Abstract
B‐cell lymphoma‐2 (Bcl‐2) is one of the most important anti‐apoptotic genes. Although Bcl-2 promotes tumor cell survival in vitro, previous studies have shown conflicting results regarding the association between Bcl-2 and breast cancer survival. The aim of this study was to assess the prognostic significance of Bcl-2 according to the molecular tumor subtype in primary invasive breast cancer patients. The relationship between immunohistochemical Bcl-2 expression and overall survival was analyzed in 2399 primary invasive breast cancer patients treated by curative surgery. Patients were classified into four subtypes based on hormone receptor (HR) and human epidermal growth factor receptor‐2 (HER2) status: HR+/HER2−, HR+/HER2+, HR−/HER2+, and HR−/HER2−. A total of 1304 patients (54.4 %) had Bcl‐2 positive (+) tumors by immunohistochemistry. Bcl‐2 (+) tumors were significantly associated with a younger age (<50 years), early stage, lower grade, positive expression of HR, and negative expression of HER2. In the HR+/HER2− group, patients with Bcl‐2 (+) tumors showed a significantly better prognosis (p < 0.001). In contrast, there was no significant prognostic effect of Bcl‐2 expression in other subtypes. In multivariate analysis, Bcl‐2 positivity remained an independent, favorable prognostic factor in the HR+/HER2− subtype (hazard ratio, 0.609; 95 % confidence interval, 0.424–0.874; p < 0.007). The prognostic significance of Bcl‐2 expression differed according to the molecular subtype of breast cancer. The expression of Bcl‐2 was an independent, favorable prognostic factor in breast cancer patients with the HR+/HER2− subtype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a heterogeneous disease that shows variation in morphological features, molecular characteristics, clinical course, and response to treatment. Because of this clinical diversity, the identification of accurate prognostic factors in breast cancer is important to allow an individualized approach to patient management. The conventional prognostic factors in breast cancer are clinicopathologic parameters such as tumor size and lymph node status, and molecular markers such as hormone receptor (HR), human epidermal growth factor receptor‐2 (HER2), and Ki67. Although such factors provide useful prognostic information in breast cancer patients, they are insufficient to accurately predict the risk of recurrence [1–4]. In the past decade, gene expression profiling has provided more reliable prognostic and predictive information [5–7]. However, even if the efficacy of this approach is accepted, it might not be widely applicable due to its high cost and technical difficulty. In contrast, immunohistochemistry (IHC) is relatively inexpensive and widely available in most clinical laboratories. Therefore, there is a clear need for novel prognostic markers using IHC in breast cancer.
B‐cell lymphoma‐2 (Bcl‐2), the protein product of which can be assessed by IHC, is one of the most important anti-apoptotic genes. The Bcl‐2 family includes at least 30 kinds of protein and is divided into three different subclasses based on structural and functional features: anti‐apoptotic regulators such as Bcl‐2 and Bcl‐xL, pro‐apoptotic regulators such as Bax and Bak, and newly identified anti‐apoptotic proteins such as Bcl‐2L10 (BOO/DIVA) and Bcl‐2L12. Interactions between these groups of Bcl‐2 family proteins determine whether apoptosis is induced in cells [8–10].
Recently, the potential prognostic importance of Bcl‐2 expression in breast cancer has been reported in several studies [11–17]. However, the role of Bcl‐2 as a prognostic factor in breast cancer continues to be debated. Most previous studies reported that Bcl‐2 expression was associated with a favorable prognosis [11–15], although others demonstrated associations between Bcl‐2 gene expression and poor clinical outcomes such as chemoresistance, recurrence, or metastasis [16, 17]. These conflicting results might be explained by the heterogeneous nature of breast cancer, which is reflected by its classification into distinct molecular subtypes, each of which is associated with distinct clinical outcomes. In clinical practice, molecular classification using estrogen receptor (ER)/progesterone receptor (PR)/HER2 expression is routinely used to predict prognosis and to decide upon a treatment strategy.
In this study, we investigated the prognostic value of Bcl-2 based on each molecular subtype in primary invasive breast cancer patients.
Patients and methods
Database and study patients
Between January 1999 and September 2007, a total of 2399 primary invasive breast cancer patients who underwent curative surgery were retrospectively analyzed. The clinical and pathologic data were obtained from a database at the Korea Cancer Center Hospital Breast Cancer Center. The database includes age at surgery; cancer stage according to the 7th edition of the American Joint Committee on Cancer Staging Manual; histological grade using the Elston and Ellis modification of the Scarff‐Bloom‐Richardson grading system; expression of ER, PR, and HER2; treatment characteristics; and patient outcomes. This study was approved by the Korea Cancer Center Hospital Institutional Review Board (K‐1307‐002‐033).
IHC and classification of molecular subtype
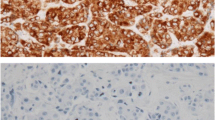
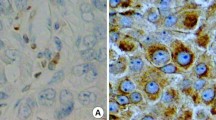
Expression of ER, PR, HER2, and Bcl-2 using IHC was evaluated by a pathologist immediately after surgery in each case. Positive staining for ER or PR was defined as staining of at least 10 % of nuclei in ten high-power fields, and HER2 positivity was defined as 3(+) on IHC staining or HER2 gene amplification by fluorescence in situ hybridization. Bcl-2 expression was detected using a streptavidin–biotin peroxidase complex IHC technique. Bcl-2-postivity was defined as the presence of cytoplasmic staining in >10 % of malignant cells, following the practice used in most previous studies [11, 12]. Patients were classified into the following 4 molecular subtypes based on tumor expression of ER, PR, and HER2: (a) HR+/HER2− (ER- and/or PR-positive and HER2-negative), (b) HR+/HER2+ (ER- and/or PR-positive and HER2-positive), (c) HR−/HER2+ (ER-negative, PR-negative, and HER2-positive), and (d) HR−/HER2− (ER-negative, PR-negative, and HER2-negative).
Statistical analysis
Analysis of clinicopathologic characteristics according to Bcl‐2 expression was performed using Student’s t test and the chi‐square test. For survival analysis, overall survival (OS) was defined as the time from the first diagnosis of primary breast cancer to death from any cause. The Kaplan–Meier method with the log‐rank test was used. The Cox proportional hazards model was used for multivariate survival analyses. In all tests, values of p < 0.05 were regarded as statistically significant.
Results
Clinicopathologic characteristics
A total of 2399 patients with a median age of 47 years (range, 22–87 years) were included in this analysis. The median follow‐up period was 99.7 months (range, 2.2–171.9 months). The clinicopathologic characteristics and treatment details of patients are summarized in Table 1. There were 1553 patients (63.9 %) with HR+ tumors, and 579 patients (24.1 %) with HER2+ tumors. Positive Bcl‐2 tumor expression was identified in 1304 patients (54.4 %). More than half of the patients (1277, 53.2 %) had HR+/HER2− type tumors, whereas 256 (10.7 %) had HR+/HER2+, 323 (13.5 %) had HR−/HER2+, and 543 (22.6 %) had HR−/HER2− tumors. Mastectomy was performed in 1720 cases (71.7 %) and breast-conserving surgery (BCS) in 679 (28.3 %) cases. Most patients (2334, 97.2 %) received chemotherapy, 1647 patients (68.7 %) underwent endocrine therapy, and 917 (38.2 %) patients underwent radiotherapy.
Bcl‐2 expression and clinicopathologic variables
There was a positive Bcl-2 tumor expression in 1304 patients (54.4 %). The associations between clinicopathologic characteristics and Bcl‐2 expression are summarized in Table 2. Bcl-2 expression was significantly associated with younger age (p = 0.001), early-stage disease (p < 0.001), lower tumor grade (p < 0.001), HR positivity (p < 0.001), and HER2 negativity (p < 0.001). HR+/HER2− type tumors most frequently expressed Bcl-2 (p < 0.001). There was no significant association between Bcl‐2 status and lymph node metastasis in this analysis.
Bcl‐2 expression and survival outcome
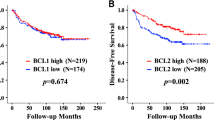
During the follow‐up period, 339 patients died (14.1 %). Bcl‐2 expression in breast cancer was significantly related to a favorable prognosis (p < 0.001) (Fig. 1). When stratified by molecular subtype, patients in the HR+/HER2− group with Bcl-2 positive tumors had a significantly longer OS (p < 0.001). There was no significant prognostic effect of Bcl‐2 tumor expression in patients with other molecular subtypes (Fig. 2). The clinicopathologic variables affecting OS in each molecular subtype are summarized in Table 3. In the HR+/HER2− group, younger age (p < 0.001), early-stage disease (p < 0.001), lower tumor grade (p < 0.001), and Bcl-2 expression (p < 0.001) were all significantly associated with better 5‐year OS. When the multivariate model included Bcl-2, age, tumor size, lymph node metastases, and histological grade, Bcl‐2 positivity remained an independent, favorable prognostic factor for the HR+/HER2− subtype (hazard ratio, 0.609; 95 % confidence interval, 0.424–0.874; p < 0.007) (Table 4).
Discussion
In the present study, we found that Bcl‐2 tumor expression was a favorable prognostic indicator in breast cancer patients, and that among patients with HR+/HER2‐tumors it was a strong independent prognostic factor for OS. We also found that Bcl‐2 expression was strongly associated with ER and/or PR positivity, young age, small tumor size, early-stage disease, low histologic grade, and negative HER‐2 status.
Bcl‐2 is one of the most potent anti-apoptotic proteins expressed on the outer mitochondrial membrane. Overexpression of Bcl-2 inhibits apoptosis induced by various cytotoxic stimuli, including cytokine deprivation, irradiation, and chemotherapeutic agents [8–10]. Some studies have demonstrated that Bcl‐2 overexpression results in enhanced tumorigenicity and metastatic potential by increased cell invasion and migration in vitro [19, 24]. Therefore, Bcl‐2 has been regarded as a potential therapeutic target in cancer. Phase II and III clinical trials have been conducted to evaluate the antisense oligonucleotide oblimersen, which down-regulates Bcl-2 mRNA and Bcl-2 protein, together with other anticancer drugs to treat chronic lymphocytic leukemia, acute myeloid leukemia, multiple myeloma, small cell lung cancer, non‐Hodgkin’s lymphoma, and melanoma [20, 21]. However, Bcl‐2 expression has been paradoxically associated with a favorable prognosis in many solid tumors, particularly in breast cancer [11, 22, 23]. These conflicting results have been consistently demonstrated in many previous studies on Bcl‐2 expression in breast cancer [11], although the reason for this inconsistency remains unclear. It is not even known whether Bcl‐2 positivity is simply a surrogate marker for other indicators of a favorable prognosis such as ER-positive and HER2-negative staining, or if Bcl‐2 directly regulates molecular mechanisms and cellular processes enhancing a favorable prognosis. Previous work suggested that Bcl‐2 may have a similar role to that of ER in promoting tumor growth, but despite this, it is actually associated with a better prognosis [25]. It has also been hypothesized that Bcl‐2 might inhibit cell proliferation regardless of its anti‐apoptotic effect [26], which was supported by clinical research showing that Bcl‐2 expression was inversely related to that of Ki‐67 [18]. An in vitro study of the effect of Bcl-2 expression on the growth of several solid tumor cell lines has suggested that it may exert a distinct biological effect in different cell types [27]. However, there are still significant unsolved questions concerning the exact mechanism by which Bcl‐2 might exert its protective effect in breast cancer.
In an effort to better understand the relationship between Bcl‐2 expression and its paradoxical effect in breast cancer, we focused on the molecular heterogeneity of this disease. The heterogeneity of breast cancer is well established at the histological level and in terms of clinical outcome, and as a result, it has been classified into clinically meaningful groups showing a relationship with survival, disease relapse, and response to treatment. At the molecular level, Perou et al. identified four breast cancer subtypes on the basis of gene‐expression profiling, which are related to different biological and prognostic features [28]. While microarray analysis is known to be the most accurate method for determining the subtype of breast cancers, IHC can be an important alternative method as it is more cost effective and readily available in most surgical pathology laboratories. It has been also shown that the molecular classification by IHC corresponds reasonably well to microarray-based classification of breast cancer [29]. In this context, we investigated whether Bcl‐2 is a prognostic marker within the different molecular subtypes of breast cancer. Our results showed that Bcl‐2 is a significant independent prognostic factor in HR+/HER2− subtype breast cancer (hazard ratio, 0.609; p = 0.007), but not for other subtypes. The HR+/HER2− subtype is generally considered to have a low risk of recurrence, and some patients with this subtype and negative lymph nodes may avoid chemotherapy [30]. Microarray‐based gene expression profiling including MammaPrint and Oncotype DX is presently used in clinical practice to help identify patients who can safely avoid adjuvant chemotherapy and its potentially harmful side effects [6, 7]. However, due to their prohibitive cost, they have not been routinely used in clinical practice. In contrast, IHC analysis of Bcl‐2 protein expression is a simple, well‐validated, inexpensive, and widely available method, and Bcl‐2 analysis in HR+/HER2− tumors could provide more accurate prognostic information and help to determine whether adjuvant or salvage chemotherapy should be administered.
This study has several limitations, the most important of which is its retrospective nature and single-institution basis using previously recorded clinical data. Another limitation is the lack of a standardized measurement of Bcl-2, as Bcl-2 status was evaluated in each case after surgery by several different pathologists. However, the results of our study are particularly meaningful because it included a large number of patients to investigate the prognostic significance of Bcl-2 and revealed that Bcl-2 is a powerful prognostic marker in patients with HR+/HER2‐tumors.
In conclusion, while it is debatable whether Bcl‐2 has significant prognostic value in breast cancer, our results demonstrate that Bcl-2 expression is associated with a favorable prognosis, and that it is a potent independent prognostic factor in patients with HR+/HER2‐tumors. We believe that identifying Bcl-2 expression in HR+/HER2− subtype tumors can help to guide more tailored therapies.
References
Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63:181–187. doi:10.1001/jama.1964.03060170107050
Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP (1980) Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer 45:2917–2924. doi:10.1002/1097-0142(19800615)45:12<2917:aid-cncr2820451203>3.0.co;2-m
Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, Symmans WF, Pusztai L, Bloom KJ (2003) The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 8:307–325. doi:10.1634/theoncologist.8-4-307
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182:311–322. doi:10.1002/(sici)1097-4652(200003)182:3<311:aid-jcp1>3.0.co;2-9
van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536. doi:10.1038/415530a
Sparano JA, Paik S (2008) Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 26:721–728. doi:10.1200/jco.2007.15.1068
Cardoso F, Van’t Veer L, Rutgers E, Loi S, Mook S, Piccart-Gebhart MJ (2008) Clinical application of the 70-gene profile: the MINDACT trial. J Clin Oncol 26:729–735. doi:10.1200/jco.2007.14.3222
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326. doi:10.1126/science.281.5381.1322
Cory S, Huang DC, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22:8590–8607. doi:10.1038/sj.onc.1207102
Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A (2012) The role of Bcl2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol 2012:524308. doi:10.1155/2012/524308
Hellemans P, van Dam PA, Weyler J, van Oosterom AT, Buytaert P, Van Marck E (1995) Prognostic value of bcl-2 expression in invasive breast cancer. Br J Cancer 72:354–360. doi:10.1038/bjc.1995.338
Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, Ellis IO, Huntsman D, Caldas C (2006) Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham prognostic index. Clin Cancer Res 12:2468–2475. doi:10.1158/1078-0432.ccr-05-2719
Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, Baglietto L, Severi G, Giles GG, McLean CA, Callagy G, Green AR, Ellis I, Gelmon K, Turashvili G, Leung S, Aparicio S, Huntsman D, Caldas C, Pharoah P (2010) BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy. Br J Cancer 103:668–675. doi:10.1038/sj.bjc.6605736
Silvestrini R, Veneroni S, Daidone MG, Benini E, Boracchi P, Mezzetti M, Di Fronzo G, Rilke F, Veronesi U (1994) The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer 86:499–504. doi:10.1093/jnci/86.7.499
Lipponen P, Pietilainen T, Kosma VM, Aaltomaa S, Eskelinen M, Syrjanen K (1995) Apoptosis suppressing protein bcl-2 is expressed in well-differentiated breast carcinomas with favourable prognosis. J Pathol 177:49–55. doi:10.1002/path.1711770109
Yu B, Sun X, Shen HY, Gao F, Fan YM, Sun ZJ (2010) Expression of the apoptosis-related genes Bcl-2 and BAD in human breast carcinoma and their associated relationship with chemosensitivity. J Exp Clin Cancer Res 29:1–7. doi:10.1186/1756-9966-29-107
Yang Q, Moran MS, Haffty BG (2009) Bcl-2 expression predicts local relapse for early stage breast cancer receiving conserving surgery and radiotherapy. Breast Cancer Res Treat 115:343–348. doi:10.1007/s10549-008-0068-4
Martinez-Arribas F, Alvarez T, Del Val G, Martin-Garabato E, Nunez-Villar MJ, Lucas R, Sánchez J, Tejerina A, Schneider J (2007) Bcl-2 expression in breast cancer: a comparative study at the mRNA and protein level. Anticancer Res 27:219–222
Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ Jr (2000) An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res 60:6101–6110
Kang MH, Reynolds CP (2009) Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res 15:1126–1132. doi:10.1158/1078-0432.CCR-08-0144
O’Brien S, Moore JO, Boyd TE et al (2009) 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol 27:5208–5212. doi:10.1200/JCO.2009.22.5748
Pezzella F, Turley H, Kuzu I, Tungekar MF, Dunnill MS, Pierce CB, Harris A, Gatter KC, Mason DY (1993) Bcl-2 protein in non-small-cell lung cancer. N Engl J Med 329:690–694. doi:10.1056/NEJM199309023291003
Manne U, Myers RB, Moron C, Poczatek RB, Dillard S, Weiss H, Brown D, Srivastava S, Grizzle WE (1997) Prognostic significance of Bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int J Cancer 74:346–358. doi:10.1002/(SICI)1097-0215(19970620)74:3<346::AID-IJC19>3.0.CO;2-9
Del Bufalo D, Biroccio A, Leonetti C, Zupi G (1997) Bcl-2 overexpression enhances the metastatic potential of a human breast cancer line. FASEB J 11:947–953
DiVito KA, Berger AJ, Camp RL, Dolled-Filhart M, Rimm DL, Kluger HM (2004) Automated quantitative analysis of tissue microarrays reveals an association between high Bcl-2 expression and improved outcome in melanoma. Cancer Res 64:8773–8777. doi:10.1158/0008-5472.CAN-04-1387
Knowlton K, Mancini M, Creason S, Morales C, Hockenberry D, Anderson BO (1998) Bcl-2 slows in vitro breast cancer growth despite its antiapoptotic effect. J Surg Res 76:22–26. doi:10.1006/jsre.1998.5277
Pietenpol JA, Papadopoulos N, Markowitz S, Willson JK, Kinzler KW, Vogelstein B (1994) Paradoxicial inhibition of solid tumor cell growth by bcl2. Cancer Res 54:3714–3717
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406:747–752. doi:10.1038/35021093
Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 29:4273–4278. doi:10.1200/JCO.2010.31.2835
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC (2006) Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 295:2492–2502. doi:10.1001/jama.295.21.2492
Acknowledgments
This research was supported by grants from the Radiological Translational Research Program (50451-2013).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study complies with the current laws of South Korea inclusive ethics approval.
Author information
Authors and Affiliations
Corresponding author
Additional information
Min-Ki Seong and Ju-Young Lee have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Seong, MK., Lee, JY., Byeon, J. et al. Bcl-2 is a highly significant prognostic marker of hormone-receptor-positive, human epidermal growth factor receptor-2-negative breast cancer. Breast Cancer Res Treat 150, 141–148 (2015). https://doi.org/10.1007/s10549-015-3305-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3305-7