Abstract
Purpose
To report our experience with full-dose 21 Gy IORT in early breast cancer patients after breast-conserving surgery to define most important selection factors.
Methods
Seven hundred and fifty eight patients, subjected to conserving surgery and IORT, were retrospectively analyzed evaluating most important clinical outcomes.
Results
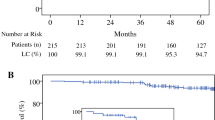
Median follow up was 5.2 years. Results from Cox analyses defined 2 groups of patients, “suitable” (age > 50 years, non lobular histology, tumour size ≤ 2 cm, pN0 or pNmic, ki67 ≤ 20%, non triple negative receptor status and G1-G2) and “unsuitable” for IORT, with a higher rate of breast related events moving from “suitable” to “unsuitable” group. The 5 year rate of IBR is 1.8% in suitable group with significant differences versus unsuitable (1.8 vs. 11.6%, p < 0.005). Same differences between two groups were evidenced in true local relapse (0.6 vs. 6.9%, p < 0.005) and in new ipsilateral BC (1.1 vs. 4.7%, p < 0.015).
Conclusions
In our current practice we consider the following preoperative factors to select patients suitable for IORT: age > 50 years, absence of lobular histology, tumor size ≤ 2 cm, pN0 or pNmic, according to APBI consensus statement, including also ki67 ≤ 20%, non triple negative receptor status and G1–G2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whole breast radiotherapy (WBRT) after breast-conserving surgery (BCS) is currently the standard of care in patients with early breast cancer (BC) younger than age 70 [1], with a 5 year local recurrence of around 5–6% [2,3,4], while for those over age 70, the standard of care is represented by BCS with or without WBRT, evaluating carefully factors related to prognostic risk [5]. Postoperative adjuvant RT is generally delivered with a 5–6 weeks’ conventional treatment. Despite the possibility to shorten the therapy duration with hypofractionated schedules [6], most of patients still undergo conventional external beam RT (EBRT) for about 30 consecutive days. Problems concerning the distance from RT centers and difficulties, such as comorbidities, to attend a daily treatment, are related to the omission of adjuvant RT in a few cases or to the choice of mastectomy to avoid postoperative RT.
During the last few years, the literature evidenced that most of local relapses are at the level of the same quadrant of the original tumor [7]. The use of accelerated partial breast irradiation (APBI) with a single session of intraoperative radiotherapy (IORT) can offer an alternative to EBRT in selected patients with the following advantages: avoidance of a long treatment duration, without waiting between surgery and radiotherapy; delineation of the tumor bed under direct visual and palpable evaluation; reduction of radiation-induced toxicity [7, 8]. Also in a subgroup of patients, such as the elderly, in which the use of postoperative RT is still controversial [9], full-dose IORT could represent an alternative to the omission of RT after BCS.
We present the results of a median 5.2 years follow-up in patients with early breast cancer after breast-conserving surgery and IORT after a stratification of all patients according to Groupe Européen de Curiethérapie-European Society for Therapeutic radiology and Oncology (GEC-ESTRO) and American Society for Radiation Oncology (ASTRO) recommendations, and results were compared and discussed. Moreove,r we considered other factors not included in previous recommendations, to access the possibility to get more reliable selection, based only on prognostic factors for patients eligible for IORT.
Materials and methods
From February 2006 to January 2016, 772 IORT procedures were performed on 758 patients at Papa Giovanni XXIII Hospital (ASST-PG23), Bergamo (Italy).
Median age was 64 (range 42–84 years).
Breast cancer was classified according to the AJCC TNM classification [10] of malignant tumors. Primary tumor size and focality were evaluated microscopically analyzing parenchima between foci. Histology and tumor grade were defined according to WHO classification [11] and the Nottingham Grading System (NGS) [12], respectively. Lympho-vascular invasion (LVI) according to Rosen’s criteria [13] was described as focal or diffuse. Regarding the presence of intraductal component (EIC), patients were divided into three groups: absent, present in a percentage <25% (focal or reduced component), and present in a percentage >25%. For Estrogen (ER) and Progesteron (PR) receptors, status was considered as two groups according to immunohistochemistry [14]: positive or negative (<10%). HER2 immunohistochemistry (IHC) scores of 0 and 1+ were considered negative. HER2 IHC 3+, and fluorescent in situ hybridization (FISH)-amplified tumors were considered positive. All IHC 2+ tumors were tested for gene amplification by FISH [15]. Concerning mitotic index Ki-67, we used a validated cutoff value according to St Gallen International Expert Consensus on primary therapy in early BC 2015 [16], and we distinguished two groups: positive (>20%) and negative (≤20%).
Finally, surgical margins were divided into three categories: negative (a layer of 2 mm from inked surface free from malignant cells), close < 2 mm (the presence of malignant cells between 1 and 2 mm from the inked margin), and positive (malignant cells in the inked margin).
For most of patients (n = 637), quadrantectomy with sentinel node biopsy alone was performed, while in 135 patients, axillary dissection was executed.
All patients underwent electron IORT (ELIOT) at the level of tumor bed. It was performed by a dedicated linear accelerator NOVAC 7 HITESYS (NRT, Italy). The single full-dose of 21 Gy was prescribed at 90%. The energy was 9 MeV (D selector) for all patients. Beam collimation was performed using Perspex cylindrical applicators mounted in exit window head. Collimator diameters were 4 cm (n = 294), 5 cm (n = 431), and 6 cm (n = 47). A simple and useful solution was developed to guarantee an optimal treatment setup: a plex disk (diameter 2 cm larger than the correspondent applicator), positioned between the inferior part of the applicator and target volume, makes the tissue more uniform and compact, permitting an easy determination of treatment volume thickness. Moreover, a dosimetric build-up effect was obtained. To verify in real time the accuracy in dose delivering, in order to define an action level, micro-mosfet detectors were used for in vivo dosimetry. The mosfet was sandwiched between the disk and the target surface.
A the end of the procedure, four clips were inserted in breast tissue along the circumference of the applicator and one at the level of the deep margin, to distinguish the in-breast tumor recurrences (IBTR) between the true local relapse (in-RT field) and the new ipsilateral recurrence (out-RT field).
Ten patients with synchronous and three with metachronous breast cancer underwent bilateral surgery followed by IORT. For one patient, ipsilateral metachronous surgery and IORT were performed.
Patients with pathological positive lymph nodes, after surgery, underwent systemic therapy.
We retrospectively stratified all patients according to GEC-ESTRO [17] and ASTRO [18] classifications. Patients were divided into three groups: for GEC-ESTRO (Table 1), “low-risk,” “intermediate risk,” and “high risk”; and for ASTRO (Table 2), “suitable,” “cautionary,” and “unsuitable.”
We evaluated the following outcomes [19]: IBTR—defined as any local relapse (LR) within the treated breast, including both true local (in the same quadrant of the primary tumor) and new ipsilateral recurrence (in quadrants other than that primarily involved); regional lymph node failure (RNF)—defined as any recurrence at the level of the ipsilateral axillary, supraclavicular, and internal mammary node areas; distant metastases (DM)—defined as any recurrence at the level of organs or structures different from ipsilateral or contralateral breast; cause-specific survival (CSS) and overall survival (OS), assessed from the date of surgery until death related to breast tumor and to the last follow-up or time of death, respectively.
For the survival analysis, the start of observation was considered the date of breast surgery.
Statistical analyses were performed by Kaplan–Meier method, and with a 5 year ratio (CI at 95%), differences among groups were made using log-rank test (p value < 0.05). Prognostic relevance of characteristics considered for the outcomes was assessed by means of Cox proportional hazard regression analysis.
Results
Median follow up was 5, 2 years (range 0–9 years).
Among patients who underwent axillary dissection (n = 135), metastatic sentinel lymph nodes were evidenced in 95 and micro-metastatic type in 15 patients, respectively.
Considering international guidelines for APBI, we stratified patients in three groups according to GEC-ESTRO and ASTRO recommendations (Tables 1, 2). Patients were inserted into each group according to the patient’s (age) and tumor’s characteristics (size, margins, grade, receptor status, histology factors, lymph nodes status).
Observing the two stratifications, most of our patients fall into the low-risk category (n = 350) for GEC-ESTRO, and into the cautionary category (n = 381) for ASTRO classification. These results can be explained considering that ASTRO is more restrictive than GEC-ESTRO particularly regarding age (≥60 vs. >50 year-old patients), tumor size (≤2 vs. ≤3 cm), and receptor status (estrogen receptor presence status vs any receptor status).
Applying GEC-ESTRO classification, we observed statistically significant differences among the three groups for IBR (p < 0.005), true local (p = 0.007), and the new ipsilateral recurrences (p < 0.005) (Table 3): moving from low-risk (3.3% IBR, 2.4% true local, and 1% new ispilateral recurrence) and intermediate-risk (9.5% IBR, 3.6% true local, and 5.9% new ispilateral recurrence) groups to high-risk group (13.4% IBR, 8.6% true local, and 4.8% new ispilateral recurrence), the incidence of breast-related events increased significantly.
Applying ASTRO classification, statistically significant differences for all the clinical outcomes, except for the OS (p = 0.088), were evidenced among the three groups (Table 4). In this case, moving from suitable group (1.2% IBR, 0% true local, and 1.2% new ispilateral recurrence) to cautionary (5.9% IBR, 3.2% true local, and 2.8% new ispilateral recurrence) and unsuitable (13.5% IBR, 8.4% true local, and 5.1% new ispilateral recurrence) groups the incidence of breast-related events increased significantly.
Evaluating most important prognostic factors for every outcome resulted from Cox analysis (Table 5) (age > 50 years, non-lobular histology, tumor size ≤2 cm, pN0 or pNmic, ki67≤20%, non-triple negative receptor status and G1–G2), we defined two groups of patients, “suitable” and “unsuitable” for IORT. We evidenced statistically significant differences in all clinical outcomes, except for OS (p = 0.231), (Table 6) as in ASTRO stratification. Besides moving from “suitable” (1.8% IBR, 0.6% true local, and 1.1% new ispilateral recurrence) to “unsuitable” (11.6% IBR, 6.9% true local, and 4.7% new ispilateral recurrence) group a significantly higher rate of breast-related events was evidenced.
Discussion
Adjuvant WBRT after BCS, currently, represents the standard of care for most of early-staged patients, reducing by 15% the 10 year risk of any recurrence and by 50% with the addition of a boost to the surgical bed [3]. However, different options can be considered, such as APBI and hormone therapy alone, but still limited data are available about patients eligible for these alternative treatments.
IORT, a type of APBI, represents an attractive option as alternative to EBRT, reducing the classical fractionated schedule to a single fraction with the same equivalent dose, delivered during surgery. Particularly, among APBI techniques, IORT showed the advantages to delineate precisely the tumor bed under visual control, to perform an immediate oncoplastic breast surgery with good cosmetic results, to decrease normal tissues toxicity, easily shielding critical organs, and to reduce treatment duration [20].
Literature reports five randomized clinical trials comparing IORT and EBRT. The Christie Hospital trial [21] and the Yorkshire Breast Cancer Group trial [22] did not evidence any efficacy of IORT in terms of local control, with a higher recurrence rate with APBI than with WBRT. On the contrary, the Budapest randomized trial [23] showed that APBI produces similar long-term results compared with conventional EBRT in a strictly selected group of breast cancer patients (inclusion criteria: negative surgical margins, unifocal tumor, pT1, pN0, cN0, pN1 with nodal micro-metastasis, G1-2; exclusion criteria: prior breast or other neoplasms, age ≤ 40 years, the presence of intraductal component, invasive lobular histology, carcinoma in situ, and bilateral breast cancer).
Other two recent large, phase III-randomized trials analyzed the results obtained from the comparison between IORT and whole breast EBRT for the targeted intraoperative radiotherapy (TARGIT-A) trial [24] and the electron intraoperative treatment (ELIOT) trial [25].
Both TARGIT-A and ELIOT trials evidenced at a 5 year median follow-up that there are no differences between IORT and EBRT in terms of overall survival, but LR rates remain significantly higher in the TARGIT (3.3 vs. 1.3%, p = 0.042) and in the ELIOT (4.4 vs. 0.4%, p < 0.0001) groups.
The 5 year LR increased from “suitable” to cautionary” to unsuitable” ASTRO groups, with a very low percentage of breast-related events in the suitable group. However, in the GEC-ESTRO, low- and intermediate-risk groups, higher percentages of IBR and LR than those in the ASTRO suitable and cautionary groups were evidenced. These results were evidenced also in the analysis of the European Institute of Oncology [19, 26] and are probably related to the differences between the two societies in the selection criteria.
In the literature, APBI generally was shown to be associated to higher frequency of local relapse compared with WBRT [27]. In our results (Table 3), the 5 year rate of IBR is 1.8% in suitable group with significant differences versus unsuitable (1.8 vs. 11.6%, p < 0.005). We evidenced results similar to those in the literature [23] regarding the incidence of LR (1.3%) rate after IORT. Same differences between the two groups were evidenced in true local relapse (0.6 vs. 6.9%, p < 0.005) and in new ipsilateral BC (1.1 vs. 4.7%, p < 0.015), with results being similar to those in the literature regarding the incidence of new primary (0.8%, CI 0.4–1.4) after whole breast RT [28].
These results are interesting also from the point of view of the possibility to use IORT in a subgroup of patients as alternative to the omission of RT. Patients with low-risk BC, particularly the elderly, constitute a special population regarding prognosis and potential comorbidities. Therefore, limiting potential radio-induced side effects and maintaining a good quality of life is extremely important. In a group of elderly patients, Kunkler et al. showed [29] a statistically significant reduction in LR at 5 years in a low-risk population with the addition of RT to adjuvant endocrine therapy after BCS (4.1% without RT vs. 1.3% with RT, p = 0.0002), with no differences regarding OS and DM. Similar results were evidenced in the CALGB trial (1.5) in which a 3% reduction of local recurrence at 5 years was noted with the addition of radiotherapy (4% without RT vs. 1% with adjuvant RT, p < 0.001). The literature shows exploratory findings [30] also in a low-risk group of postmenopausal women with early-staged luminal A BC who may be spared breast irradiation, but conclusion is that further studies are needed. In an extended low-risk population (women under 60 years-old), literature [31,32,33] has evidenced that, at 5 year follow-up, RT after BCS is advisable even when selected adjuvant systemic therapy is given. Fyles et al. [31] showed that the addition of RT to hormone therapy results in a significantly lower rate of breast relapse than administering hormone therapy alone (0.6% with RT vs. 7.7% without RT, p < 0.001), with no differences regarding DM and OS. Same results were evidenced in the Austrian trial [34], where the 5-year recurrence rate was 0.4% with RT and 5.1% without RT (p = 0.0001). Therefore, it is still difficult to identify the characteristics of patients with lowest risk of LR who could avoid RT after BCS [35]. In this contest, APBI, with its potential advantages compared with EBRT (shorter therapy time, improved cosmesis, and cost reduction), could be currently considered as alternative to the omission of RT in a favorable prognosis patients [29, 35].
Regarding axillary dissection (n = 135), during the initial years that we performed IORT, we included also patients with clinical positive nodes and we did not use to wait for the lymph node sentinel histology result. Literature widely demonstrated the influence of metastatic lymph nodes on prognosis in patients undergoing IORT [18], and we changed our practice, confirming the importance of performing IORT in patients with preoperative negative nodes (clinical and imaging assessment) and waiting intraoperative histology assessment of sentinel lymph node.
An unresolved problem regards the LVI that, from our statistical analyses, confirmed to be a fundamental predictive factor. Nevertheless, it can be evaluable only in the postoperative setting.
In conclusion, from our analysis, focused on evaluating an appropriate selection of early BC patients undergoing exclusively IORT, not generically APBI procedures as per GEC-ESTRO and ASTRO guidelines, we confirmed the importance of an adequate preoperative selection to define patients eligible for IORT.
In our current practice, we consider the following selection factors to make BC patients suitable for full-dose IORT: age > 50 years; the absence of lobular histology; tumor size ≤ 2 cm; pN0 or pNmic, according to APBI consensus statement [17, 18]; we include also non-triple negative status, G1-2, and a new factor—not inserted in GEC-ESTRO/ASTRO classifications—ki67 ≤ 20%.
In this contest, IORT is a feasible and safe technique, with results eing similar to EBRT regarding breast-related events in selected patients without significant late toxicities.
References
Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, Muss HB, Smith BL, Hudis CA, Winer EP, Wood WC (2013) Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 31:2382–2387
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y, Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15 year survival: an overview of the randomised trials. Lancet 366:2087–2106
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R (2011) Effect of radiotherapy after breast-conserving surgery on 10 year recurrence and 15 year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707–1716
Esposito E, Anninga B, Harris S, Capasso I, D’Aiuto M, Rinaldo M, Douek M (2015) Intraoperative radiotherapy in early breast cancer. Br J Surg 102:599–610
Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, Wheeler J, Champion LA, Smith TJ, Smith BL, Shapiro C, Muss HB, Winer E, Hudis C, Wood W, Sugarbaker D, Henderson IC, Norton L (2004) Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 351:971–977
Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C (2010) Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 362:513–520
Lehman M, Hickey BE, Francis DP, See AM (2014) Partial breast irradiation for early breast cancer. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007077
Lemanski C, Azria D, Gourgou-Bourgade S, Ailleres N, Pastant A, Rouanet P, Fenoglietto P, Dubois JB, Gutowski M (2013) Electrons for intraoperative radiotherapy in selected breast-cancer patients: late results of the Montpellier phase II trial. Radiat Oncol 8:191
Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, Reed M, Ciatto S, Voogd AC, Brain E, Cutuli B, Terret C, Gosney M, Aapro M, Audisio R (2012) Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 13:148–160
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2009) AJCC cancer staging manual. Springer, New York, pp 419–460
Tavassoli FA, Devilee P (2003) World Health Organization classification of tumours. Pathology and genetics tumours of the breast and female genital organs. IARC Press, Lyon, pp 19–23
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Rosen PP (1983) Tumour emboli in intramammary lymphatics in breast carcinoma: pathologic criteria for diagnosis and clinical significance. Pathol Annu 2:215–232
Rhodes A, Jasani B, Balaton AJ, Barnes DM, Miller KD (2000) Frequency of oestrogen and progesterone receptor positivity by immunohistochemical analysis in 7016 breast carcinomas: correlation with patient age, assay sensitivity, threshold value, and mammographic screening. J Clin Pathol 53:688–696
Munzone E, Botteri E, Sciandivasci A, Curigliano G, Nolè F, Mastropasqua M, Rotmensz N, Colleoni M, Esposito A, Adamoli L, Luini A, Goldhirsch A, Viale G (2012) Prognostic value of Ki-67 labeling index in patients with node-negative, triple negative breast cancer. Breast Cancer Res Treat 134:277–282
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn HJ (2015) Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 26:1533–1546
Polgár C, Van Limbergen E, Pötter R, Kovács G, Polo A, Lyczek J, Hildebrandt G, Niehoff P, Guinot JL, Guedea F, Johansson B, Ott OJ, Major T, Strnad V (2009) Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Group Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence. Radiother Oncol 94:264–273
Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, Julian TB, Marks LB, Todor DA, Vicini FA, Whelan TJ, White J, Wo JY, Harris JR (2009) Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 74:987–1001
Leonardi MC, Maisonneuve P, Mastropasqua MG, Morra A, Lazzari R, Dell’Acqua V, Ferrari A, Rotmensz N, Sangalli C, Luini A, Veronesi U, Orecchia R (2013) Accelerated partial breast irradiation with intraoperative electrons: using GEC-ESTRO recommendations as guidance for patient selection. Radiother Oncol 106:21–27
Orecchia R, Leonardi MC (2011) Intraoperative radiation therapy: is it a standard now? Breast 20(S3):S111–S115
Ribeiro GG, Magee B, Swindell R, Harris M, Banerjee SS (1993) The Christie Hospital breast conservation trial: an update at 8 years from inception. Clin Oncol 5:278–283
Dodwell DJ, Dyker K, Brown J, Hawkins K, Cohen D, Stead M, Ash D (2005) A randomised study of whole-breast vs tumour-bed irradiation after local excision and axillary dissection for early breast cancer. Clin Oncol 17:618–622
Polgár C, Fodor J, Major T, Sulyok Z, Kásler M (2013) Breast-conserving therapy with partial or whole breast irradiation: ten-years results of the Budapest randomized trial. Radiother Oncol 108:197–202
Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, Flyger HL, Massarut S, Alvarado M, Saunders C, Eiermann W, Metaxas M, Sperk E, Sütterlin M, Brown D, Esserman L, Roncadin M, Thompson A, Dewar JA, Holtveg HM, Pigorsch S, Falzon M, Harris E, Matthews A, Brew-Graves C, Potyka I, Corica T, Williams NR, Baum M, TARGIT trialists’ group (2014) Risk-adapted targeted intraoperative radiotherapy vs whole breast radiotherapy for breast cancer: 5 years results for local control and overall survival from the TARGIT-A randomized trial. Lancet 383:603–613
Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, Luini A, Veronesi P, Galimberti V, Zurrida S, Leonardi MC, Lazzari R, Cattani F, Gentilini O, Intra M, Caldarella P, Ballardini B (2013) Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomized controlled equivalence trial. Lancet Oncol 14:1269–1277
Leonardi MC, Maisonneuve P, Mastropasqua MG, Morra A, Lazzari R, Rotmensz N, Sangalli C, Luini A, Veronesi U, Orecchia R (2012) How do the ASTRO consensus statement guidelines for the application of accelerated partial breast irradiation fit intraoperative radiotherapy? A retrospective analysis of patients treated at the European Institute of Oncology. Int J Radiat Oncol Biol Phys 83:806–813
Marta GN, Macedo CR, Carvalho Hde A, Hanna SA, da Silva JL, Riera R (2015) Accelerated partial breast irradiation for breast cancer: systematic review and metanalysis of 8653 women in eight randomized trials. Radiother Oncol 114:42–49
Gujral DM, Sumo G, Owen JR, Ashton A, Bliss JM, Haviland J, Yarnold JR (2011) Ipsilateral breast tumor relapse: local recurrence versus new primary tumor and the effect of whole-breast radiotherapy on the rate of new primaries. Int J Radiat Oncol Biol Phys 79:19–25
Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM (2015) Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 16:266–273
Liu FF, Shi W, Done SJ, Miller N, Pintilie M, Voduc D, Nielsen TO, Nofech-Mozes S, Chang MC, Whelan TJ, Weir LM, Olivotto IA, McCready DR, Fyles AW (2015) Identification of a low-risk luminal a breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol 33:2035–2040
Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, Weir LM, Olivotto IA (2004) Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 351:963–970
Forrest AP, Stewart HJ, Everington D, Prescott RJ, McArdle CS, Harnett AN, Smith DC, George WD (1996) Randomised controlled trial of conservation therapy for breast cancer: 6 year analysis of the Scottish trial. Scottish Cancer Trials Breast Group. Lancet 348:708–713
Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, Margolese RG, Nesbitt L, Paik S, Pisansky TM, Wolmark N (2002) Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 20:4141–4149
Pötter R, Gnant M, Kwasny W, Tausch C, Handl-Zeller L, Pakisch B, Taucher S, Hammer J, Luschin-Ebengreuth G, Schmid M, Sedlmayer F, Stierer M, Reiner G, Kapp K, Hofbauer F, Rottenfusser A, Pöstlberger S, Haider K, Draxler W, Jakesz R (2007) Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys 68:334–340
Poortmans PM, Arenas M, Livi L (2016) Over-irradiation. Breast S0960–9776(16):30132–301331
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Takanen, S., Gambirasio, A., Gritti, G. et al. Breast cancer electron intraoperative radiotherapy: assessment of preoperative selection factors from a retrospective analysis of 758 patients and review of literature. Breast Cancer Res Treat 165, 261–271 (2017). https://doi.org/10.1007/s10549-017-4321-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4321-6