Abstract
Purpose
Regulatory T cells (Tregs) impair the clinical benefit of cancer immunotherapy. To optimize the antitumor efficacy of therapeutic dendritic cell (DC) vaccines, we aimed to inhibit Foxp3, a transcription factor required for Treg function.
Methods
Mice bearing established syngeneic LM3 and 4T1 breast tumors were treated with antitumor DC vaccines and a synthetic peptide (P60) that has been shown to inhibit Foxp3.
Results
Treatment with P60 improved the therapeutic efficacy of DC vaccines in these experimental models. In addition, monotherapy with P60 inhibited tumor growth in immunocompetent as well as in immuno-compromised animals bearing established tumors. We found expression of Foxp3 in human and murine breast tumor cells. P60 inhibited IL-10 secretion in breast cancer cells that expressed Foxp3.
Conclusions
Our results suggest that Foxp3 blockade improves the therapeutic efficacy of DC vaccines by inhibition of Tregs and through a direct antitumor effect. This strategy could prove useful to neutralize the immunosuppressive microenvironment and to boost antitumor immunity in breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The goal of immunotherapy is to specifically target and eradicate tumor cells by enhancing antitumor immunity. The field of immunotherapy for breast cancer has exponentially grown over the last decade mainly due to the detection of immunomodulators in breast tumors, such as CTLA-4, PD-1, IDO, IL-10, TGF-β, and tumor infiltrating regulatory T cells (Tregs), all of which constitute antitumor therapeutic targets [28]. Dendritic cells (DCs) have been extensively used in the development of antitumor vaccines. Although they trigger antitumor immunity in cancer patients, this response is not enough to induce tumor regression [35]. This lack of efficacy has been related to the expansion of immunosuppressive cells and to the upregulation of immunological checkpoints that generate T cell exhaustion. Indeed, antitumor DC vaccination in cancer patients promotes not only the expansion of effector lymphocytes, but also of Tregs [4, 6, 13, 31, 50, 53].
Tregs, which are the main cellular mediators of peripheral tolerance, play a central role in tumor pathogenesis and immunological escape. In fact, it has been well documented that oncological patients exhibit high levels of circulating Tregs [14, 23]. In breast cancer, both tumor infiltrating and circulating Tregs increase with the stage of the disease [33]. This apparent enrichment is very important because Tregs seem to suppress antitumor effector T cells in a dose-dependent manner [33]. The presence of high proportions of Tregs within tumor infiltrating lymphocytes (TILs) and in particular, the low ratio between CD8+ T cells and Tregs, have been associated with poor prognosis in breast cancer [5, 32].
The expression of the transcription factor Forkhead box P3 (Foxp3) is a distinctive feature of Tregs. Foxp3 is critical for the suppressor activity of Tregs as it triggers a transcriptional program that leads to upregulation of CD25, CTLA-4, and LAG3, and suppresses the expression of proinflammatory genes [55]. Since Foxp3 seems to be specifically expressed in Tregs, it constitutes an attractive target to inhibit Treg function. Because Foxp3 is an intracellular target, it cannot be targeted by antibody-based strategies. Foxp3 can be efficiently blocked with the cell-penetrating peptide (CPP) P60 [9]. CPPs are amphipathic and positively charged peptides with less than 30 aminoacids, which can penetrate cell membranes [30]. P60 is a 15 aminoacid-peptide that binds Foxp3 and inhibits its homodimerization, nuclear translocation as well as its interaction with other transcription factors, i.e. NF-κB, NFAT [9], and AML1/Runx1 [24]. P60 reduces the suppressor activity of murine and human Tregs and improves the efficacy of prophylactic vaccines in murine models of colon cancer [9].
Our previous results indicate that prophylactic antitumor DC vaccines exert antitumor and antimetastatic effects in murine models of breast cancer [29]. However, these vaccines lacked efficacy in therapeutic settings. Thus, we aimed to improve the antitumor effect of DC vaccines using the Foxp3 blocking peptide P60 in HER2+ LM3 tumor-bearing mice and in the 4T1 triple negative breast cancer (TNBC) model, which have been previously shown to respond to immunotherapeutic strategies [1, 22, 51]. Immunocompetent tumor-bearing mice are ideal models for translational immunotherapy because the host immune system is conserved as well as the interactions between host and tumor. Our tumor models also develop spontaneous metastases upon s.c. tumor inoculation, which resembles the physiopathological metastatic process better than the i.v. inoculation of tumor cells [3]. We found that P60 inhibited the expansion of Tregs induced by DC vaccines in immunocompetent mice bearing established breast tumors. Consequently, P60 administration improved the antitumor and antimetastatic effect of therapeutic DC vaccines. Nevertheless, monotherapy with P60 also exerted antitumor efficacy in this setting. Although Foxp3 seems to be a specific marker of Tregs within the immune system, Foxp3 has also been detected in tumor cells in breast cancer specimens [20, 46, 54]. In order to rule out whether the antitumor effect of P60 was an immune-mediated or a direct antitumor effect, we administered monotherapy with P60 into immuno-compromised animals bearing established LM3 tumors, finding that P60 delayed tumor growth in these animals. P60 treatment in vitro reduced IL-10 release from tumor cells that expressed Foxp3. Our results suggest that Foxp3 blockade is a powerful tool to improve the efficacy of antitumor vaccines. Moreover, inhibition of Foxp3 could have a direct impact over tumor Foxp3-expressing cells.
Materials and methods
Drugs
IL-4 and GM-CSF were purchased from BioLegend (Cat# 574304 and 576304, respectively, San Diego, CA). TLR9 agonist CpG1826 oligodeoxynucleotide was purchased from Eurofins Genomics (Louisville, KY).
Animals
Adult female BALB/c and athymic N:NIH Swiss mice (6–8 weeks old) were purchased at the vivarium of Facultad de Ciencias Veterinarias, Universidad Nacional de La Plata, and kept in controlled conditions of light (12 h light–dark cycles) and temperature (20–25 °C). Mice were fed with standard lab chow and water ad libitum and all efforts were made to minimize distress. All animal work was conducted according to the NIH guidelines and was approved by the Institutional Ethical Committee, Facultad de Medicina, Universidad de Buenos Aires (CD Res. Nº120/2011 and 2071/2015).
Synthesis of P60
Therapeutic peptide P60 (RDFQSFRKMWPFFAM) and control peptide P301 (MKMFFDAFPQRRSWF) were synthesized by the solid phase method of Merrifield using the fluorenylmethyloxycarbonyl alternative, as previously described [7]. The purity of peptides was 90% as judged by HPLC.
Breast cancer cell lines
Culture and maintenance of mammary cell lines, as well as ELISA assays, flow cytometry, immunocytochemistry, and cell viability assessment were described in Supplementary Material.
In vivo experimental breast cancer models
BALB/c mice or athymic N:NIH Swiss were inoculated s.c. into the flank with 3 × 105 tumor cells. Tumor size was measured 3 times per week using a caliper. The tumor volume was calculated with the following formula: (width2 × long)/2. When tumors were macroscopic (day 9–18 after tumor inoculation) immunocompetent animals were inoculated s.c. with a DC vaccine, generated as previously described [29] (see Supplementary Data). Tumor-bearing mice received daily i.p. injections of Foxp3 blocking peptide P60 or control peptide P301 (100 µg) for 6–7 days. Mice were monitored daily and when the first signs of distress appeared, they were euthanized by cervical dislocation. Then, lungs were dissected and fixed with Bouin’s fixative solution [71% picric acid (saturated), 24% formaldehyde (37–40%), 5% glacial acetic acid], and spontaneous metastases were counted under a binocular stereoscopic microscope.
In a group of mice, the content of lymphocytes was assessed in spleen and tumor as previously described [8, 29] (see Supplementary Data).
In a group of nude mice, tumors were dissected and Foxp3 content was analyzed by Western blot on the last day of P60 administration (see Supplementary Data).
A group of LM3 and 4T1 tumor-bearing mice were euthanized by terminal perfusion under deep anesthesia with Tyrode solution (NaCl 132 mM, CaCl2 1.8 mM, NaH2PO4 0.32 mM, glucose 5.56 mM, NaHCO3 11.6 mM, and KCl 2.68 mM) followed by 4% PFA. The presence of immune cells was assessed in tumor sections by immunocytochemistry as previously described [8] (see Supplementary Data).
Statistical analysis
Data were graphed and analyzed using GraphPad Prism version 5.00 software (GraphPad Software). The number of lymphocytes and spontaneous metastases per mouse was evaluated by ANOVA followed by Tukey’s test. Tumor growth was analyzed by multiple regression analysis. Kaplan Meier survival curves were analyzed using Mantel log rank test. Percentage of cytoplasmic or nuclear high intensity Foxp3+ cells was analyzed by χ 2 test. Western Blot, MTT assay, IL-10, and BrDU ELISA data were analyzed by Student’s t test. Differences between groups were considered significant when p < 0.05. All the experiments were performed at least twice.
Results
Antitumor DC vaccines induce the expansion of splenic and tumor infiltrating Tregs in murine models of breast cancer
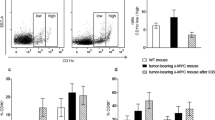
We first evaluated whether a therapeutic DC vaccination protocol would induce the expansion of Tregs in mice bearing an experimental breast cancer model, as it has been described in patients with different types of cancer [6, 13, 31, 53]. Immunocompetent mice were inoculated with syngeneic HER2+ LM3 mammary tumor cells [48] and 20 days later they received a s.c. antitumor DC vaccine loaded with tumor cell lysates and activated with the TLR9 agonist CpG1826 [29] (Fig. 1a). We evaluated the content of lymphocytes in the spleen and the tumor by flow cytometry at different time points after vaccination (Fig. 1b, c). We observed an increase in the number of CD4+ and CD8+ T cells in the spleen at day 3 and 9 after vaccination that was accompanied by a similar increase in the content of Tregs (Fig. 1b). However, the ratio Tregs/CD8+ T cells exhibited a rapid rise at day 3, returning to basal levels at day 9 after DC vaccination. We did not observe differences in the ratio between Tregs/CD4+ cells at any time point. When we evaluated the lymphocyte populations infiltrating the tumor microenvironment, we found that the number of tumor infiltrating Tregs, CD4+ and CD8+ T cells increased along with tumor growth, as it was significantly higher 9 days after DC vaccination (Fig. 1c).
Effect of antitumor DC vaccination on the content of T lymphocytes in spleen and tumor of experimental breast cancer models. a Mice bearing LM3 tumors received a CpG-activated DC vaccine loaded with tumor cell lysates, at day 20 post tumor inoculation. b The number of Tregs, CD4+ and CD8+ T cells was evaluated by flow cytometry in spleen at days 0, 3, and 9 post-vaccination. Ratio of Tregs/CD8+ and Tregs/CD4+ was calculated. *p < 0.05 versus d0, ^ p < 0.05 versus d4 (ANOVA followed by Tukey test), n = 3, Representative dot plots are displayed. The numbers indicate the percentage of each cell population. c Tumor infiltrating lymphocytes (TILs) were evaluated at day 0 and 9 after DC vaccination (left panel). Representative dot plots are shown (right panel). The numbers indicate the percentage of each cell population. *p < 0.05 versus d0 (Student’s t test), n = 3
Efficacy of antitumor DC vaccines in combination with Foxp3 blockade in immunocompetent mice bearing metastatic mammary adenocarcinomas
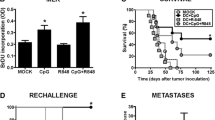
Since our results indicated that antitumor vaccination induced not only the expansion of effector lymphocytes, but also of Tregs, we aimed to improve the efficacy of this therapy by specifically blocking Treg function using the cell-penetrating peptide P60, which binds and inhibits Foxp3 [9]. Immunocompetent mice were inoculated s.c. with syngeneic LM3 breast cancer cells and the characterization of the immune cell infiltrates was performed by immunocytochemistry and flow cytometry. Immune cells were readily detected in LM3 tumor sections by immunofluorescence using specific antibodies against CD45, CD8, and MHCII, indicating that T lymphocytes and antigen presenting cells (APCs) infiltrate these tumors (Fig. 2a). The infiltration of CD4+ T cells and Tregs was assessed by flow cytometry and they accounted for 8% ± 3.2 and 1.6% ± 0.6 of CD45+ tumor-infiltrating cells, respectively (Fig. 2a). When the tumors were macroscopic (day 18), animals were vaccinated with a s.c. injection of DC vaccine loaded with tumor cell lysates and activated with CpG1826 [29]. Mice received a daily injection of therapeutic peptide P60 or control peptide P301 (100 µg/dose) for 6 days starting on the day of vaccination (Fig. 2b). The content of splenic and tumor infiltrating Tregs was evaluated by flow cytometry 3 days after DC vaccination. P60 administration reduced the number of Foxp3+ T cells in spleen and tumor of vaccinated mice (Fig. 2c). When we evaluated the efficacy of the therapy, we found that therapeutic DC vaccines did not significantly impair tumor growth. However, the treatment with P60 alone or in combination with DC vaccine inhibited tumor growth (Fig. 2d). While monotherapy with DC vaccine or with P60 led to long-term survival in 20–30% of mice, combination of DC vaccine with P60 led to 70% long-term survival (Fig. 2e). Although the analysis of spontaneous lung metastases did not show significant differences within experimental groups (Fig. 2f), 3/8 mice treated with vaccine + P60 did not develop lung metastases.
Efficacy of DC vaccination in combination with Foxp3 blockade in mice bearing s.c. syngeneic LM3 mammary tumors. a Representative microphotographs show immune cells as assessed by immunofluorescence in LM3 tumor sections using antibodies against CD45, CD8, and MCH II (left panels). Insets depict higher magnification microphotographs of immunopositive cells. Tumor infiltrating mononuclear cells were purified and CD4 T cells and Tregs were assessed by flow cytometry in the CD45+ population (right panels). Representative dot plots are depicted. The numbers indicate the percentage of each cell population. b Immunocompetent mice bearing LM3 breast tumors received CpG-activated DC vaccine loaded with tumor cell lysate at day 18 post tumor inoculation. Mice received daily i.p. injections of 100 µg Foxp3 blocking peptide (P60) or control peptide (P301) for 6 days starting on the date of DC vaccination. c Spleen and tumor infiltrating Tregs (CD4+/Foxp3+) were evaluated 3 days after vaccination. *p < 0.05 versus P301; ^ p < 0.05 versus vaccine + P301 (ANOVA followed by Tukey test), n = 4–5. d Tumor growth was measured with caliper 3 times a week. *p < 0.05 versus control + P301; ^ p < 0.05 versus vaccine + P301, (Multiple regression analysis) n = 9–10. e Kaplan Meyer survival curves. *p < 0.05 versus P301; ^ p < 0.05 versus vaccine + P301 (Log Rank test), n = 9–10. f Spontaneous lung metastases (ANOVA followed by Tukey test)
Next, we assessed the efficacy of the combination of DC vaccines and Foxp3 blockade in the TNBC model 4T1. Immunocompetent mice were s.c. inoculated with syngeneic 4T1 cells and the immune cell infiltrates were assessed by immunocytochemistry and flow cytometry. Profuse infiltration of T lymphocytes was detected in these tumors (Fig. 3a). APCs were also found, although at a lesser extent. CD4+ T cells and Tregs were detected by flow cytometry, accounting for 23% ± 6.4 and 5% ± 1.6 of CD45+ tumor-infiltrating cells, respectively (Fig. 3a). When the tumors were macroscopic (day 9), mice were inoculated with antitumor DC vaccine. Animals received daily i.p. injections of therapeutic or control peptides for 7 days. We observed that monotherapy with DC vaccines or with P60 reduced tumor growth in comparison with the control group treated with P301 peptide. However, combination of DC vaccination with P60 exhibited higher efficacy than the monotherapies, exerting a more powerful inhibition of tumor growth (Fig. 3b). Moreover, only the combined therapy improved the survival of tumor-bearing mice (Fig. 3c) and reduced the number of spontaneous lung metastasis when compared to control mice (Fig. 3d).
Efficacy of DC vaccination and Foxp3 blockade in 4T1 breast tumor model. a Representative microphotographs show immune cells in 4T1 tumor sections as assessed by immunofluorescence using antibodies against CD45, CD8, and MCH II (left panels). Insets depict higher magnification microphotographs of immunopositive cells. Tumor infiltrating mononuclear cells were purified and CD4+ T cells and Tregs were assessed by flow cytometry in the CD45+ population (right panels). Representative dot plots are depicted. The numbers indicate the percentage of each cell population. b Immunocompetent mice bearing syngeneic 4T1 breast tumors received CpG-activated DC vaccine loaded with tumor cell lysate at day 10 post tumor inoculation. Mice received daily i.p. injections of 100 µg Foxp3 blocking peptide (P60) or control peptide (P301) for 6 days starting on the date of DC vaccination. Tumor growth was measured with caliper 3 times a week. *p < 0.05 versus control + P301; ^ p < 0.05 versus vaccine + P301 (Multiple regression analysis) n = 6. c Kaplan Meyer survival curves. *p < 0.05 versus control + P301 (Log Rank test). d Spontaneous lung metastases. *p < 0.05 versus P301 (ANOVA followed by Tukey test)
Expression and function of Foxp3 in murine mammary carcinoma cell lines
Our experiments in vivo indicated that monotherapy P60 exerts antitumor effects. Considering that this effect could be related to an inhibitory effect of P60 on Tregs or could be a direct effect on tumor cells, we aimed to evaluate the expression and function of Foxp3 in murine breast tumor cells. We evaluated the expression of Foxp3 in murine LM3 and 4T1 cell lines by immunofluorescence (Fig. 4a) and flow cytometry (Fig. 4b). We found that although both murine cell lines expressed Foxp3, LM3 cells exhibited higher nuclear and cytoplasmic expression of Foxp3 when compared to 4T1 cells (Fig. 4c). We then evaluated the effect of Foxp3 blockade on the viability and proliferation of LM3 and 4T1 cells in vitro. Cells were incubated with the therapeutic peptide P60 or with the control peptide P301 for 24 h. During which viability and proliferation were evaluated by MTT and BrDU incorporation, respectively (Fig. 5a, b). We observed that P60 reduced the viability (Fig. 5a) and the proliferation (Fig. 5b) of LM3 cells in about 25%, whereas we did not find any effect over 4T1 cells. Taking into account that Foxp3 modulates the production of IL-10 in Tregs, a key cytokine in the maintenance of an immunosuppressive tumor microenvironment [39], we evaluated the effect of P60 on IL-10 secretion in LM3 and 4T1 cells by ELISA. P60 exerted a robust inhibitory effect on IL-10 production from LM3 cells, without affecting 4T1 cells (Fig. 5c).
Foxp3 expression in murine breast cancer cells. Expression of Foxp3 was assessed in LM3 (left panel) and 4T1 cells (right panel) by indirect immunofluorescence (a) and flow cytometry (b). Representative microphotographs show Foxp3 expression (green). Nuclei were counterstained with DAPI (blue). Representative histograms are shown (isotype is depicted in gray). c Percentage of cytoplasmic or nuclear high intensity Foxp3 staining was determined using ImageJ. *p < 0.05 (χ 2 test)
Foxp3 blockade in murine breast cancer cell lines in vitro. LM3 (left panel) and 4T1 (right panel) cells were cultured in the presence of 50 µM control peptide P301 or Foxp3 blocking peptide (P60) for 24 h. a Viability was evaluated by MTT assay. b Proliferation was measured by BrDu incorporation and assessed by ELISA. c The content of IL-10 was evaluated in cell culture supernatants by ELISA. *p < 0.05 versus P301 (Student’s t test)
Direct antitumor effect of Foxp3 blockade in immunosuppressed breast tumor models
Considering that P60 exerted a direct antitumor effect on the murine LM3 mammary adenocarcinoma cells in vitro, we next evaluated the efficacy of Foxp3 blockade in an immunosuppressed in vivo tumor model that would allow us to discard the effect of P60 on the immune system. We inoculated LM3 tumors in nude mice, which lack Tregs and all the components of a functional T lymphocyte system [36]. When the tumors were detected (day 10), animals started receiving daily i.p. injections of P60 or control peptide for 7 days (Fig. 6a). At the last day of treatment, we evaluated the content of Foxp3 in the tumor by western blot. Treatment with P60 significantly reduced tumor content of Foxp3 (Fig. 6b). As shown in Fig. 6c, Foxp3 blockade inhibited tumor growth compared to control animals. However, we did not observe an increase in the overall survival of nude mice treated with P60 (Fig. 6d).
Foxp3 blockade in immunosuppressed mice bearing LM3 tumors. a Nude mice were inoculated with murine LM3 mammary cancer cells. At day 10 post tumor inoculation, mice started receiving daily i.p. injections of 100 µg Foxp3 blocking peptide (P60) or control peptide (P301) for 7 days. b Tumor Foxp3 protein levels were evaluated by western blot at day 16 post tumor inoculation. Representative blots are displayed. *p < 0.05 (Student’s t test). c Tumor growth was measured with caliper 3 times a week. *p < 0.05 (Multiple regression analysis) n = 12. d Kaplan Meyer survival curves
Evaluation of Foxp3 expression and function in human breast cell lines
In order to evaluate the translational value of our observations in murine models of breast cancer, we evaluated the expression and function of Foxp3 in human breast carcinoma cell lines. We assessed Foxp3 expression by immunofluorescence in MCF7 cells, a tumor cell line that expresses ER, PR, and HER2 [43] and MDA-MB-231 cells, as a model of triple negative human tumors [43] by immunofluorescence. We also evaluated Foxp3 expression in MCF-10 cells, a human non-tumoral mammary epithelial cell line [42]. We found expression of Foxp3 in MCF7 cells, but not in MDA-MB-231 (Fig. 7a) or MCF-10 cells (not shown). Accordingly, when we incubated MCF7 and MDA-MB-231 cells in the presence of P60 for 24 h, we observed a 20% decrease in the viability of MCF7, without changes in MDA-MB 231 cells (Fig. 7b).
Foxp3 in human breast cancer cells. a Expression of Foxp3 was assessed in MCF7 and MDA-MB-231 cells by immunofluorescence. Representative microphotographs show Foxp3 expression (green). Nuclei were counterstained with DAPI (blue). b MCF7 and MDA-MB-231 cells were incubated in presence of 50 µM control peptide (P301) or Foxp3 blocking peptide (P60) for 24 h. Viability was assayed by MTT. *p < 0.05 versus P301 (Student’s t test)
Discussion
The expansion of Tregs has been proposed as one of the mechanisms that impair the efficacy of antitumor vaccines [13, 31]. We found that DC vaccination not only increased the levels of CD4+ and CD8+ T cells, but also induced the expansion of Tregs in spleen and tumor, with a rapid increase in the ratio of splenic Tregs/CD8+ T cells. The expansion of Tregs following DC vaccination has been involved in the reduced efficacy of these vaccines in patients with renal and cervical cancer [6, 53]. Expansion of Tregs has also been involved in the lack of efficacy of tumor peptide vaccines in melanoma patients [31] and subsets of these cells exhibit robust antigen-specific immunosuppressive activity [13]. Thus, blockade of Tregs arises as an essential step to improve the clinical benefit of antitumor vaccines.
Several approaches have been employed to deplete Tregs in breast cancer patients undergoing vaccination protocols [28], such as daclizumab, a humanized antibody against IL-2 receptor CD25 [37] and metronomic therapy with cyclophosphamide [41, 52]. However, these strategies also entail disadvantages. The systemic depletion of Tregs entails the risk of developing autoimmune lesions [18, 19]. In addition, anti-CD25 antibodies could additionally target effector T cells that transiently express CD25 during activation, which has recently led to the development of newer generation anti-CD25 antibodies [2]. Also, long-term metronomic therapy can lead to an accumulation of chemotherapeutic drugs, increasing the risk of secondary neoplasias [28]. Here, we found that the systemic administration of P60 inhibits the expansion of splenic and tumor-infiltrating Tregs induced by DC vaccination in mice bearing experimental breast tumors. Accordingly, P60 administration improved the therapeutic efficacy of antitumor DC vaccines in the tumor models tested, leading to inhibition of tumor growth and metastasis development, as well as long-term survival. P60 has been previously shown to enhance the immune response of mice bearing CT26 colon cancer that received prophylactic peptide vaccines [9]. It is worth mentioning that P60 allows the blockade of Foxp3 without depleting the cells [9], which is an advantageous feature since it has been shown that depletion of Tregs is counteracted by conversion of CD4+ T cells to CD4+CD25+Foxp3+ in a homeostatic mechanism that restores de immune-tolerance [49]. In addition, drugs designed to block Foxp3 must be able to penetrate the tumor microenvironment and the cell membrane [25], which constitutes a challenge for antibody-based strategies [34].
The tumor models used in our study have been previously shown to respond to immunotherapeutic strategies. LM3 tumor-bearing mice responded to attenuated Salmonella vaccine with an antitumor Th1-type response characterized by the expansion of IFN-γ-secreting T cells and a reduction in the number of Tregs in the draining lymph nodes [51]. In addition, the inhibition of Treg expansion in LM3 tumor-bearing mice has been proposed to mediate the antitumor immunomodulatory effect of type 2 ribosome-inactivating protein Pulchellin [11]. IL-12-producing tumor cell vaccines also induced a tumor-specific Th1 response, leading to antitumor efficacy and CD8+ T cell-mediated immunological memory in LM3 tumor-bearing mice [1]. Our previous findings indicate that prophylactic DC vaccines exert antitumor and antimetastatic efficacy in mice bearing LM3 tumors, which develop long-term antitumor immunological memory [29]. The 4T1 tumor model has been extensively employed for the preclinical evaluation of antitumor immunotherapy. 4T1 is considered an immunogenic tumor model, because it exhibits upregulation of T cell and DC activation markers [22]. This immunogenic profile correlates with a strong response to immunotherapeutic strategies, such as administration of tumor targeted chemokine CCL16 combined with metronomic chemotherapy and antitumor DC vaccines, when evaluated within a panel of high and low immunogenic tumor models [22]. T cell-mediated antitumor efficacy has also been reported in 4T1 tumor-bearing mice treated by attenuated Listeria vaccines [21] and irradiated tumor cell vaccines [45].
When we evaluated the immune cell infiltrates, we found that all immune cell populations studied were present in tumor samples from both breast tumor models. The presence of TILs in breast tumors has been lately considered an indicator of immunogenicity and warrants the use of immunotherapeutic approaches in such patients [28]. When we assessed the efficacy of antitumor vaccines combined with Foxp3 blockade, higher antitumor efficacy was achieved in LM3 tumor-bearing mice than in animals bearing 4T1 tumors. We have previously reported that LM3 tumor-bearing mice also respond better than 4T1 tumor-bearing mice to prophylactic antitumor vaccines [29]. Since clinical investigation has lately shown that breast tumors that are infiltrated with T lymphocytes and Tregs have increased expression of immunological checkpoint PD-L1 [28], it is likely that its inhibition using blocking antibodies would further improve the efficacy of antitumor vaccines combined with P60 administration. 4T1 tumor cells up-regulate PD-L1 during tumor progression [16] and treatment of 4T1 tumor-bearing mice with specific anti-PD-L1 antibodies leads to an expansion of tumor antigen specific T cells accompanied by tumor regression when combined with radiotherapy [40] and antibodies with agonistic activity towards the T cell costimulatory molecule CD137 [16]. In addition, other immune cell populations have also been implicated in the reduced efficacy of immunotherapeutic strategies in 4T1 tumor models. It has been described that myeloid-derived suppressor cells facilitate 4T1 tumor progression [10], and thus, this cell population could constitute an additional therapeutic target to improve the therapeutic efficacy of antitumor vaccines and other immunotherapeutic approaches. Expression of immunosuppressive molecules has also been detected in 4T1 tumors, such as IDO [22], which is upregulated by IFN-gamma [17]. In fact, IDO blockade leads to synergistic antitumor effect when combined with immunological checkpoint blockade in 4T1 tumor-bearing mice [17]. Taken together, these findings support the notion that combinatorial immunotherapeutic approaches that boost antitumor immunity while inhibiting immunosuppressive targets are required for antitumor efficacy in breast cancer.
Monotherapy with P60 inhibited tumor growth in all the tumor models evaluated. The antitumor effect of P60 can be explained, at least in part, by the blockade of Tregs that infiltrate the tumors in immunocompetent mice [34]. In addition, our findings indicate that P60 could exert a direct inhibitory effect on tumor cells that express Foxp3. Tumor cells seem to resemble Tregs in terms of their expression of Foxp3 and their ability to secrete IL-10, one of the main immunosuppressive cytokines in the tumor microenvironment. In LM3 cells, IL-10 secretion was inhibited by 80% when Foxp3 was blocked with P60. Tallying with our findings, a correlation between the expression levels of Foxp3 and immunosuppressive cytokines, including IL-10 and TGF-β, has been reported in human cell lines of colon and breast cancer, melanoma, and acute erythroid leukemia [20]. It is interesting to mention that Hinz et al. showed that Foxp3-expressing human pancreatic cell lines are able to inhibit the proliferation of anti-CD3/anti-CD28-activated T cells [15]. All these results suggest that tumor cells can resemble Tregs, and that blocking tumor Foxp3 may inhibit the maintenance of an immunosuppressive tumor microenvironment.
Lately, Foxp3 has been detected in different types of tumor cells and cancer specimens. Although there is enough consensus on the role of Foxp3 in normal mammary gland, where Foxp3 appears to act as a tumor suppressor factor [38], its function in cancer cells remains controversial. Preclinical studies suggest that Foxp3 plays a tumor suppressor role in breast, prostate, and ovarian cancer [20]. However, the expression of Foxp3 in tumor cells has also been postulated as a mechanism that facilitates the progression of various types of tumors. Foxp3 expression correlates with tumor growth in preclinical models of melanoma [27]. In addition, the transcriptional inhibition of Foxp3 in melanoma cells reduces tumor growth and inhibits the maintenance of the immunosuppressive microenvironment [12, 47]. Foxp3 expression in pancreatic carcinoma cells has been involved in the suppression of effector T cells [15]. Analysis of human carcinoma biopsies has found a strong association between the expression of Foxp3 in tumor cells and poor prognosis [54]. Foxp3 expression in breast cancer biopsies has also been associated with worse chance of overall survival [26]. These authors proposed that the expression of Foxp3 could be linked to the metastatic potential of the tumor and not to suppression of antitumor immunity [26]. On the other hand, our findings indicate that P60 treatment transiently inhibits tumor growth, but it does not improve long-term survival in nude mice, an effect that it was observed in immunocompetent mice. This observation suggests that the immune-mediated effects of this therapy are required to achieve appropriate antitumor efficacy.
The cellular location of Foxp3 has been proposed to ultimately determine if this transcription factor indicates good or bad prognosis in cancer patients [44]. Further studies are required to understand the role of Foxp3 in the pathogenesis of breast cancer, such as its complete transcriptional network in tumor cells. The evidence that blocking this transcription factor with shRNA [12, 47] or P60 leads to direct antitumor effects in vitro and in preclinical in vivo cancer models, suggests that Foxp3 blockade could hold therapeutic potential in Foxp3-expressing tumors. The fact that tumor cells express Foxp3 suggests caution when analyzing Foxp3 expression in tumor specimens. Increased levels of Foxp3 mRNA in tumor biopsies may not only reflect an influx of Tregs, but also an increase in the expression of Foxp3 in tumor cells. Also, therapies that target Foxp3 can also affect the tumor cells themselves, in which Foxp3 function seems to depend on the type of tumor [25].
Our findings suggest that Foxp3 blockade with P60 could neutralize the immunosuppressive tumor microenvironment and boost antitumor immunity. Thus, this strategy could improve the therapeutic efficacy of antitumor vaccines as well as other immunotherapeutic approaches that are counteracted by Treg-mediated immunosuppression.
References
Adris S et al (2000) Mice vaccination with interleukin 12-transduced colon cancer cells potentiates rejection of syngeneic non-organ-related tumor cells. Can Res 60:6696–6703
Arce Vargas F et al (2017) Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 46:577–586. doi:10.1016/j.immuni.2017.03.013
Asad AS et al (2017) Viral gene therapy for breast cancer: progress and challenges. Expert Opin Biol Ther 17:945–959. doi:10.1080/14712598.2017.1338684
Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM (2006) Expansion of FOXP3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood 108:2655–2661. doi:10.1182/blood-2006-03-011353
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380. doi:10.1200/JCO.2006.05.9584
Berntsen A, Brimnes MK, thor Straten P, Svane IM (2010) Increase of circulating CD4+CD25highFoxp3+ regulatory T cells in patients with metastatic renal cell carcinoma during treatment with dendritic cell vaccination and low-dose interleukin-2. J Immunother 33:425–434. doi:10.1097/CJI.0b013e3181cd870f
Borras-Cuesta F et al (1991) Insights on the amino acid side-chain interactions of a synthetic T-cell determinant. Biologicals 19:187–190
Candolfi M et al (2011) B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia 13:947–960
Casares N et al (2010) A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. J Immunol 185:5150–5159. doi:10.4049/jimmunol.1001114
Condamine T, Ramachandran I, Youn JI, Gabrilovich DI (2015) Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 66:97–110. doi:10.1146/annurev-med-051013-052304
de Matos DC, de Ribeiro LC, Tansini A, Ferreira LS, Placeres MC, Colombo LL, Carlos IZ (2012) Immunological response in mice bearing LM3 breast tumor undergoing pulchellin treatment. BMC Complement Altern Med 12:107. doi:10.1186/1472-6882-12-107
Franco-Molina MA et al (2016) Silencing of Foxp3 delays the growth of murine melanomas and modifies the tumor immunosuppressive environment. Onco Targets Ther 9:243–253. doi:10.2147/OTT.S90476
Francois V et al (2009) The CD4(+) T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Can Res 69:4335–4345. doi:10.1158/0008-5472.CAN-08-3726
Gobert M et al (2009) Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Can Res 69:2000–2009. doi:10.1158/0008-5472.CAN-08-2360
Hinz S et al (2007) Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Can Res 67:8344–8350. doi:10.1158/0008-5472.CAN-06-3304
Hirano F et al (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Can Res 65:1089–1096
Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP (2013) Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210:1389–1402. doi:10.1084/jem.20130066
Jacob JB, Kong YC, Meroueh C, Snower DP, David CS, Ho YS, Wei WZ (2007) Control of Her-2 tumor immunity and thyroid autoimmunity by MHC and regulatory T cells. Can Res 67:7020–7027. doi:10.1158/0008-5472.CAN-06-4755
Jacob JB, Kong YC, Nalbantoglu I, Snower DP, Wei WZ (2009) Tumor regression following DNA vaccination and regulatory T cell depletion in neu transgenic mice leads to an increased risk for autoimmunity. J Immunol 182:5873–5881. doi:10.4049/jimmunol.0804074
Karanikas V et al (2008) Foxp3 expression in human cancer cells. J Transl Med 6:19. doi:10.1186/1479-5876-6-19
Kim SH, Castro F, Paterson Y, Gravekamp C (2009) High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Can Res 69:5860–5866. doi:10.1158/0008-5472.CAN-08-4855
Lechner MG et al (2013) Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother 36:477–489. doi:10.1097/01.cji.0000436722.46675.4a
Liyanage UK et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
Lozano TGM, Lasarte-Cía A, Ruiz M, Rabal O, Oyarzabal J, Hervás-Stubbs S, Llopiz D, Sarobe P, Prieto J, Casares N, Lasarte JJ (2017) Blockage of FOXP3 transcription factor dimerization and FOXP3/AML1 interaction inhibits T regulatory cell activity: sequence optimization of a peptide inhibitor. Oncotarget. doi:10.18632/oncotarget.17845
Lu H (2009) FOXP3 expression and prognosis: role of both the tumor and T cells. J Clin Oncol 27:1735–1736. doi:10.1200/JCO.2008.20.0675
Merlo A et al (2009) FOXP3 expression and overall survival in breast cancer. J Clin Oncol 27:1746–1752. doi:10.1200/JCO.2008.17.9036
Miranda-Hernandez DF et al (2013) Expression of Foxp3, CD25 and IL-2 in the B16F10 cancer cell line and melanoma is correlated with tumor growth in mice. Oncol Lett 6:1195–1200. doi:10.3892/ol.2013.1526
Moreno Ayala MA, Gottardo MF, Asad AS, Zuccato C, Nicola A, Seilicovich A, Candolfi M (2017) Immunotherapy for the treatment of breast cancer. Expert Opin Biol Ther. doi:10.1080/14712598.2017.1324566
Moreno Ayala MA et al (2017) Dual activation of Toll Like Receptors 7 and 9 impairs the efficacy of antitumor vaccines in murine models of metastatic breast cancer. J Cancer Res Clin Oncol. doi:10.1007/s00432-017-2421-7
Morris MC, Deshayes S, Heitz F, Divita G (2008) Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell 100:201–217. doi:10.1042/BC20070116
Nicholaou T et al (2009) Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res 15:2166–2173. doi:10.1158/1078-0432.CCR-08-2484
Nishikawa H, Sakaguchi S (2014) Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 27:1–7. doi:10.1016/j.coi.2013.12.005
Oleinika K, Nibbs RJ, Graham GJ, Fraser AR (2013) Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol 171:36–45. doi:10.1111/j.1365-2249.2012.04657.x
Pallasch CP et al (2014) Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell 156:590–602. doi:10.1016/j.cell.2013.12.041
Palucka K, Banchereau J (2012) Cancer immunotherapy via dendritic cells. Nat Rev Cancer 12:265–277. doi:10.1038/nrc3258
Pelleitier M, Montplaisir S (1975) The nude mouse: a model of deficient T-cell function. Methods Achiev Exp Pathol 7:149–166
Rech AJ, Vonderheide RH (2009) Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci 1174:99–106. doi:10.1111/j.1749-6632.2009.04939.x
Recouvreux MS et al (2016) RUNX1 and FOXP3 interplay regulates expression of breast cancer related genes. Oncotarget 7:6552–6565. doi:10.18632/oncotarget.6771
Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR (2011) Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res 51:170–182. doi:10.1007/s12026-011-8262-6
Sharabi AB et al (2015) Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res 3:345–355. doi:10.1158/2326-6066.CIR-14-0196
Soriano JL et al (2011) Metronomic cyclophosphamide and methotrexate chemotherapy combined with 1E10 anti-idiotype vaccine in metastatic breast cancer. Int J Breast Cancer 2011:710292. doi:10.4061/2011/710292
Soule HD et al (1990) Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Can Res 50:6075–6086
Subik K et al (2010) The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer 4:35–41
Takenaka M et al (2013) FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol 1:625–632. doi:10.3892/mco.2013.107
Tkach M et al (2012) Targeting Stat3 induces senescence in tumor cells and elicits prophylactic and therapeutic immune responses against breast cancer growth mediated by NK cells and CD4+ T cells. J Immunol 189:1162–1172. doi:10.4049/jimmunol.1102538
Triulzi T, Tagliabue E, Balsari A, Casalini P (2013) FOXP3 expression in tumor cells and implications for cancer progression. J Cell Physiol 228:30–35. doi:10.1002/jcp.24125
Tsai BY, Suen JL, Chiang BL (2010) Lentiviral-mediated Foxp3 RNAi suppresses tumor growth of regulatory T cell-like leukemia in a murine tumor model. Gene Ther 17:972–979. doi:10.1038/gt.2010.38
Urtreger A, Ladeda V, Puricelli L, Rivelli A, Vidal M, Delustig E, Joffe E (1997) Modulation of fibronectin expression and proteolytic activity associated with the invasive and metastatic phenotype in two new murine mammary tumor cell lines. Int J Oncol 11:489–496
Valzasina B, Piconese S, Guiducci C, Colombo MP (2006) Tumor-induced expansion of regulatory T cells by conversion of CD4+ CD25- lymphocytes is thymus and proliferation independent. Can Res 66:4488–4495. doi:10.1158/0008-5472.CAN-05-4217
Vasir B et al (2008) Fusions of dendritic cells with breast carcinoma stimulate the expansion of regulatory T cells while concomitant exposure to IL-12, CpG oligodeoxynucleotides, and anti-CD3/CD28 promotes the expansion of activated tumor reactive cells. J Immunol 181:808–821
Vendrell A et al (2011) A novel Salmonella Typhi-based immunotherapy promotes tumor killing via an antitumor Th1-type cellular immune response and neutrophil activation in a mouse model of breast cancer. Vaccine 29:728–736. doi:10.1016/j.vaccine.2010.11.017
Wang X et al (2016) Prospective study of cyclophosphamide, thiotepa, carboplatin combined with adoptive DC-CIK followed by metronomic cyclophosphamide therapy as salvage treatment for triple negative metastatic breast cancers patients (aged < 45). Clin Transl Oncol 18:82–87. doi:10.1007/s12094-015-1339-2
Welters MJ et al (2008) Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 14:178–187. doi:10.1158/1078-0432.CCR-07-1880
Wolf D et al (2005) The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 11:8326–8331. doi:10.1158/1078-0432.CCR-05-1244
Xie X, Stubbington MJ, Nissen JK, Andersen KG, Hebenstreit D, Teichmann SA, Betz AG (2015) The regulatory T cell lineage factor Foxp3 regulates gene expression through several distinct mechanisms mostly independent of direct DNA binding. PLoS Genet 11:e1005251. doi:10.1371/journal.pgen.1005251
Acknowledgements
This work was supported by Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET-PIP114-201101-00353 to M.C.; PIP11220120100261 to A.S.); Doctoral Fellowship to M.A.M.A., M.F.G. and A.S.A.); ANPYCT (PICT-2012-0830; PICT-2013-0310, PICT-2015-3309 to M.C.; PICT 2014-0334 to A.S.); Fundación Bunge y Born (“Jorge Oster” fellowship to M.A.M.A) and LALCEC fellowship to M.A.M.A. and by grants from Ministerio de Educación y Ciencia de España (SAF2016-78568-R), Fundación Ramón Areces and Gobierno de Navarra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moreno Ayala, M.A., Gottardo, M.F., Imsen, M. et al. Therapeutic blockade of Foxp3 in experimental breast cancer models. Breast Cancer Res Treat 166, 393–405 (2017). https://doi.org/10.1007/s10549-017-4414-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4414-2