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer treated with ET ± targeted agents.
Results
We identified 19 studies reporting 10 clinical trials. Efficacy was similar in age-stratified subsets. There was a reduced disease progression risk for ET + everolimus, palbociclib, or ribociclib versus ET alone. In the first-line setting, median progression-free survival (mPFS) in older patients was 8.5, 26.2 months, and not reached with letrozole + temsirolimus, palbociclib, and ribociclib, respectively, and in younger patients was 9.0, 18.8 months, and not reached, respectively. In the second-line setting, older patients had mPFS of 6.8 and 9.9 months with everolimus + exemestane and palbociclib + fulvestrant, respectively, and younger patients had mPFS of 8.1 and 9.5 months, respectively. Tolerability was worse for combination therapy versus monotherapy. No age-related differences in discontinuations were observed for CDK4/6 inhibitors, although a higher rate of treatment discontinuation was observed for patients ≥ 70 years receiving everolimus + exemestane. Adverse event rates were similar in age-stratified subsets.
Conclusions
ET + CDK4/6 or mTOR inhibitors are likely safe and effective in older patients with HR+, HER2− advanced breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endocrine therapies are the standard first-line treatment for hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer [1, 2]. Evidence from Phase 3 trials shows that the combination of endocrine therapy and targeted agents, such as the mammalian target of rapamycin (mTOR) inhibitor everolimus and the cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors palbociclib, ribociclib, and abemaciclib, prolongs progression-free survival (PFS) compared with endocrine monotherapy in patients with metastatic HR+/HER2− breast cancer in the first- or second-line setting [3,4,5,6].
Although more than 40% of new breast cancer cases are diagnosed in patients aged ≥ 65 years [7], patients in this age group who have cancer are frequently underrepresented in oncology clinical trials [8]. One meta-analysis of Alliance for Clinical Trials in Oncology breast cancer trials from 1985 to 2012 showed that < 20% of enrolled patients were ≥ 65 years of age [9]. The reasons for underrepresentation of older patients in clinical trials are multifactorial and often occur because of enrollment criteria that exclude older patients, poorer performance status, patient and provider preferences, and a higher incidence of comorbidities and prescriptions with unknown interactions with study agents [8]. Thus, the benefits and risks of combination treatment with endocrine and targeted therapies are not well understood but are important to examine given the widespread use of novel therapeutics in clinical practice and the fact that the vast majority of breast cancers in older patients are HR+ [10].
To address the gap in prospective evidence, we reviewed the available literature reporting age-stratified PFS and safety data from randomized trials comparing endocrine monotherapy versus endocrine therapy in combination with targeted therapy in patients with HR+/HER2− advanced breast cancer.
Methods
We conducted a systematic search of PubMed using keywords to identify randomized trials reporting PFS or disease-free survival in patients with advanced or metastatic breast cancer treated with endocrine therapies in combination with targeted agents. The specific search terms for endocrine and targeted agents were anastrozole, exemestane, letrozole, palbociclib, ribociclib, abemaciclib, tamoxifen, fulvestrant, goserelin, leuprolide, everolimus, toremifene, Fareston, raloxifene, megestrol, BKM120, buparlisib, BYL719, GDC0941, everolimus, temsirolimus, entinostat, bevacizumab, and Avastin. The search was conducted on June 28, 2017. No restriction was placed on the article publication date. Only English-language articles were included.
The results of the literature search were manually filtered to include only Phase 2 or Phase 3 trials comparing endocrine monotherapy versus combination of endocrine plus targeted therapies in patients with HR+/HER2− advanced breast cancer that reported efficacy and safety in a subset of older patients. The age cutoff for older patients differed across the identified studies but was ≥ 65 years of age in most publications (age-group comparisons for each identified study are described in Table 1). We also manually searched for relevant abstracts from the annual meetings for the American Society of Clinical Oncology (ASCO), European Cancer Congress (ECCO), European Society for Medical Oncology (ESMO), Miami Breast Cancer Conference (MBCC), and San Antonio Breast Cancer Symposium (SABCS) published from January 1, 2015, through June 28, 2017, using the search terms and criteria described above. Data extracted from the selected publications included demographics, PFS, and safety in age-stratified patient subsets. No statistical testing was performed given the differences in patient populations, lines of therapy, and age cutoffs provided in each study.
Results
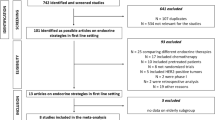
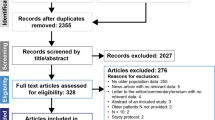
The literature search identified 551 publications, which were manually filtered to 19. The reasons for exclusion included publication did not report a Phase 2 or Phase 3 study; study design did not include endocrine monotherapy versus endocrine therapy in combination with targeted therapy; there was no older patient subset analysis; and participants were HER2+. The 19 identified publications were reports of 10 clinical trials (some trials had multiple secondary publications identified in the search; Fig. 1), 5 of which investigated first-line combination treatment, with the other 5 investigating second-line combination treatment. These publications included 8 Phase 3 and 2 Phase 2 trials of endocrine therapy combined with CDK4/6 inhibitors (n = 5; PALOMA-1, -2, and -3; MONALEESA-2; and MONARCH 2), estrogen synthesis inhibitors [n = 2; SOFEA and NCT00075764 (unnamed)], mTOR inhibitors [n = 2; BOLERO-2 and NCT00083993 (unnamed)], and an androgen synthesis inhibitor [n = 1; NCT01381874 (unnamed); Table 1] [4,5,6, 11,12,13,14,15,16,17,18,19,20,21,22]. Among the trials reporting baseline characteristics stratified by age, 24.8–46.1% of patients within an individual trial were aged ≥ 65 years. In the 2013 report of the BOLERO-2 trial from Pritchard et al., which used 70 years of age as the cutoff for older patients, 22.7% of patients were aged ≥ 70 years [17].
Patient characteristics
Three publications reported baseline characteristics according to the age-based subsets [BOLERO-2 (≥ 70 vs. < 70 years), PALOMA-1 (≥ 65 vs. < 65 years), and MONALEESA-2 (≥ 65 vs. < 65 years)], showing similar baseline disease characteristics in older and younger patients. In these studies, the proportions of patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 and 1 ranged from 51 to 56% and 39 to 47% in older patients, respectively, and from 55 to 68% and 32 to 45% in younger patients [12, 17, 19]. The percentage of patients with < 3 metastatic sites ranged from 63 to 65% in older patients and 49 to 69% in younger patients [17, 19]. Across all 3 studies, the proportions of patients with visceral disease sites (older, 49–72%; younger 40–59%) were higher than those with bone-only sites (older, 12–23%; younger, 7–24%) [12, 17, 19].
Efficacy in age-stratified subgroups
Median PFS stratified by age was reported for patients treated in the first-line setting in PALOMA-1, MONALEESA-2, and the Phase 3 trial of letrozole ± temsirolimus (> 65 vs. ≤ 65 years) [12, 19, 21]. Responses to treatment were similar in older and younger patients (Fig. 2). Among older patients treated in the first-line setting, median PFS was 10.1–18.4 months with letrozole monotherapy across studies and 8.5 months, 26.2 months, and not reached (with 15.3 months median follow-up) with letrozole + temsirolimus, palbociclib, and ribociclib, respectively [5, 12, 19, 21]. In younger patients, median PFS with endocrine monotherapy was 5.6–13.0 months across studies and was 9.0 months, 18.8 months, and not reached with letrozole + temsirolimus, palbociclib, and ribociclib, respectively. Progression-free survival benefits were also observed with second-line combination treatment for both older and younger patients in BOLERO-2 and PALOMA-3 (≥ 65 vs. < 65 years) [11, 17]. In older patients, median PFS was 1.5 and 3.9 months with endocrine monotherapy and 6.8 and 9.9 months with everolimus + exemestane and palbociclib + fulvestrant, respectively. In the younger patient subsets, median PFS was 4.0 and 5.6 months with endocrine monotherapy and 8.1 and 9.5 months with everolimus + exemestane and palbociclib + fulvestrant, respectively. Overall, patients experienced improved PFS with combination therapy, although no clear benefit was observed for patients treated with letrozole in combination with temsirolimus [21].
Median progression-free survival with endocrine monotherapy versus combination therapy stratified by age [11,12,13, 17, 19, 21]. NR not reached. aPatients ≥ 65 years old were classified as older. bPatients ≥ 65 years old were classified as older. cPatients ≥ 70 years old were classified as older. dProgression-free survival in PALOMA-3 was reported by subgroup analysis that stratified patients as pre/perimenopausal versus postmenopausal
The relative efficacy of endocrine monotherapy and combination therapy with targeted agents is shown in a forest plot for PFS across trials in Fig. 3. Hazard ratios indicate reduced risk of disease progression in both younger and older patients receiving endocrine therapy in combination with everolimus, palbociclib, ribociclib, or abemaciclib compared with those receiving endocrine monotherapy [4, 11,12,13, 15, 17, 19, 20, 23]. In the Phase 3 trial of letrozole ± temsirolimus, a reduced risk of progression was observed for combination treatment in younger patients but not in older patients [21]. No improvement in the risk of progression was seen for combination treatment versus endocrine monotherapy in trials of fulvestrant ± anastrozole or exemestane ± abiraterone [14,15,16]. Overall, younger patients tended to have a greater or as good a benefit with combination treatment as with monotherapy relative to older patients.
Forest plot of efficacy of combination therapy versus monotherapy stratified by age [4, 6, 11,12,13,14,15,16,17, 19,20,21, 23] CI confidence interval. aUpdated efficacy data from MONALEESA-2 with an additional 11 months of follow-up reported a hazard ratio of 0.658 (95% CI 0.466–0.392) in patients ≥ 65 years of age and 0.518 (95% CI 0.392–0.684) in patients < 65 years of age. bPrior treatment was allowed in the adjuvant setting only. cThe median age for the exemestane + abiraterone treatment arm was 64 years. The median age for the exemestane-alone treatment arm was 63 years
Tolerability and safety in age-stratified subgroups
Among the 10 trials that analyzed older subgroups, age-stratified safety data were published for BOLERO-2, MONALEESA-2, and the PALOMA trials. Overall, combination treatment led to substantially higher rates of dose interruptions/reductions and permanent treatment discontinuation due to adverse events (AEs) compared with monotherapy [12, 17, 19]. The tolerability of combination therapy for older patients versus younger patients varied. In the everolimus + exemestane treatment arm in BOLERO-2, similar rates of everolimus dose interruptions/reductions were observed in both age subsets (≥ 70 years, 66.9%; < 70 years, 66.8%) [17]. However, in this trial, everolimus + exemestane therapy led to a higher rate of AE-related discontinuations in the subset of patients ≥ 70 years old (17.4%) versus those < 70 years old (6.3%). In comparison, reports from studies of CDK4/6 inhibitors in combination with endocrine therapy show similar rates of dose interruptions/reductions and treatment discontinuations in older and younger patient subsets. In PALOMA-1/TRIO-18, 38% of patients ≥ 65 years of age and 39% of patients < 65 years of age receiving endocrine + palbociclib combination treatment had AE-related dose reductions; 16 and 13% of patients, respectively, discontinued study treatment because of AEs [12]. In MONALEESA-2, 71% of patients ≥ 65 years of age and 66% < 65 years of age treated with ribociclib + endocrine therapy had AE-related dose interruptions, 53 and 49% had dose reductions, and 9 and 7% had a permanent treatment discontinuation, respectively [19].
Common AEs (incidence ≥ 35% in either the older or the younger population for the respective study) associated with combination therapy are shown in Table 2. In BOLERO-2, patients ≥ 70 years of age treated with everolimus + exemestane reported a higher incidence of decreased appetite (36 vs. 29%) and a lower incidence of stomatitis (49 vs. 62%) and rash (31 vs. 42%) compared with younger patients [17]. In the MONALEESA-2 trial of ribociclib + letrozole, older patients had higher rates of diarrhea (41 vs. 30%) and vomiting (35 vs. 25%) compared with younger patients [19]. In PALOMA-1, older patients treated with palbociclib + letrozole reported higher rates of neutropenia (81 vs. 70%), leukopenia (54 vs. 35%), fatigue (46 vs. 37%), and anemia (43 vs. 28%) compared with younger patients [12].
Discussion
Understanding how to optimally manage cancer in older patients is increasingly important as the population ages and the prevalence of breast cancer among older patients increases [24]. By 2030, an estimated 57% increase in the number of patients ≥ 65 years of age with breast cancer is expected [24]. Furthermore, because most breast cancers in older patients are HR+ , understanding the efficacy and safety of available endocrine therapy–based treatment options for older patients with breast cancer is crucial for addressing the therapeutic needs of this growing population. In this review, we evaluated the results of available clinical trials investigating combinations of endocrine therapies and targeted therapies in patients with HR+ , HER2− advanced breast cancer with data analyzed in age-stratified subsets [4,5,6, 11,12,13,14,15,16,17,18,19,20,21,22]. Overall, there were similar durations of PFS and reductions in progression risk in older and younger patient populations in the reviewed studies [4, 11,12,13, 15, 17, 19, 20, 23]. Across ages, the greatest reductions in disease progression risk were seen for treatment with CDK4/6 inhibitors (i.e., palbociclib, ribociclib, and abemaciclib) and mTOR inhibitors in combination with endocrine therapies versus endocrine monotherapy [4, 12, 17, 19, 20], and treatment with letrozole + CDK4/6 inhibitors provided the longest duration of PFS relative to endocrine monotherapy [4, 12, 19, 20].
Although it is reassuring that the benefits of combination therapy were observed regardless of age in BOLERO-2; PALOMA-1, -2, and -3; MONALEESA-2; and MONARCH 2, we acknowledge that these older patient subsets may not be fully representative of older breast cancer patients in the general population, as patients with poor performance status or significant comorbidities (both more commonly seen with increasing age) were excluded from enrollment [25, 26]. For example, the reviewed trials required ECOG performance status < 2 or < 3 and excluded patients with comorbidities such as gastrointestinal or cardiac dysfunction or history of malignancies within the past 3–5 years [4, 6, 12, 27,28,29,30,31,32]. As such, the safety and efficacy of combination treatment with endocrine therapies + CDK4/6 inhibitors or mTOR inhibitors in older patients with poor performance status or serious comorbidities is unknown, although the reviewed results do support the efficacy of these agents regardless of age and should help promote comfort with their use in practice.
In the 3 identified trials that reviewed the tolerability of combination treatment versus endocrine monotherapy in older and younger patients, tolerability varied according to treatments received [12, 17, 19]. As expected, endocrine monotherapy was better tolerated than the combination treatments in both age subsets. Reassuringly, the rates of common AEs associated with the addition of CDK4/6 or mTOR inhibition were also similar for older and younger patients. However, it is of note that everolimus-treated older patients were more likely to discontinue study therapy for AEs compared with younger patients, suggesting different thresholds for treatment cessation according to age. Additional efficacy/safety information in older patients with metastatic disease will likely be available from the ongoing MONALEESA-3 and COMPLEEMENT-1 trials of ribociclib + endocrine therapy (fulvestrant and letrozole, respectively) [33, 34]. However, additional trials, such as smaller studies specifically for older populations, may be needed to understand the optimal management of breast cancer in older patients with comorbidities or poor functional status. The current need for more prospective data on optimal treatment of advanced breast cancer in older patients requires a commitment to their enrollment in clinical trials with novel therapeutic regimens. One potential strategy to address this challenge is to mandate extensions of trials that have proven efficacy and tolerability to subsets of populations who are not well represented in initial clinical trial populations (e.g., older patients and underrepresented minority groups). This strategy will require policy changes on a national level, with many calls to action already underway to support such efforts [35]. It is only through robust evidence-based approaches that we can fully understand how to tailor treatment across subgroups and clinical situations.
Despite the limitations of the available data, including limited numbers of older patients across studies and a lack of statistical comparisons for toxicity, the results of the identified trials in this review suggest that combination treatment with endocrine therapy + CDK4/6 or mTOR inhibitors is safe and effective for patients with HR+, HER2− advanced breast cancer regardless of age and should be considered for all patients when clinically appropriate.
References
National Comprehensive Cancer Network (2017) NCCN clinical practice guidelines in oncology: breast cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 12 July 2017
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F et al (2014) ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 23(5):489–502. https://doi.org/10.1016/j.breast.2014.08.009
Baselga J, Campone M, Piccart M, Burris HA III, Rugo HS, Sahmoud T et al (2012) Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med 366(6):520–529
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936. https://doi.org/10.1056/NEJMoa1607303
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S et al (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375(18):1738–1748. https://doi.org/10.1056/NEJMoa1609709
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X et al (2017) MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884
SEER Cancer Stat Facts: female breast cancer. National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/statfacts/html/breast.html. Accessed 25 April 2017
Aapro MS, Köhne C-H, Cohen HJ, Extermann M (2005) Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist 10(3):198–204
Freedman RA, Foster JC, Seisler DK, Lafky JM, Muss HB, Cohen HJ et al (2016) Accrual of older patients with breast cancer to alliance systemic therapy trials over time: protocol A151527. J Clin Oncol 35(4):421–431
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106(5). https://doi.org/10.1093/jnci/dju055
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439. https://doi.org/10.1016/S1470-2045(15)00613-0
Finn RS, Crown JP, Ettl J, Schmidt M, Bondarenko IM, Lang I et al (2016) Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res 18(1):67. https://doi.org/10.1186/s13058-016-0721-5
Hart LL, Souami F, Sutradhar S, Miller M, Germa C, Burris HA (2017) Efficacy and safety of ribociclib (LEE011) + letrozole in elderly patients with hormone receptor–positive, HER2-Negative advanced breast cancer in MONALEESA-2 study. Poster presented at the 34th Annual Miami Breast Cancer Conference, Miami Beach, FL, March 9–12
Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L et al (2013) Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 14(10):989–998. https://doi.org/10.1016/S1470-2045(13)70322-X
Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR et al (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367(5):435–444. https://doi.org/10.1056/NEJMoa1201622
O’Shaughnessy J, Campone M, Brain E, Neven P, Hayes D, Bondarenko I et al (2016) Abiraterone acetate, exemestane or the combination in postmenopausal patients with estrogen receptor-positive metastatic breast cancer. Ann Oncol 27(1):106–113. https://doi.org/10.1093/annonc/mdv487
Pritchard KI, Burris HA 3rd, Ito Y, Rugo HS, Dakhil S, Hortobagyi GN et al (2013) Safety and efficacy of everolimus with exemestane vs. exemestane alone in elderly patients with HER2-negative, hormone receptor-positive breast cancer in BOLERO-2. Clin Breast Cancer 13(6):421–432. https://doi.org/10.1016/j.clbc.2013.08.011
Rugo HS, Turner NC, Finn RS, Joy AA, Verma S, Harbeck N et al (2016) Palbociclib in combination with endocrine therapy in treatment-naive and previously treated elderly women with HR+, HER2− advanced breast cancer: a pooled analysis from randomized phase 2 and 3 studies. Poster presented at the 2016 San Antonio Breast Cancer Symposium, San Antonio, TX, December 6–10
Sonke GS, Hart LL, Campone M, Erdkamp F, Janni W, Verma S et al (2017) Efficacy and safety of ribociclib + letrozole in elderly patients with HR+, HER2- advanced breast cancer in MONALEESA-2. Paper presented at the European Cancer Congress, Amsterdam, The Netherlands, January 27–30
Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H (2015) Palbociclib in Hormone-receptor-positive advanced breast cancer. N Engl J Med 373(3):209–219. https://doi.org/10.1056/NEJMoa1505270
Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, Chow L et al (2013) Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 31(2):195–202. https://doi.org/10.1200/JCO.2011.38.3331
Yardley DA, Noguchi S, Pritchard KI, Burris HA 3rd, Baselga J, Gnant M et al (2013) Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 30(10):870–884. https://doi.org/10.1007/s12325-013-0060-1
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S et al (2017) Updated results from MONALEESA-2, a phase 3 trial of first-line ribociclib + letrozole in hormone receptor-positive (HR+), HER2-negative (HER2−), advanced breast cancer (ABC). J Clin Oncol 35:1038
Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA (2009) Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 27(17):2758–2765
Braithwaite D, Satariano WA, Sternfeld B, Hiatt RA, Ganz PA, Kerlikowske K et al (2010) Long-term prognostic role of functional limitations among women with breast cancer. J Natl Cancer Inst 102(19):1468–1477
Land L, Dalton S, Jensen M, Ewertz M (2012) Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br J Cancer 107(11):1901–1907
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S et al (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738–1748. https://doi.org/10.1056/NEJMoa1609709. https://www.nejm.org/doi/suppl/10.1056/NEJMoa1609709/suppl_file/nejmoa1609709_protocol.pdf. Accessed 13 July 2017
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936. https://doi.org/10.1056/NEJMoa1607303. https://www.nejm.org/doi/suppl/10.1056/NEJMoa1607303/suppl_file/nejmoa1607303_protocol.pdf. Accessed 13 July 2017
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X et al (2017) MONARCH 2: Abemaciclib in combination with fulvestrant in patients with HR +/HER2- advanced breast cancer who progressed on endocrine therapy. J Clin Oncol 35(25):2875–2884. https://doi.org/10.1200/JCO.2017.73.7585. https://ascopubs.org/doi/suppl/10.1200/JCO.2017.73.7585/suppl_file/protocol_2017.737585.pdf. Accessed 18 July 2017
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T et al (2012) Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med 366(6):520–529 https://doi.org/10.1056/NEJMoa1109653. https://www.nejm.org/doi/suppl/10.1056/NEJMoa1109653/suppl_file/nejmoa1109653_protocol.pdf. Accessed 13 July 2017
US National Institutes of Health Palbociclib (PD-0332991) Combined with fulvestrant in hormone receptor + HER2-negative metastatic breast cancer after endocrine failure (PALOMA-3). https://clinicaltrials.gov/ct2/show/NCT01942135. Accessed 13 July 2017
US National Institutes of Health study of Letrozole with or without Palbociclib (PD-0332991) for the first-line treatment of hormone-receptor positive advanced breast cancer. https://clinicaltrials.gov/ct2/show/NCT00721409. Accessed 13 July 2017
US National Institutes of Health study to assess the safety and efficacy of Ribociclib (LEE011) in combination with letrozole for the treatment of men and pre/postmenopausal women with HR+ HER2− aBC. https://clinicaltrials.gov/ct2/show/NCT02941926. Accessed 13 July 2017
US National Institutes of Health study of efficacy and safety of LEE011 in men and postmenopausal women with advanced breast cancer. (MONALEESA-3). https://clinicaltrials.gov/ct2/show/NCT02422615. Accessed 13 July 2017
Hurria A, Dale W, Mooney M, Rowland JH, Ballman KV, Cohen HJ et al (2014) Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 32(24):2587–2594
Acknowledgements
Editorial assistance was provided under the direction of the authors by Jonathan Morgan, Ph.D., and David Boffa, ELS, MedThink SciCom.
Funding
This study was funded by Novartis Pharmaceuticals Corporation. Rachel Freedman receives funding from Susan G. Komen and the American Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rachel Freedman receives research funding (institutional) from Eisai and Puma Biotechnology. Sara Tolaney receives research funding (institutional) from Novartis Pharmaceuticals Corporation, Eli Lilly and Co, Pfizer, Exelixis, Eisai, Merck, Genentech, AstraZeneca, Cyclacel, and Nektar Therapeutics, and she serves as a consultant to Eli Lilly and Co, Novartis Pharmaceuticals Corporation, Pfizer, Eisai, Sanofi, Merck, AstraZeneca, and Nektar Therapeutics. This study was supported by Novartis Pharmaceuticals Corporation.
Informed consent
Not applicable.
Research involving human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Freedman, R.A., Tolaney, S.M. Efficacy and safety in older patient subsets in studies of endocrine monotherapy versus combination therapy in patients with HR+/HER2− advanced breast cancer: a review. Breast Cancer Res Treat 167, 607–614 (2018). https://doi.org/10.1007/s10549-017-4560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4560-6