Abstract
Adding a taxane to anthracycline-based adjuvant chemotherapy prolongs survival in node-positive early breast cancer. However, which is the preferable taxane in a dose-dense regimen remains unknown. We conducted a randomized study to compare the efficacy of dose-dense paclitaxel versus docetaxel following 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) as adjuvant chemotherapy in women with node-positive early breast cancer. Following surgery women with HER2-negative breast cancer and at least one infiltrated axillary lymph node were randomized to receive four cycles of FEC (700/75/700 mg/m2) followed by four cycles of either paclitaxel (175 mg/m2) or docetaxel (75 mg/m2). All cycles were administered every 14 days with G-CSF support. The primary endpoint was disease-free survival (DFS) at 3 years. Between 2004 and 2007, 481 women were randomized to paclitaxel (n = 241) and docetaxel (n = 240). After a median follow-up of 6 years, 51 (21 %) and 48 (20 %) women experienced disease relapse (p = 0.753) and there was no significant difference in DFS between the paclitaxel- and docetaxel-treated groups (3-year DFS 87.4 vs. 88.3 %, respectively; median DFS not reached; p = 0.633). Toxicities were manageable, with grade 2–4 neutropenia in 21 versus 31 % (p = 0.01), thrombocytopenia 0.8 versus 3.4 % (p = 0.06), any grade neurotoxicity 17 versus 7.5 % (p = 0.35) and onycholysis 4.9 versus 12.1 % (p = 0.03) for patients receiving paclitaxel and docetaxel, respectively. There were no toxic deaths. Dose-dense paclitaxel versus docetaxel after FEC as adjuvant chemotherapy results in a similar 3-year DFS rate in women with axillary node-positive early breast cancer. Due to its more favorable toxicity profile, paclitaxel is the taxane of choice in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adjuvant chemotherapy substantially reduces the risk of disease recurrence and death among women with early breast cancer [1]. The addition of taxane to an anthracycline-containing regimen, either sequentially or concurrently, further reduces the risk of relapse. Two studies in which patients received four cycles of paclitaxel every 3 weeks after four cycles of doxorubicin and cyclophosphamide (AC regimen) established a new standard of care for operable breast cancer patients and led to regulatory approval of paclitaxel for axillary lymph node-positive early breast cancer [2, 3]. Another study demonstrating that the concurrent administration of docetaxel with doxorubicin and cyclophosphamide was more effective than fluorouracil, doxorubicin, and cyclophosphamide led to regulatory approval of docetaxel for node-positive disease [4]. Recently, BCIRG 001 investigators provided evidence that the initial therapeutic benefit of the docetaxel-containing regimen seen at the 5-year follow-up was maintained at 10 years, both in terms of disease-free [hazard ratio (HR) 0.80, 95 % CI 0.68–0.93; p = 0.043] and overall survival (HR 0.74, 95 % CI 0.61–0.90; p = 0.02) [5]. Nowadays, taxanes in combination with anthracyclines are considered a standard treatment approach for women with either node-negative or node-positive disease, based on the results of the aforementioned studies as well as others [6–10].
Initial preclinical and indirect clinical evidence suggested that docetaxel was more effective than paclitaxel and that weekly paclitaxel was better than the conventional 3-weekly schedule of administration [11]. In the metastatic setting, phase III trials demonstrated that docetaxel every 3 weeks [12] or weekly paclitaxel [13] were indeed superior to 3-weekly paclitaxel. However, in the adjuvant setting, weekly paclitaxel after standard adjuvant chemotherapy with AC has been shown to improve disease-free and overall survival compared to docetaxel (administered either weekly or every 3 weeks) and 3-weekly paclitaxel [14].
Administration of adjuvant chemotherapy on an accelerated administration (dose-dense therapy) aims to improve treatment results over standard dosing schedules. A meta-analysis of dose-dense versus standard dosing regimens including data from ten trials and over 11,000 women, reported that dose-dense treatment was associated with an improvement in both disease-free and overall survival [15].
Thus far, no study has compared head-to-head the two taxanes in the dose-dense adjuvant setting. To address this question we conducted a randomized trial of dose-dense G-CSF-supported paclitaxel versus docetaxel, administered every 2 weeks following 5-fluorouracil-epirubicin-cyclophosphamide (FEC) regimen as adjuvant chemotherapy in women with axillary lymph node-positive early breast cancer.
Patients and methods
This randomized study was conducted mainly at 11 sites of the Hellenic Oncology Research Group (HORG). The protocol and related materials were approved by the institutional review boards and independent ethics committees and registered under the NCT00431080 identifier at the clinicaltrials.gov website. The study was conducted in compliance with Good Clinical Practice and the Declaration of Helsinki. Written informed consent was required from all patients to enter the study.
Eligible patients should have undergone either lumpectomy or modified radical mastectomy with tumor-free surgical margins plus axillary node dissection. The tumor had to be invasive adenocarcinoma with at least one positive axillary lymph node on pathologic examination. Determination of the estrogen receptor (ER) and progesterone receptor (PR) status of the primary tumor had to be performed before random assignment and patients with HER2-positive tumors (as determined by local institutional laboratories) were not eligible for this study. Normal hematologic parameters and adequate hepatic, renal, and cardiac function were mandatory. Patients with previous history of invasive breast cancer or ductal carcinoma-in situ (in either breast) were ineligible, as were patients who had received any prior radiation, chemotherapy, or hormonal therapy.
Chemotherapy
All women received upfront epirubicin (75 mg/m2 of body-surface area, given by slow intravenous (iv) push during a period of 5–15 min), cyclophosphamide (700 mg/m2 by iv infusion for 30–60 min), and 5-fluorouracil (700 mg/m2 by slow iv push during a period of 5–15 min) every 2 weeks for four cycles. This therapy was followed by the taxane treatment with the randomization performed before the commencement of FEC. Women were randomly assigned to 175 mg/m2 of paclitaxel given by iv infusion over 3 h every 2 weeks for four doses or 75 mg/m2 of docetaxel given by iv infusion for 1 h every 2 weeks for four doses. All patients received primary prophylaxis with filgrastim (5 μg/kg rounded to either 300 or 480-μg total dose) on days 3–10 of each cycle.
Hormonal & radiation therapy & follow-up
Patients who had breast-conserving surgery received standard radiotherapy after the completion of chemotherapy. Women who had a modified radical mastectomy were also permitted to receive radiotherapy after completion of chemotherapy, if they had large (>5 cm) primary tumors and/or more than three infiltrated axillary nodes. Patients with hormone receptor-positive disease received 20 mg of tamoxifen daily or an aromatase inhibitor both for a 5-year period depending on the menopausal status at diagnosis. Premenopausal patients treated with tamoxifen were also given an LHRH agonist for the initial 2–3 years at the discretion of the treating physician.
Patients were followed every 3–4 months for the first 2 years, every 6 months for the subsequent 3 years and yearly thereafter. History, physical examination and routine labs were performed at each visit, surveillance mammograms were done yearly and imaging studies were ordered when clinically indicated and at the discretion of the treating physician.
Study endpoints
The primary endpoint of the study was to compare the 3-year disease-free survival (DFS) rates between the paclitaxel- and docetaxel-treated groups. The DFS was defined as the time from randomization to the date of breast cancer recurrence (either locoregional or distant), contralateral breast cancer diagnosis, non-breast second primary cancer or death from any cause, whichever occurred first. Secondary end-points were overall survival (OS), defined as the time from the date of random assignment to death from any cause and the toxicity profile of the regimens. Toxicity grading used the Common Terminology Criteria for Adverse Events of the National Cancer Institute version 3.0.
Statistical analysis
Based on the assumption of a 3-year DFS rate equal to 85 % for the paclitaxel arm, 239 patients were required to enroll on each arm for the trial to detect an 8 % difference between the two arms with 80 % power and a type I error of 5 % (two sided). Stratification parameters for the random assignment were the menopausal status (pre versus post), the number of infiltrated axillary nodes (1–3 vs. 4–10 vs. >10) and the tumor hormone receptor status (ER and/or PR positive vs. both negative). All patients who received at least one cycle of treatment were included in the analysis. The DFS and OS rates were calculated by the Kaplan–Meier method. The comparison of the treatment arms was assessed using the log-rank test. The independent effect of treatment and other prognostic factors on DFS and OS was analyzed by Cox’s proportional hazards model. Quantitative factors were compared by Pearson’s χ2 contingency table analysis or Fisher’s test whenever appropriate. All p values <0.05 were considered statistically significant for all comparisons. Clinical data were held centrally (Clinical Trial Office, Hellenic Oncology Research Group) and analyzed using the SPSS (version 22.0) program. Data were current as of March 2014.
Results
Patients
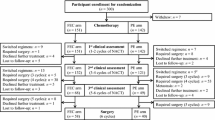
Between September 2004 and December 2007, 495 patients were assessed for enrollment, of whom 481 (97 %) were eligible for the study. Eight patients did not meet all the eligibility criteria and six patients withdrew their consent. Therefore 481 patients were centrally randomized to either paclitaxel (n = 241 patients) or docetaxel (n = 240 patients) (Fig. 1; CONSORT diagram of the study). The two patient groups were well balanced regarding their prognostic characteristics (Table 1). The median age was 55 years (range 26–75). One-third of the patients were premenopausal at diagnosis. Approximately, 55 % had 1–3 positive nodes, while 13 % had more than ten axillary nodes with tumor involvement. The tumor was positive for estrogen receptor, progesterone receptor, or both in roughly 85 % of patients and negative for both hormonal receptors in 15 %.
Treatment
The proportion of women who received all four cycles of FEC were 99.6 % versus 99.2 % for the paclitaxel and docetaxel arm, respectively (p = 0.560). Similarly, the proportion of women who received all four taxane cycles was 90.0 %, and 96.3 %, for the paclitaxel and docetaxel group, respectively (p = 0.007). The mean number of taxane cycles received was 3.7 for the paclitaxel group and 3.8 for the docetaxel group (p = 0.074). The reasons for treatment discontinuation in the paclitaxel group were adverse events (n = 16), patient refusal to continue (n = 7), and disease progression (n = 1) while in the docetaxel group were adverse events (n = 4), and patient refusal to continue (n = 5). Treatment was administered on time without delay in 93.6 % and 92.6 % of cycles in the paclitaxel and docetaxel group, respectively (p = 0.251). Similarly, dose reduction due to toxicity was required in 2.8 % and 1.7 % of administered cycles in the paclitaxel and docetaxel group, respectively (p = 0.025).
Disease-free and overall survival
After a median follow-up of 73 and 72.7 months, 51 (21 %) and 48 (20 %) patients had experienced disease recurrence (p = 0.753), while 27 (11 %) and 25 (10 %) patients had died in the paclitaxel and docetaxel arm, respectively (p = 0.781). Although the median DFS has not been reached yet, there was no significant difference in DFS between the paclitaxel- and the docetaxel-treated group (HR 1.101; 95 % CI 0.742–1.633; p = 0.633). Figure 2a illustrates the Kaplan–Meier curves for disease-free survival in the two treatment groups. The 3-year DFS rates were 87.4 % for the group treated with paclitaxel and 88.3 % for the docetaxel group. Similarly, there was no difference in overall survival between the two groups (p = 0.814; Fig. 2b).
Adverse events
Fifty-three percent of patients receiving paclitaxel developed grade 2–4 adverse events, compared to 60.4 % of those treated with docetaxel (p = 0.106). Table 2 shows the toxicity profile of each taxane arm. The higher proportion of toxicity in the group receiving docetaxel was mainly due to the 31 % incidence of neutropenia as compared with 21 % for the paclitaxel group (p = 0.01). However, this did not result in a higher rate of febrile neutropenia (5 and 4 cases, respectively), thanks to prophylaxis with filgrastim. The incidence of grade 3 or 4 neuropathy in the two groups was quite low (2.1 vs. 0.8 %), but overall the group receiving paclitaxel had a numerically higher incidence of neuropathy of any grade (17 %) compared to the docetaxel treatment group (7.5 %) (p = 0.35). Finally, onycholysis grade 2–4 was the only non-hematologic toxicity with significant difference between the two groups (1.2 % for the paclitaxel and 4.6 % for the docetaxel group, p = 0.03). Hopefully, there were no toxic deaths.
Discussion
This trial was designed to compare the efficacy of dose-dense paclitaxel with that of docetaxel in nearly 500 women with axillary lymph node-positive early breast cancer. After a median follow-up of nearly 6 years, no significant difference in disease-free or overall survival between the two groups was found. In comparison with patients treated with paclitaxel, those who received docetaxel had significantly more severe neutropenia and nail toxicity. The higher toxicity observed in the docetaxel arm is consistent with a previous study that compared docetaxel, epirubicin, and cyclophosphamide every 3 weeks with dose-dense 2-weekly schedules of epirubicin and cyclophosphamide followed by docetaxel or the reverse sequence [16]. As in our study, they found that the most frequent hematologic toxicity was neutropenia; also it was more frequent in the group receiving epirubicin and cyclophosphamide followed by docetaxel.
For patients with node-positive early breast cancer, the combination of a taxane with an anthracycline as adjuvant therapy has been investigated in several studies [2–10, 14, 17, 18]. Most of the studies have been focusing on the optimal sequence of the anthracyclines and taxanes as well as the preferable taxane and the optimal schedule of administration. Some of those studies concluded that the dose-dense regimens (2-week intervals) not only prolong disease-free and overall survival but also that they are as safe and as well tolerated as the 3-weekly conventional regimens [19–21]. Two important aspects of current adjuvant chemotherapy including taxane in early breast cancer, have been addressed in a study reported by Sparano et al. [14]. The 2 × 2 factorial design of the trial allowed the comparison of paclitaxel every 3 weeks for four cycles with three experimental regimens: paclitaxel every week for 12 cycles, docetaxel every 3 weeks for four cycles, or docetaxel every week for 12 cycles. Each regimen was given after standard AC. The comparison of the three experimental groups with the group receiving standard paclitaxel treatment showed that the group of weekly paclitaxel had significantly improved disease-free and overall survival while the group receiving docetaxel every 3 weeks had significantly improved disease-free survival only. This came at the cost of more moderate-to-severe neuropathy for the patients treated with weekly paclitaxel and more severe neutropenia and its associated complications for the group receiving docetaxel every 3 weeks.
More recently, breast cancer patients with node-positive or high-risk node-negative disease have been shown to obtain the same disease control benefit with weekly low-dose paclitaxel (80 mg/m2 for 12 cycles) as compared with a higher dose given every 2 weeks (175 mg/m2 for six cycles) [22]. That study also had a 2 × 2 factorial design to compare additionally the weekly administration of doxorubicin/cyclophosphamide versus the dose-dense schedule. The estimated 5-year survival rate was 82 % among patients receiving weekly paclitaxel and 81 % among those treated with the dose-dense regimen. The rates of grade 3–4 adverse events were also similar, but the profiles differed. The weekly regimen was associated with more neutropenia (6 vs. 1 %), whereas the dose-dense regimen with more allergy-related reactions (14 vs. 6 %), musculoskeletal pain (11 vs. 3 %), and neuropathy (17 vs. 10 %). Overall, the toxicity profile favored weekly paclitaxel, but we have to note that the higher toxicity profile of dose-dense paclitaxel might be due to the higher cumulative dose (six cycles of therapy instead of the usual four).
Our study has certain limitations that should be taken into consideration. Since this was not conducted as a conventional non-inferiority study, we cannot exclude that there is a small difference in the 3-year DFS. However, the Kaplan–Meier curves and computed hazard ratios suggest very little, if any difference between the two arms. In addition, at the time when the study was commenced, none of the arms could have been considered as standard treatment for node-positive early breast cancer in Europe. This was in contrast to the practice in the USA where the dose-dense AC followed by paclitaxel regimen was quickly adopted as a standard regimen, based on the report of CALGB 9741 in 2003 [19]. Following the report of the studies by Sparano et al. [14] and the SO121 by SWOG [22], we believe that our study completes the puzzle of taxanes in the adjuvant setting of women with early breast cancer. Weekly paclitaxel after dose-dense AC remains the regimen of choice due to its more favorable toxicity profile. However, when a shorter course of treatment is important for patients (e.g., young professionals) the dose-dense administration of paclitaxel should be preferred over docetaxel, due to less toxicity.
In conclusion, treatment with dose-dense paclitaxel or docetaxel after dose-dense FEC results in a similar 3-year DFS rate as adjuvant treatment for women with node-positive early breast cancer. However, the toxicity of the two taxanes is different with docetaxel causing more neutropenia, thrombocytopenia, and onycholysis and paclitaxel more neurotoxicity. Therefore due its more favorable toxicity profile, paclitaxel remains the taxane of choice in this setting.
References
Radcliffe Infirmary CTSU (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM et al (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23:3686–3696
Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ et al (2003) Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976–983
Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313
Mackey JR, Martin M, Pienkowski T, Rolski J, Guastalla JP et al (2013) Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol 14:72–80
Roche H, Fumoleau P, Spielmann M, Canon JL, Delozier T et al (2006) Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol 24:5664–5671
Martin M, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L et al (2008) Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst 100:805–814
Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER et al (2010) Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 362:2053–2065
Eiermann W, Pienkowski T, Crown J, Sadeghi S, Martin M et al (2011) Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol 29:3877–3884
Swain SM, Tang G, Geyer CE Jr, Rastogi P, Atkins JN et al (2013) Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: the NSABP B-38 trial. J Clin Oncol 31:3197–3204
Saloustros E, Mavroudis D, Georgoulias V (2008) Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother 9:2603–2616
Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L et al (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551
Seidman AD, Berry D, Cirrincione C, Harris L, Muss H et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 non overexpressors: final results of cancer and leukemia group B protocol 9840. J Clin Oncol 26:1642–1649
Sparano JA, Wang M, Martino S, Jones V, Perez EA et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Bonilla L, Ben-Aharon I, Vidal L, Gafter-Gvili A, Leibovici L et al (2010) Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst 102:1845–1854
Piedbois P, Serin D, Priou F, Laplaige P, Greget S et al (2007) Dose-dense adjuvant chemotherapy in node-positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol 18:52–57
Trudeau M, Charbonneau F, Gelmon K, Laing K, Latreille J et al (2005) Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol 6:886–898
Polyzos A, Malamos N, Boukovinas I, Adamou A, Ziras N et al (2010) FEC versus sequential docetaxel followed by epirubicin/cyclophosphamide as adjuvant chemotherapy in women with axillary node-positive early breast cancer: a randomized study of the Hellenic Oncology Research Group (HORG). Breast Cancer Res Treat 119:95–104
Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup trial C9741/cancer and leukemia group B trial 9741. J Clin Oncol 21:1431–1439
Jones RL, Walsh G, Ashley S, Chua S, Agarwal R et al (2009) A randomised pilot phase II study of doxorubicin and cyclophosphamide (AC) or epirubicin and cyclophosphamide (EC) given 2 weekly with pegfilgrastim (accelerated) vs 3 weekly (standard) for women with early breast cancer. Br J Cancer 100:305–310
Burnell M, Shepherd K, Gelmon K, Bramwell V, Walley B, et al (2012) A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: results of the final relapse free survival analysis. Cancer Res 72 [24 Suppl 3, abstr P1-13-01]
Budd GT, Barlow WE, Moore HCF, Hobday TJ, Stewart JA, et al (2013) Comparison of two schedules of paclitaxel as adjuvant therapy for breast cancer. J Clin Oncol 31 (suppl; abstr CRA1008)
Acknowledgments
We acknowledge the assistance of Dora Hatzidaki and Vasso Athanasaki in the preparation of this manuscript.
Conflict of interest
The authors have declared no pertinent conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov Identifier: NCT00431080.
Rights and permissions
About this article
Cite this article
Saloustros, E., Malamos, N., Boukovinas, I. et al. Dose-dense paclitaxel versus docetaxel following FEC as adjuvant chemotherapy in axillary node-positive early breast cancer: a multicenter randomized study of the Hellenic Oncology Research Group (HORG). Breast Cancer Res Treat 148, 591–597 (2014). https://doi.org/10.1007/s10549-014-3202-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3202-5