Abstract
The aim of this study is to investigate the efficacy of combining a histone deacetylase inhibitor (LBH589) and a breast cancer stem cells (BCSC)-targeting agent (salinomycin) as a novel combination therapy for triple-negative breast cancer (TNBC). We performed in vitro studies using the TNBC cell lines to examine the combined effect. We used the mammosphere and ALDEFLUOR assays to estimate BCSC self-renewal capacity and distribution of BCSCs, respectively. Synergistic analysis was performed using CalcuSyn software. For in vivo studies, aldehyde dehydrogenase 1 ALDH1-positive cells were injected into non-obese diabetic/severe combined immunodeficiency gamma (NSG) mice. After tumor formation, mice were treated with LBH589, salinomycin, or in combination. In a second mouse model, HCC1937 cells were first treated with each treatment and then injected into NSG mice. For mechanistic analysis, immunohistochemistry and Western blot analysis were performed using cell and tumor samples. HCC1937 cells displayed BCSC properties including self-renewal capacity, an ALDH1-positive cell population, and the ability to form tumors. Treatment of HCC1937 cells with LBH589 and salinomycin had a potent synergistic effect inhibiting TNBC cell proliferation, ALDH1-positive cells, and mammosphere growth. In xenograft mouse models treated with LBH589 and salinomycin, the drug combination effectively and synergistically inhibited tumor growth of ALDH1-positive cells. The drug combination exerted its effects by inducing apoptosis, arresting the cell cycle, and regulating epithelial–mesenchymal transition (EMT). Combination of LBH589 and salinomycin has a synergistic inhibitory effect on TNBC BCSCs by inducing apoptosis, arresting the cell cycle, and regulating EMT; with no apparent associated severe toxicity. This drug combination could therefore offer a new targeted therapeutic strategy for TNBC and warrants further clinical study in patients with TNBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the second leading cause of cancer-related deaths among women in the United States with over 232,000 new diagnoses expected in 2014 and approximately 40,000 deaths [1]. Triple-negative breast cancer (TNBC) accounts for approximately 15 % of all breast cancers [2] and is defined by absence of the estrogen receptor (ER) and progesterone receptor (PR), and no amplification [based on immunohistochemistry (IHC) analysis] of the human epidermal growth factor receptor 2 (HER2) [3–5]. TNBC is an aggressive breast cancer subtype that does not respond to targeted therapies [6], and patients with TNBC have a poorer prognosis than patients with hormone receptor-positive or HER2-positive cancers [7].
The aggressive nature of TNBC may be explained in part by the presence of breast cancer stem cells (BCSCs) within TNBC tumors. Cancer stem cells (CSCs) have the ability to self-renew [8], invade [9], and metastasize [10], and are resistant to conventional chemotherapy and radiation therapy causing cancer recurrence after treatment [11–13]. BCSCs are defined by expression of the CD44 cell-surface marker and low or no expression of CD24 (CD44+ CD24−/low) as well as expression of aldehyde dehydrogenase 1 (ALDH1) [14, 15]. BCSCs have been shown to possess the same qualities as CSCs, forming tumors in vivo [14, 15] and playing an important role in epithelial–mesenchymal transition (EMT) [16–18], a process that leads to invasion and metastasis [19, 20]. Development of effective novel therapies that target BCSCs in TNBC may therefore provide new therapeutic options for patients with TNBC.
Histone deacetylase (HDAC) inhibitors are a new class of anti-cancer drugs [21] being trialed in solid tumors [22]. They act by modifying gene expression to restore normal differentiation or apoptotic pathways to transformed cancer cells [23]. This class of drugs may also be useful to treat TNBC. We previously showed that the HDAC inhibitor LBH589 (panobinostat) can suppress aromatase expression in a promoter-specific manner [24] and inhibit aromatase inhibitor (AI)-resistant tumor growth [25]. LBH589 has also been shown to suppress TNBC cell growth [26]. Combining LBH589 with drugs that target BCSCs may further improve the efficacy of treatment for TNBC.
Salinomycin (an antibiotic used in farm animals [27]), was identified as an effective anti-BCSC compound by high-throughput screening [28]. Salinomycin alone has been shown to inhibit BCSCs in TNBC [28, 29] and when used in combination with HDAC inhibitors in glioblastoma, it enhanced lethality in stem-like glioblastoma cells [30]. These studies suggest that combining an HDAC inhibitor such as LBH589 with salinomycin in TNBC could provide an effective targeted therapy for patients with TNBC. However, a better understanding of the efficacy and potential side effects of this novel combination therapy is still required.
In the current study, we performed in vitro and in vivo studies to determine the synergy between LBH589 and salinomycin in TNBC and establish the clinical utility of this novel targeted combination therapy in TNBC.
Materials and methods
Cell culture
The HCC1937, MDA-MB-231, MCF7, and SK-BR-3 breast cancer cell lines were obtained from the American Type Culture Collection (ACTT) (Manassas, VA, USA). HCC1937 cells were maintained in 1:1 DMEM supplemented with 10 % fetal bovine serum and the Mammary Epithelial Cell Growth Medium (MEGM) Bullet Kit (Lonza, Walkersville, MD, USA). MDA-MB-231, MCF7, and SK-BR-3 cells were maintained in RPMI 1640 supplemented with 10 % fetal bovine serum and 1 % penicillin/streptomycin. Cells were maintained at 37 °C under 5 % CO2.
Mammosphere culture
A mammosphere assay was used to estimate BCSC self-renewal capacity in HCC1937, MDA-MB-231, MCF7, and SK-BR-3 breast cancer cells. Please see supplementary materials for detailed procedures.
ALDEFLUOR assay and cell sorting
ALDEFLUOR analysis was used to monitor the distribution of TNBC BCSCs in LBH589 and/or salinomycin treated samples by detecting cells that expressed ALDH1. The ALDEFLUOR assay was carried out using the ALDEFLUOR kit according to the manufacturer’s instructions (Stem Cell Technologies, Vancouver, BC, Canada). Please see supplementary materials for detailed procedures.
MTT assay and synergistic analysis
To determine the effect of HDAC inhibitors on TNBC cell proliferation, we performed the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. HCC1937 and MDA-MB-231 cells were seeded in 96-well plates at a concentration of 2000 cells per well in 100 μl of appropriate culture media for each cell line (as described above). After 24 h incubation at 37 °C, cells were treated with either LBH589 (4, 8, 16, 32, and 64 nM), entinostat (2.4, 4.8, 9.6. 19.2, 38.4 nM, 9.357, 18.75, 37.5, 75, and 150 µM), or the drugs described below; dimethyl sulfoxide (DMSO) was added as a negative control. After treatment, cells were cultured for 6 days. On days 3 and 6, MTT reagent was added to each well and incubated for 1 h at 37 °C. The absorbance was measured at 570 nm on days 3 and 6 using a 96-well plate reader. A synergistic analysis was performed using CalcuSyn software, version 2 (Biosoft, Cambridge, UK) and the Chou–Talalay method [31].
The Notch, Hsp90, Hedgehog, and NFκB pathways have been shown to be important for BCSCs [32]. Based on this information we chose to test drugs that inhibited these pathways and may have a synergistic effect on inhibiting TNBC cell proliferation when combined with HDAC inhibitors. We tested MK0752 (Selleckchem, Houston, TX, USA) (Notch pathway inhibitor), 17-DMAG (Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, National Cancer Institute, Bethesda, MD, USA) (Hsp90 inhibitor), GDC0449 (Selleckchem, Houston, TX, USA) (Hedgehog pathway inhibitor), and parthenolide (Sigma-Aldrich, St. Louis, MO, USA) (NFκB inhibitor). We also tested salinomycin (Sigma-Aldrich, St. Louis, MO, USA) based on the study by Gupta et al. [28].
Mouse xenograft treatment models
All animal research procedures were approved by the institutional animal care and use committee (IACUC) at City of Hope for assessment and accreditation of laboratory animal care, and were in accordance with NIH guidelines. To determine how LBH589 and salinomycin affected tumorigenicity of TNBC BCSCs, two mouse models were generated. In the first model, ALDH1-positive and -negative cells from the HCC1937 cell culture were identified using the ALDEFLUOR assay and sorted using the FACSAria III. 1 × 105 ALDH1-positive or -negative HCC1937 cells were then each injected into the fourth mammary pads of NOD/SCID/interleukin 2 gamma-receptor-null (NSG) mice. According to previously described methods [28], after confirming tumor growth, mice were divided into four treatment groups: vehicle (5 % dextrose), LBH589 (10 mg/kg), salinomycin (5 mg/kg), or a combination of both drugs. Drugs were administered by intraperitoneal injection 3 times per week. Tumor size was measured once a week using calipers, and tumor volume was calculated using the formula 3/4π × L × W2×1/8 (L: length; W: width; mm). Food intake and body weight were measured weekly to monitor toxicity associated with the treatments. In the second mouse model, according to previously described methods [28], HCC1937 cells were treated in vitro with vehicle (DMSO), LBH589 (32 nM), salinomycin (120 nM), or a combination treatment of both drugs for 3 days; drugs were removed by changing medium for 4 days for cell recovery. Cells (1 × 105 or 1 × 104) were then injected into NSG mice. Tumor size was measured once a week for 12 weeks.
Western blot and IHC analysis
Please see supplementary materials for procedures.
Statistical analysis
Statistical analyses were performed using Excel 2010 software (Microsoft, Redmond, WA, USA). All analyses were subjected to unpaired student’s t test. p < 0.05 was considered statistically significant.
Results
HCC1937 TNBC cells display BCSC properties
To determine which breast cancer cell lines had BCSC properties, we performed the mammosphere and ALDEFLUOR assays using the HCC1937, MDA-MB-231, MCF7, and SK-BR-3 cell lines (Fig. 1 and Supplementary Fig. 1). Of the four cell lines tested, only HCC1937 cells formed mammospheres (Fig. 1a) and expressed ALDH1 (12 % positive cells) (Fig. 1b). To determine the tumor-forming ability of ALDH1-positive versus ALDH1-negative cells, ALDH1-positive and -negative HCC1937 cells were each injected into NSG mice. Only ALDH1-positive HCC1937 cells formed tumors (Supplementary Fig. 1).
Breast cancer cell lines with BCSC properties. a Mammosphere formation assay in MCF7, SK-BR-3, MDA-MB-231, and HCC1937 cells. Images show representative mammospheres in each cell line at day 5 of incubation. Original magnification: ×200. b ALDEFLUOR assay in breast cancer cell lines (HCC1937, MDA-MB-231, MCF7, and SK-BR-3). The upper panels show the negative controls (cells treated with DEAB, a specific inhibitor of ALDH1). Based on the negative control, the gated area was assigned as ALDH1-positive. Percentages of ALDH1-positive cell populations of each cell lines are shown in the lower panels
HDAC inhibitors suppress TNBC cell proliferation and self-renewal
To determine the effect of HDAC inhibitors on TNBC cell proliferation, we performed the MTT assay. LBH589 and entinostat, which have previously been tested in clinical trials [33], were tested in this assay. The effects of these two HDAC inhibitors on the proliferation of HCC1937 and MDA-MB-231 cells (both TNBC cell lines) were determined (Fig. 2). Both drugs inhibited the proliferation of both cell lines in a dose-dependent manner (Fig. 2a, b). The IC50 values of LBH589 and entinostat were 68.8 and 3.93 nM (MDA-MB-231 cells) and 13.1 nM and 1.35 μM (HCC1937 cells). To determine the effect of HDAC inhibitors on TNBC BCSC cell self-renewal, HCC1937 mammospheres were treated with LBH589 and entinostat alone. Both LBH589 and entinostat significantly suppressed mammosphere growth (Fig. 2c, d).
Effect of HDAC inhibitors on TNBC cell proliferation and mammosphere growth. HCC1937 and MDA-MB-231 cells treated with a LBH589 and b entinostat and cell viabilities assessed by MTT assay. c, d Single mammospheres collected and treated by DMSO, LBH589 (20 nM), and entinostat (300 nM). Pictures were taken on day 0, 2, 5, 7, 9, and 12. d Change in mammosphere volume ratio to day 0. The volume was calculated based on the radius of the mammosphere. Original magnification was ×200. The error bars represent mean ± SD. *p < 0.05; ***p < 0.0005
Combination of LBH589 and salinomycin synergistically inhibits TNBC cell proliferation
To determine which drug combinations improved the efficacy of LBH589 against TNBC, we first examined the effects of MK0752, 17-DMAG, GDC0449, parthenolide, and salinomycin alone on cell proliferation using the MMT assay (Fig. 3 and Supplementary Fig. 2). Only 17-DMAG, parthenolide, and salinomycin inhibited TNBC cell proliferation in a dose-dependent manner (Fig. 3a and Supplementary Fig. 2A). Parthenolide (NFκB inhibitor) and salinomycin were selected for the further study in combination with LBH589 because NFκB inhibitors have been shown to inhibit BCSCs in mouse xenograft models [34], and salinomycin was previously identified as the most effective anti-BCSC drug among 16,000 compounds by high-throughput screening [28]. Both drug combinations inhibited TNBC cell proliferation in a dose-dependent manner (Fig. 3b). However, analysis of the synergistic effect revealed that only LBH589 and salinomycin synergistically inhibited HCC1937 and MDA-MB-231 cell proliferation (Fig. 3c and Supplementary Fig. 2B).
Effect of LBH589 and salinomycin on TNBC cell proliferation. a HCC1937 and MDA-MB-231 cells treated with parthenolide and salinomycin and cell viabilities assessed by MTT assay. b HCC1937 and MDA-MB-231 cells treated with a combination of parthenolide or salinomycin and LBH589 in different concentrations and cell viabilities assessed by MTT assay. c Synergistic analysis based on the MTT assay. The horizontal axis shows fraction affected (Fa) and the vertical axis shows combination index (CI). CI offers quantitative definition for additive effect (CI = 1), synergism (CI < 1), and antagonism (CI > 1) in drug combinations
Combination of LBH589 and salinomycin inhibits TNBC BCSC cell self-renewal
To determine the effect of LBH589 and salinomycin on TNBC BCSC cell self-renewal, we performed the mammosphere and ALDEFLUOR assays using HCC1937 cells (Fig. 4). LBH589 and salinomycin alone both reduced the number of HCC1937 mammospheres in a dose-dependent manner; and combination of LBH589 and salinomycin significantly reduced the number of mammospheres compared with the control or other treatment groups (Fig. 4a, b). LBH589 and salinomycin alone also both reduced the ALDH1-positive cell population in HCC1937 cells in a dose-dependent fashion; and combination of LBH589 and salinomycin had the strongest effect on reducing ALDH1-positive cells compared with the control and other treatment groups (Fig. 4c, d).
Effect of LBH589 and salinomycin on TNBC BCSC self-renewal capacity and the TNBC BCSC population. a, b HCC1937 cells treated with DMSO, LBH589, salinomycin, or a combination of LBH589 and salinomycin in mammosphere culture conditions. a Images show representative mammospheres in each treatment at day 5. b Number of mammospheres >70 μm in each treatment group. c, d HCC1937 cells treated with DMSO, LBH589, salinomycin, or combination of LBH589 and salinomycin after ALDEFLUOR assays. c The panels show representative flow cytometry of ALDEFLUOR assays in each treatment condition. d The graph shows the ALDH1-positive cell population in each treatment condition. Original magnifications were ×200. The error bars represent mean ± SD. *p < 0.05; **p < 0.005; ***p < 0.005
Combination of LBH589 and salinomycin reduces TNBC BCSC tumorigenicity
To determine the effect of LBH589 and salinomycin on the tumorigenicity of TNBC BCSCs, we generated xenograft mouse models (Fig. 5 and Supplementary Fig. 3). Combination of LBH589 and salinomycin significantly reduced tumor growth compared with the treatment of LBH589 or salinomycin alone (p < 0.05) (Fig. 5a); tumor weight was also lowest in the combination treatment group (Fig. 5b). There was no significant difference in body weight and food intake between the four treatment groups (Fig. 5c, d).
Effect of LBH589 and salinomycin on tumorigenicity of TNBC cells. a Tumor volume change in NSG mice. Mice were treated with vehicle, LBH589, salinomycin, or a combination of LBH589 and salinomycin. Error bars represent mean ± SEM. b Final weight (g) of harvested tumors. Images show the tumors in each treatment condition. Scale bar: 2 cm. The error bars represent mean ± SEM. *p < 0.05. (c, d) Body weight (g) and food intake (g) per mouse were measured weekly. Error bars represent mean ± SEM. e IHC staining of Ki67 from harvested formalin-fixed tumors in each treatment condition. The upper panel shows representative images of each treatment condition. The lower panel shows the percentage of Ki67-positive cells in each treatment condition. Original magnifications were ×400. Error bars represent mean ± SD. *p < 0.05; **p < 0.005; ***p < 0.005
We harvested tumor tissue from all mice and performed IHC analysis to examine tumor cell proliferation. Expression of Ki67, a marker of cell proliferation, was significantly lower in mice treated with both LBH589 and salinomycin compared with the control and single-treatment groups (Fig. 5e). Furthermore, in mice injected with HCC1937 cells pretreated with LBH589, salinomycin, or both drugs, the tumors resulting from HCC1937 cells pretreated with a combination of LBH589 and salinomycin were significantly smaller than those of the other treatment groups (Supplementary Fig. 3).
Combination of LBH589 and salinomycin induces apoptosis and cell cycle arrest in TNBC BCSCs
LBH589 and salinomycin alone can each induce apoptosis in cancer cells [26, 35]. To evaluate the combined effect of LBH589 and salinomycin on apoptosis of TNBC BCSCs, we performed IHC analysis using HCC1937 cells (Fig. 6 ). A larger amount of nuclear fragmentation, indicative of apoptosis [36], was detected in cells treated with LBH589 and salinomycin alone compared with control cells; and combination of the two drugs further increased nuclear fragmentation (Fig. 6a). Furthermore, activated PARP, a specific marker of apoptosis, increased in HCC1937 cells after treatment with LBH589 and salinomycin alone in a dose-dependent manner compared with control cells; and combination of the two drugs resulted in the highest PARP expression observed (Fig. 6b).
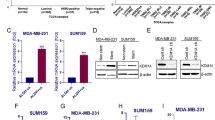
Effect of LBH589 and salinomycin on cell cycle arrest, apoptosis, and EMT-related markers in TNBC BCSCs. a HCC1937 cells treated with vehicle (DMSO), LBH589 (4, 16, 64 nM), salinomycin (SAL) (15 nM, 60 nM, 240 nM), or the drugs combined. After 5 days of treatment, cells were stained with 10 ug/ml of DAPI. Representative images from each treatment condition are shown. Original magnification was ×200. b HCC1937 cells treated with LBH589, SAL, or the drugs combined for 48 h. Cell extracts were analyzed by Western blotting with anti-cleaved PARP and anti-GAPDH antibodies. c HCC1937 cells treated with LBH589, SAL, or the drugs combined for 48 h. Cell extracts were analyzed by Western blotting with anti-acetylated histone H3 and H4, anti-p21, anti-cyclin D1, and anti-GAPDH antibodies. d HCC1937 ALDH1-positive and -negative cell extracts were analyzed by Western blotting with anti-E-cadherin, anti-vimentin, anti-N-cadherin, anti-SLUG, and anti-actin antibodies. e Proteins extracted from snap-frozen mouse tumors treated by vehicle, LBH589, SAL, or the drugs combined for 12 weeks and analyzed by Western blotting with anti-E-cadherin, anti-vimentin, and anti-actin antibodies
The p21 gene is a tumor suppressor gene that regulates the cell cycle by binding to the cyclin D1/CDK4/6 complex. To evaluate the combined effect of LBH589 and salinomycin on cell cycle regulation in TNBC BCSCs, we performed IHC analysis using HCC1937 cells. Expression of p21 was up-regulated in cells treated with LBH589 or salinomycin alone in a dose-dependent manner compared with control cells; and combination of the two drugs resulted in the highest p21 expression observed. Cyclin D1 was also down-regulated by combination treatment with LBH589 and salinomycin (Fig. 6c).
We also examined histone acetylation because LBH589 is thought to induce the acetylation of histone H3 and H4, and acetylated histone H3 and H4 activates transcription of p21 [37, 38]. Expression of acetylated histone H3 and H4 was increased in cells treated with LBH589 or salinomycin alone in a dose-dependent manner compared with control cells; and combination of the two drugs further increased expression of acetylated histone H3 and H4 (Fig. 6c).
Combination of LBH589 and salinomycin suppress TNBC BCSCs by regulating EMT
To examine the effect of LBH589 and salinomycin on the “stemness” of TNBC BCSCs, we analyzed expression of EMT-related genes (E-cadherin, vimentin, N-cadherin, and SLUG) by IHC analysis in ALDH1-positive and -negative HCC1937 cells because cancer cell “stemness” has been associated with EMT [16–18]. Prior to treatment, expression of E-cadherin was low and vimentin was high in ALDH1-positive cells compared with ALDH1-negative cells (Fig. 6d). After treatment, E-cadherin was up-regulated by LBH589 in a dose-dependent manner and was further up-regulated by the combination of LBH589 and salinomycin in HCC1937 cells. Vimentin was down-regulated by salinomycin in a dose-dependent manner, and was slightly down-regulated by the combination of LBH589 and salinomycin in a dose-dependent manner compared to treatment with LBH589 alone in HCC1937 cells (Fig. 6e). Finally, N-cadherin and SLUG were each down-regulated by LBH589 and salinomycin alone in a dose-dependent manner; and combination of the two drugs further decreased expression of each of these EMT-related genes in HCC1937 cells (Fig. 6e).
To determine if combination of LBH589 and salinomycin regulates EMT-related markers in vivo, we analyzed expression of E-cadherin and vimentin in tumor tissue from xenograft mouse models treated with LBH589 and salinomycin. E-cadherin was up-regulated in tumors from mice treated with LBH589 and salinomycin alone compared with controls; and combination of the two drugs further increased tumor expression of E-cadherin. Vimentin was down-regulated in tumors from mice treated with LBH589 and salinomycin alone compared with controls; and combination of the two drugs decreased tumor expression of vimentin compared with treatment with LBH589 alone (Fig. 6f).
Discussion
The dysregulation of HDAC in cancer cells leads to changes in gene expression and promotes cancer cell viability [39]. Drugs that inhibit HDAC in cancer cells (HDAC inhibitors) therefore offer promise as anti-cancer agents [23, 33, 40, 41]. LBH589 (panobinostat) is a potent HDAC inhibitor that blocks pan-deacetylase and has demonstrated activity against all Class I, II, and IV HDAC enzymes [42]. In preclinical studies, LBH589 inhibited TNBC cell growth, decreased tumorigenesis in mice, and partially reversed EMT [26, 43]. Combination of LBH589 with chloroquine inhibited TNBC cell growth and increased survival of TNBC cell xenografts [44]. In clinical studies, LBH589 has been trialed as a single agent in patients with solid tumors [23] and has also been evaluated in Phase I trials in combination with other drugs [45, 46]. In patients with metastatic breast cancer, LBH589 in combination with trastuzumab was well-tolerated and resulted in a 29 % tumor reduction in 2/18 patients [45], and LBH589 in combination with letrozole was well-tolerated (LBH589 at 20 mg), and 1/12 patients had a confirmed partial response [46].
It has been suggested that HDAC inhibitors may be effective in the treatment of breast cancer because of their ability to target BCSCs decreasing proliferation of ALDH1-positive cells and decreasing the formation of breast cancer mammospheres [47]. In light of all of these studies, we determined the effect of LBH589 on TNBC cell lines with BCSC properties. We showed that the HCC1937 TNBC cell line has BCSC properties including self-renewal capacity, an ALDH1-positive cell population, and the ability to form tumors. We also showed that LBH589 not only effectively inhibited proliferation and mammosphere formation and growth in these cells, but also decreased the number of ALDH1-positive cells.
BCSC population was first identified by Al-Hajj et al. using the combination of cell-surface markers CD44 and CD24 [14]. They showed CD44+ CD24−/low population could form tumors in NOD/SCID mice even in the low cell numbers and concluded that this small population had tumor initiating capacity, which can be defined as BCSC population. Furthermore, ALDH1 was reported as a novel BCSC marker by Ginestier et al. [15]. According to their report, only ALDH1 + population could make tumors in mice even in low cell numbers whereas CD44+ CD24− could not. Based on these findings, in many recent reports, ALDH1 has been used for BCSC assays, it can predict prognosis in patients, and it is thought to be a potent BCSC marker [48–53]. In addition, ALDH1 is associated with basal-like subtype which is roughly equal to TNBC. On the other hand, CD44+ CD24− is reported to be associated with luminal B subtype [54]. Therefore, in our study, we adopted ALDH1 as a BCSC marker in TNBC.
Developing novel therapies that target BCSCs has recently become one of the most exciting areas of pharmaceutical research. The Hedgehog, Notch, and Wnt signaling pathways have been suggested as attractive therapeutic targets because of their demonstrated roles in oncogenesis regulating CSC renewal and maintenance, and selective inhibition of specific pathway components using BCSC-targeted drugs has been reported [32, 55]. Use of heat shock protein 90 (Hsp90) inhibitors have also been investigated as BCSC-targeted therapies because Hsp90 is over-expressed in ALDH1-positive BCSCs, and Hsp90 inhibitors have been shown to reduce this cell population [56]. Drugs that inhibit the NFκB pathway, such as parthenolide and pyrrolidinedithiocarbamate, may also be effective as they have been shown to preferentially inhibit BCSCs in mouse xenograft models [34]. Salinomycin was also recently identified as a potent selective inhibitor of BCSCs, reducing BCSCs by >100-fold relative to paclitaxel [28]. Taking the reported data into consideration, we chose several candidate drugs to combine with LBH589 to target BCSCs in TNBC. Of all the drug combinations tested, we found that treatment of HCC1937 cells with LBH589 and salinomycin had a potent synergistic effect inhibiting TNBC cell proliferation and mammosphere growth. Furthermore, in xenograft mouse models treated with a combination of LBH589 and salinomycin, this drug combination effectively and synergistically inhibited tumor growth of ALDH1-positive cells (BCSCs) with no significant difference in body weight and food intake; thus, suggesting that the dosage and schedule of the drugs are not associated with severe toxicity.
Salinomycin is a monocarboxylic polyether organic anion used widely in poultry as an anti-coccidiosis antibiotic and is fed to ruminant animals to improve feed efficiency [27, 57]. Salinomycin has not typically been used in humans because of associated toxicities observed in other mammals such as horses, pigs, cats, and alpacas [58, 59]. The first therapeutic use of salinomycin in humans was in a pilot clinical trial with a small cohort of patients with metastatic breast, ovarian, and head and neck cancers [60]. Intravenous administration of 200–250 μg/kg salinomycin every second day for 3 weeks in metastatic breast cancer patients resulted in partial regression of metastasis and showed only minor acute and long-term side effects [60]. This data supports our preclinical findings and suggests that this drug may be a useful addition to the TNBC armamentarium.
To understand how the combination of LBH589 and salinomycin target BCSCs in TNBC, we examined the mechanisms underlying inhibition of BCSC proliferation and “stemness”. We showed that combination of LBH589 and salinomycin effectively and synergistically inhibited tumor growth of ALDH1-positive cells (BCSCs) by inducing apoptosis, arresting the cell cycle and regulating EMT. Apoptosis was observed by high levels of 4′,6-diamidino-2-phenylindole (DAPI) staining that identified DNA fragmentation and high levels of cleaved PARP expression that serves as a marker of cells undergoing apoptosis [61]. HDACs inactivate transcription of the cyclin-dependent kinase inhibitor p21 [62, 63]; p21 promotes cell cycle arrest in response to stimuli, including salinomycin [64, 65]; and HDAC inhibitors have been shown to induce acetylation of histones H3 and H4, which leads to activation of p21 and cell cycle regulation [37]. We showed that LBH589 increased the acetylation of histone H3 and H4 in a dose-dependent manner and that p21 expression was up-regulated by the combination of LBH589 and salinomycin more highly than with either drug alone, suggesting that this drug combination synergistically induces cell cycle arrest and apoptosis through the up-regulation of p21. The synergistic inhibitory effect of the drug combination on cell proliferation was also confirmed by our finding that expression of Ki67, a marker of cell proliferation, was significantly decreased in vivo by the combination of LBH589 and salinomycin compared with either drug alone. Finally, it has been suggested that EMT plays a role in cancer “stemness” [16–18] by down-regulating epithelial markers and up-regulating mesenchymal markers; and HDAC inhibitors and salinomycin have each been shown to reduce EMT [28, 43]. Our in vivo and in vitro mechanistic studies demonstrated that a combination of LBH589 and salinomycin up-regulates epithelial markers and down-regulates mesenchymal markers, and this combination suppresses BCSC through regulating EMT-related markers.
Conclusions
Combination of LBH589 and salinomycin has a synergistic inhibitory effect on TNBC cell proliferation, as well as on BCSC properties such as self-renewal capacity and ALDH1 activity. This drug combination has a potent synergistic inhibitory effect on tumor growth of ALDH1-positive cells, does not appear to have any severe associated toxicity, and exerts its synergistic effects by inducing apoptosis, arresting the cell cycle, and regulating EMT in BCSCs. While neither of these drugs is currently used clinically, our study suggests that combination of an HDAC inhibitor and a BCSC-targeting agent, such as those described, could offer a new targeted therapeutic strategy for TNBC, and this drug combination warrants further clinical study in patients with TNBC.
Abbreviations
- TNBC:
-
Triple-negative breast cancer
- BCSC:
-
Breast cancer stem cell
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HDAC:
-
Histone deacetylase
- ALDH1:
-
Aldehyde dehydrogenase 1
- EMT:
-
Epithelial–mesenchymal transition
- DMSO:
-
Dimethyl sulfoxide
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NSG:
-
Non-obese diabetic/severe combined immunodeficiency gamma
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29. doi:10.3322/caac.21208
Anders CK, Carey LA (2009) Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer 9(Suppl 2):S73–S81. doi:10.3816/CBC.2009.s.008
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15 Pt 1):4429–4434. doi:10.1158/1078-0432.CCR-06-3045
Carey L, Winer E, Viale G, Cameron D, Gianni L (2010) Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 7(12):683–692. doi:10.1038/nrclinonc.2010.154
Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA (2008) Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res 14(24):8010–8018. doi:10.1158/1078-0432.CCR-08-1208
Oakman C, Viale G, Di Leo A (2010) Management of triple negative breast cancer. Breast 19(5):312–321. doi:10.1016/j.breast.2010.03.026
Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO (2007) Prognostic markers in triple-negative breast cancer. Cancer 109(1):25–32. doi:10.1002/cncr.22381
Dalerba P, Cho RW, Clarke MF (2007) Cancer stem cells: models and concepts. Annu Rev Med 58:267–284. doi:10.1146/annurev.med.58.062105.204854
Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S, Nakshatri H (2006) CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast cancer Res 8(5):R59. doi:10.1186/bcr1610
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS (2009) Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 69(4):1302–1313. doi:10.1158/0008-5472.CAN-08-2741
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100(9):672–679. doi:10.1093/jnci/djn123
Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5(4):275–284. doi:10.1038/nrc1590
Gangemi R, Paleari L, Orengo AM, Cesario A, Chessa L, Ferrini S, Russo P (2009) Cancer stem cells: a new paradigm for understanding tumor growth and progression and drug resistance. Curr Med Chem 16(14):1688–1703
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988. doi:10.1073/pnas.0530291100
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1(5):555–567. doi:10.1016/j.stem.2007.08.014
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715. doi:10.1016/j.cell.2008.03.027
Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV, Battula VL, Herschkowitz JI, Guerra R, Chang JT, Miura N, Rosen JM, Mani SA (2013) FOXC2 expression links epithelial–mesenchymal transition and stem cell properties in breast cancer. Cancer Res 73(6):1981–1992. doi:10.1158/0008-5472.CAN-12-2962
Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang Z, Yang CJ, Yuan L, Ouyang G (2011) Twist2 contributes to breast cancer progression by promoting an epithelial–mesenchymal transition and cancer stem-like cell self-renewal. Oncogene 30(47):4707–4720. doi:10.1038/onc.2011.181
Gunasinghe NP, Wells A, Thompson EW, Hugo HJ (2012) Mesenchymal–epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev 31(3–4):469–478. doi:10.1007/s10555-012-9377-5
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9(4):265–273. doi:10.1038/nrc2620
Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC (2005) Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol 45:495–528. doi:10.1146/annurev.pharmtox.45.120403.095825
Slingerland M, Guchelaar HJ, Gelderblom H (2014) Histone deacetylase inhibitors: an overview of the clinical studies in solid tumors. Anticancer Drugs 25(2):140–149. doi:10.1097/CAD.0000000000000040
Bose P, Dai Y, Grant S (2014) Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharmacol Ther 143(3):323–336. doi:10.1016/j.pharmthera.2014.04.004
Chen S, Ye J, Kijima I, Evans D (2010) The HDAC inhibitor LBH589 (panobinostat) is an inhibitory modulator of aromatase gene expression. Proc Natl Acad Sci USA 107(24):11032–11037. doi:10.1073/pnas.1000917107
Kubo M, Kanaya N, Petrossian K, Ye J, Warden C, Liu Z, Nishimura R, Osako T, Okido M, Shimada K, Takahashi M, Chu P, Yuan YC, Chen S (2013) Inhibition of the proliferation of acquired aromatase inhibitor-resistant breast cancer cells by histone deacetylase inhibitor LBH589 (panobinostat). Breast Cancer Res Treat 137(1):93–107. doi:10.1007/s10549-012-2332-x
Tate CR, Rhodes LV, Segar HC, Driver JL, Pounder FN, Burow ME, Collins-Burow BM (2012) Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat. Breast Cancer Res 14(3):R79. doi:10.1186/bcr3192
Miyazaki Y, Shibuya M, Sugawara H, Kawaguchi O, Hirsoe C (1974) Salinomycin, a new polyether antibiotic. J Antibiot 27(11):814–821
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES (2009) Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138(4):645–659. doi:10.1016/j.cell.2009.06.034
King TD, Suto MJ, Li Y (2012) The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem 113(1):13–18. doi:10.1002/jcb.23350
Booth L, Roberts JL, Conley A, Cruickshanks N, Ridder T, Grant S, Poklepovic A, Dent P (2014) HDAC inhibitors enhance the lethality of low dose salinomycin in parental and stem-like GBM cells. Cancer Biol Ther 15(3):305–316. doi:10.4161/cbt.27309
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res 70(2):440–446. doi:10.1158/0008-5472.CAN-09-1947
Gangopadhyay S, Nandy A, Hor P, Mukhopadhyay A (2013) Breast cancer stem cells: a novel therapeutic target. Clin Breast Cancer 13(1):7–15. doi:10.1016/j.clbc.2012.09.017
West AC, Johnstone RW (2014) New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 124(1):30–39. doi:10.1172/JCI69738
Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y (2008) NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat 111(3):419–427. doi:10.1007/s10549-007-9798-y
Huczynski A (2012) Salinomycin: a new cancer drug candidate. Chem Biol Drug Des 79(3):235–238. doi:10.1111/j.1747-0285.2011.01287.x
Dini L, Coppola S, Ruzittu MT, Ghibelli L (1996) Multiple pathways for apoptotic nuclear fragmentation. Exp Cell Res 223(2):340–347. doi:10.1006/excr.1996.0089
Gui CY, Ngo L, Xu WS, Richon VM, Marks PA (2004) Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA 101(5):1241–1246. doi:10.1073/pnas.0307708100
Richon VM, Sandhoff TW, Rifkind RA, Marks PA (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA 97(18):10014–10019. doi:10.1073/pnas.180316197
Dawson MA, Kouzarides T (2012) Cancer epigenetics: from mechanism to therapy. Cell 150(1):12–27. doi:10.1016/j.cell.2012.06.013
Wagner JM, Hackanson B, Lubbert M, Jung M (2010) Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenetics 1(3–4):117–136. doi:10.1007/s13148-010-0012-4
Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R (2007) FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 12(10):1247–1252. doi:10.1634/theoncologist.12-10-1247
Atadja P (2009) Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 280(2):233–241. doi:10.1016/j.canlet.2009.02.019
Rhodes LV, Tate CR, Segar HC, Burks HE, Phamduy TB, Hoang V, Elliott S, Gilliam D, Pounder FN, Anbalagan M, Chrisey DB, Rowan BG, Burow ME, Collins-Burow BM (2014) Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators. Breast Cancer Res Treat 145(3):593–604. doi:10.1007/s10549-014-2979-6
Rao R, Balusu R, Fiskus W, Mudunuru U, Venkannagari S, Chauhan L, Smith JE, Hembruff SL, Ha K, Atadja P, Bhalla KN (2012) Combination of pan-histone deacetylase inhibitor and autophagy inhibitor exerts superior efficacy against triple-negative human breast cancer cells. Mol Cancer Ther 11(4):973–983. doi:10.1158/1535-7163.MCT-11-0979
Conte P, Campone M, Pronzato P, Amadori D, Frank R, Schuetz F, Rea D, Wardley A, Britten C, Elias A (2009) Phase I trial of panobinostat (LBH589) in combination with trastuzumab in pretreated HER2-positive metastatic breast cancer (mBC): Preliminary safety and tolerability results. J Clin Oncol 27(15S):1081
Winston TJB, Alvaro MA, Donald WN, James NI, Edith AP (2012) Phase I study of panobinostat (LBH589) and letrozole in post-menopausal women with metastatic breast cancer. J Clin Oncol 30:e13501
Salvador MA, Wicinski J, Cabaud O, Toiron Y, Finetti P, Josselin E, Lelievre H, Kraus-Berthier L, Depil S, Bertucci F, Collette Y, Birnbaum D, Charafe-Jauffret E, Ginestier C (2013) The histone deacetylase inhibitor abexinostat induces cancer stem cells differentiation in breast cancer with low Xist expression. Clin Cancer Res 19(23):6520–6531. doi:10.1158/1078-0432.CCR-13-0877
Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS (2008) BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA 105(5):1680–1685. doi:10.1073/pnas.0711613105
Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, Badve S (2010) Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat 123(1):97–108. doi:10.1007/s10549-009-0619-3
Charafe-Jauffret E, Ginestier C, Bertucci F, Cabaud O, Wicinski J, Finetti P, Josselin E, Adelaide J, Nguyen TT, Monville F, Jacquemier J, Thomassin-Piana J, Pinna G, Jalaguier A, Lambaudie E, Houvenaeghel G, Xerri L, Harel-Bellan A, Chaffanet M, Viens P, Birnbaum D (2013) ALDH1-positive cancer stem cells predict engraftment of primary breast tumors and are governed by a common stem cell program. Cancer Res 73(24):7290–7300. doi:10.1158/0008-5472.CAN-12-4704
Bane A, Viloria-Petit A, Pinnaduwage D, Mulligan AM, O’Malley FP, Andrulis IL (2013) Clinical-pathologic significance of cancer stem cell marker expression in familial breast cancers. Breast Cancer Res Treat 140(1):195–205. doi:10.1007/s10549-013-2591-1
Alamgeer M, Ganju V, Kumar B, Fox J, Hart S, White M, Harris M, Stuckey J, Prodanovic Z, Schneider-Kolsky ME, Watkins DN (2014) Changes in aldehyde dehydrogenase-1 expression during neoadjuvant chemotherapy predict outcome in locally advanced breast cancer. Breast Cancer Res 16(2):R44. doi:10.1186/bcr3648
Marcato P, Dean CA, Liu RZ, Coyle KM, Bydoun M, Wallace M, Clements D, Turner C, Mathenge EG, Gujar SA, Giacomantonio CA, Mackey JR, Godbout R, Lee PW (2015) Aldehyde dehydrogenase 1A3 influences breast cancer progression via differential retinoic acid signaling. Mol Oncol 9(1):17–31. doi:10.1016/j.molonc.2014.07.010
Tsang JY, Huang YH, Luo MH, Ni YB, Chan SK, Lui PC, Yu AM, Tan PH, Tse GM (2012) Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Res Treat 136(2):407–417. doi:10.1007/s10549-012-2271-6
Debeb BG, Lacerda L, Xu W, Larson R, Solley T, Atkinson R, Sulman EP, Ueno NT, Krishnamurthy S, Reuben JM, Buchholz TA, Woodward WA (2012) Histone deacetylase inhibitors stimulate dedifferentiation of human breast cancer cells through WNT/beta-catenin signaling. Stem Cells 30(11):2366–2377. doi:10.1002/stem.1219
Lee CH, Hong HM, Chang YY, Chang WW (2012) Inhibition of heat shock protein (Hsp) 27 potentiates the suppressive effect of Hsp90 inhibitors in targeting breast cancer stem-like cells. Biochimie 94(6):1382–1389. doi:10.1016/j.biochi.2012.02.034
Danforth HD, Ruff MD, Reid WM, Johnson J (1977) Anticoccidial activity of salinomycin in floor-pen experiments with broilers. Poult Sci 56(3):933–938
Plumlee KH, Johnson B, Galey FD (1995) Acute salinomycin toxicosis of pigs. J Vet Diagn Invest 7(3):419–420
Kosal ME, Anderson DE (2004) An unaddressed issue of agricultural terrorism: a case study on feed security. J Anim Sci 82(11):3394–3400
Naujokat C, Steinhart R (2012) Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol 2012:950658. doi:10.1155/2012/950658
Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM (1998) Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem 273(50):33533–33539
Fang JY, Lu YY (2002) Effects of histone acetylation and DNA methylation on p21(WAF1) regulation. World J Gastroenterol 8(3):400–405
Wilson AJ, Byun DS, Popova N, Murray LB, L’Italien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM (2006) Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem 281(19):13548–13558. doi:10.1074/jbc.M510023200
Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9(6):400–414. doi:10.1038/nrc2657
Al Dhaheri Y, Attoub S, Arafat K, Abuqamar S, Eid A, Al Faresi N, Iratni R (2013) Salinomycin induces apoptosis and senescence in breast cancer: upregulation of p21, downregulation of survivin and histone H3 and H4 hyperacetylation. Biochim Biophys Acta 1830(4):3121–3135. doi:10.1016/j.bbagen.2013.01.010
Acknowledgments
We thank Sofia Loera for assistance with IHC staining, Lucy Brown for assistance with the flow cytometry analyses, Donna Isbell and Lauren Ratcliffe for animal care, and Nicola Solomon, Ph.D., for assistance in writing and editing the manuscript. The research was supported by Susan G. Komen for the Cure (KG080161), the National Cancer Institute (P30 CA033572), the City of Hope Women’s Cancers Program Idol Research Project Award (NK and TL), and the Carr-Baird family.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kai, M., Kanaya, N., Wu, S.V. et al. Targeting breast cancer stem cells in triple-negative breast cancer using a combination of LBH589 and salinomycin. Breast Cancer Res Treat 151, 281–294 (2015). https://doi.org/10.1007/s10549-015-3376-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3376-5