Abstract
The purpose of this study was to assess the safety and efficacy of progressive resistance training (PRT) in breast cancer. Randomized controlled trials (RCTs) published to November 2013 that reported on the effects of PRT (>6 weeks) on breast cancer-related lymphedema (BCRL) (incidence/exacerbation, arm volume, and symptom severity), physical functioning (upper and lower body muscular strength), and health-related quality of life (HRQoL) in breast cancer patients were included. Of 446 citations retrieved, 15 RCTs in 1,652 patients were included and yielded five studies on BCRL incidence/exacerbation (N = 647), four studies on arm volume (N = 384) and BCRL symptom severity (N = 479), 11 studies on upper body muscular strength (N = 1,252), nine studies on lower body muscular strength (N = 1,079), and seven studies on HRQoL (N = 823). PRT reduced the risk of BCRL versus control conditions [OR = 0.53 (95 % CI 0.31–0.90); I 2 = 0 %] and did not worsen arm volume or symptom severity (both SMD = −0.07). PRT significantly improved upper [SMD = 0.57 (95 % CI 0.37–0.76); I 2 = 58.4 %] and lower body muscular strength [SMD = 0.48 (95 % CI 0.30–0.67); I 2 = 46.7 %] but not HRQoL [SMD = 0.17 (95 % CI −0.03 to 0.38); I 2 = 47.0 %]. The effect of PRT on HRQoL became significant in our sensitivity analysis when two studies conducted during adjuvant chemotherapy [SMD = 0.30 (95 % CI 0.04–0.55), I 2 = 37.0 %] were excluded. These data indicate that PRT improves physical functioning and reduces the risk of BCRL. Clinical practice guidelines should be updated to inform clinicians on the benefits of PRT in this cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequently diagnosed cancer in women globally (1.68 million new cases estimated in 2012) [1, 2]. The 5-year relative survival rate in many developed countries has improved steadily in recent decades [1, 3]. As the prevalent breast cancer survivor population continues to grow [4], important questions remain regarding long-term standard of care, physical functioning, and health-related quality of life (HRQoL) in this patient group.
Treatment of breast cancer can include surgery to the breast and axilla and adjuvant chemotherapy, radiotherapy, and endocrine therapies. These interventions have increased survival [5–7] but can induce chronic side effects such as breast cancer-related lymphedema (BCRL) [8], upper body functional impairment [9], chronic fatigue [10], weight gain [11, 12], bone loss [13], inflammation [14], immunosuppression [15], peripheral neuropathy [16], and psychological impairments (e.g., depression) [17]. The adverse effects of breast cancer treatment are often associated with decreased physical activity [18] and fitness [9, 19], and impairments of physical functioning [20] and HRQoL [21]. Low physical functioning and HRQoL, in turn, contribute to greater mortality in this population [22, 23].
Progressive resistance training (PRT) is an anabolic exercise modality that can potentially target many of the adverse effects of breast cancer treatment, improving physical functioning and HRQoL [24]. However, there have been concerns regarding the safety of strenuous upper body PRT in women treated for breast cancer, particularly on the risk of BCRL [25]. Since 2006, several randomized controlled trials (RCTs) have investigated the safety and efficacy of PRT regimens involving upper body exertion [26–40]. However, these data have not yet been systematically reviewed; accordingly, recommendations for PRT (and prescribed exercise training in general) remain absent from clinical guidelines [41–43]. We therefore systematically assessed the total body of RCT evidence on the safety and efficacy of PRT to inform clinical guidelines and practice.
Methods
Search strategy and study selection
Search strategy: A systematic review of all published literature using the following electronic databases was conducted up to November 2013: MEDLINE (OvidSP, Wolters Kluwer), PubMed (NCBI, U.S. National Library of Medicine), ScienceDirect (SciVerse, Elsevier), SPORTDiscus (EBSCOhost, EBSCO), Scopus (SciVerse, Elsevier), Web of Science (Web of Knowledge, Thomson Reuters), the Cochrane Library (John Wiley & Sons), Embase (OvidSP, Wolters Kluwer), CINAHL, and Google Scholar. Search syntaxes were developed in consultation with an experienced university librarian taking into account a broad range of terms and phrases used in definitions related to breast cancer (e.g., breast cancer, breast neoplasm, breast carcinoma, breast tumor, mammary carcinoma, etc.) and resistance training (e.g., resistance training, resistance exercise, weight training, weight lifting, strength training, etc.). A sample search strategy (PubMed and Scopus) has been presented in the Electronic Supplementary Material (Appendix S1). Reference lists of retrieved full-text articles and recent reviews were examined to identify additional articles not found by our search strategy.
Study selection: Electronic references were compiled in an Endnote X6© (Thomson Reuters) file, and duplicates were identified and deleted. Two authors (BSC and EA) independently reviewed the titles and abstracts of each reference for potential inclusion. Each reviewer then performed a second screening on the full-text version of these articles, and disagreements were resolved by discussion. RCTs that investigated the isolated effects of PRT on BCRL (number of cases of incidence or exacerbation, arm volume, and severity of BCRL symptoms) and/or upper body strength, and/or lower body strength and/or HRQoL in women surgically treated for primary tumor of the breast were included. PRT interventions may have included but were not restricted to, any form of resistive type exercise using body weight (calisthenics), equipment (machine weights, free weights) or apparatus (elastic bands), and had to have been at least 6 weeks in duration. Studies that prescribed aerobic training plus PRT were excluded, unless a comparison group undertook the same dosage of aerobic training in isolation (i.e., to control for confounding effect of aerobic training). Studies that prescribed flexibility training plus PRT were included given that PRT involves aspects of flexibility training, i.e., loaded movements throughout a complete range of motion. Where multiple PRT prescriptions were tested, higher intensity regimens were prioritised over lower intensity regimens. The review was restricted to articles published in English.
Primary outcomes (safety)
Primary outcomes assessed the effect of PRT on BCRL outcomes, including: (1) cases of BCRL incidence or exacerbation during the trial, (2) arm volume outcomes, and (3) BCRL symptom severity between the treatment and control group. Where multiple measures of BCRL incidence or exacerbation were reported, we prioritized clinician-defined diagnosis with objective physical measurements over other methods. Data for BCRL incidence and exacerbation were combined given that the physiologic mechanism between cases is identical (i.e., a decrease in lymphatic transport capacity relative to lymphatic load) [44]. Where multiple arm volume outcomes were reported, we prioritized measurements of the interlimb volume difference, followed by volume of the affected limb. Where multiple BCRL symptom severity outcomes were reported, we prioritized assessments using the arm symptoms subscale of the European Organization for Research and Treatment of Cancer Breast Cancer Module (QLQ-BR23) [45].
Data about additional PRT-related adverse events (beyond BCRL) were also included for a descriptive synthesis.
Secondary outcomes (efficacy)
Secondary outcomes assessed efficacy data of PRT and included: (1) upper body strength, (2) lower body strength, and (3) HRQoL after intervention (post-treatment) between the treatment and control group. Where multiple upper body muscular strength outcomes were reported, we prioritized the most common measure (i.e., bench press) followed by shoulder press, shoulder flexion, and wrist flexion. Where assessments of upper body strength were completed bilaterally, we prioritized measures of the ipsilateral (affected) extremity over those of the contralateral extremity. Where multiple lower body muscular strength outcomes were reported, we prioritized leg press followed by knee extension. Where multiple HRQoL outcomes were reported, we prioritized domain and then summary scale scores of physical functioning, followed by global scores of HRQoL.
Data extraction
Data extraction of included studies was performed and/or verified independently by three reviewers (BSC, SLK, and PF). Discrepancies were resolved through discussion. Authors of relevant studies were contacted, where possible, for data that could not be extracted from the published articles.
Quality assessment
The following data were extracted from included studies using a standard proforma: study population characteristics, PRT intervention (e.g., specific exercises, number of sets per exercise, number of repetitions per set, intensity (load), frequency, and duration of training and loading progression). Our quality checklist was designed based on established criteria for the assessment of RCTs [46]. Quality items for RCT studies reviewed were (each worth 1.0 numerical point) as follows: (1) evidence of randomization and concealment of treatment allocation, (2) statistical similarity of groups at baseline, (3) specification of eligibility criteria, (4) blinding of outcomes assessors, (5) reporting of compliance, (6) supervision of exercise sessions, (7) reporting of dropouts, (8) presenting data for primary and secondary outcomes, (9) use of intention-to-treat analysis (if data for >90 % of baseline sample were analyzed, a score of 1.0 was given), and (10) reporting of adverse events. Summated scores ranged from 0 to 10 points with higher scores reflecting better quality. The quality assessment was completed and checked by two reviewers (BSC and SLK).
Data synthesis
Three reviewers (BSC, SLK, and EA) independently collated and/or verified extracted data to present a descriptive synthesis of important study characteristics and a quantitative synthesis of effect estimates.
Statistical methods
We pooled and weighted studies first using random effects meta-analysis models and second using fixed effects models for verification [47]. The effect was measured as the difference between groups after the treatment period without correction for possible baseline differences between groups. The mean and standard deviation of the pre- to post-treatment improvement in outcome were unavailable for the majority of papers. While these statistics could have been estimated from the pre- and post-treatment statistics [48], such estimation requires the pre–post correlation. We computed point estimates of correlation for those few papers which provided pre-, post-, and change means and standard deviations [48]. However, as the number of studies with full information was small and the estimated correlations from these studies were not fully consistent, we opted to restrict the analyses to the known post-treatment statistics without correction for possible baseline differences. We checked all studies for differences between groups at baseline and where statistically significant differences were found, we used sensitivity analysis to check the impact of these differences on the pooled results.
To examine the incidence/exacerbation of lymphedema cases, we reported the pooled odds ratio between treatment and control groups and associated 95 % confidence interval (95 % CI). Where articles reported 0 cases in either the treatment or control group, the Haldane continuity correction was applied by adding 0.5 to all four cells [49]. Articles which reported 0 cases in both the treatment and control groups were excluded from the analysis as differences in group size would produce bias in the continuity correction [50]. To examine the effects of PRT on arm swelling, lymphedema severity, upper and lower body strength, and HRQoL outcomes, the standardized mean difference (SMD) from each study was pooled to produce an overall estimate of effect and associated 95 % CI between treatment and control groups. For each meta-analysis model, the degree of heterogeneity was assessed by visual inspection, the I-squared statistic (I 2) (moderate being <50 %) [51], and the χ 2-test of goodness of fit [52]. When evidence of heterogeneity was observed, we checked data extracted from individual outlier studies, qualitatively investigated reasons for their different results, and explored the effects of study exclusion in sensitivity analyses.
We also used sensitivity analysis to investigate the robustness of the meta-analyses models. We variously excluded studies that combined PRT with other exercise modalities or physical therapies, studies that did not include a no-treatment control group, studies that prescribed PRT during the adjuvant chemotherapy treatment phase, studies conducted outside the US, studies of shorter duration (≤12 weeks), studies in older cohorts (≥60 years), studies in which BCRL was an entry criterion, and studies of lower quality (score ≤6.0). Publication bias, which reflects the tendency for smaller studies to be published in the literature only when findings are positive, was assessed visually using funnel plots [53]. All calculations were performed in Stata version 12 (StataCorp, College Station, TX, USA) using the ‘metan’ and ‘metafunnel’ commands. A two-tailed P value <0.05 was considered statistically significant throughout the analyses.
Results
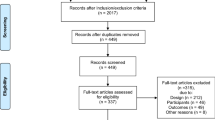
Figure 1 presents a flowchart summarizing identification of potentially relevant studies and those included. Our search strategy identified 446 citations after duplicates were removed. Of these, 392 citations were excluded after the first screening of titles and/or abstracts for inclusion and exclusion criteria. After further assessment of the remaining 54 citations, 40 were excluded (Electronic Supplementary Material, Appendix S2) for reasons listed in Fig. 1. An expert in the field provided one recent citation not captured by our search. Fifteen citations were included in the present review. Most citations were excluded due to being a conference abstract only or due to being redundant citations of the same study.
Descriptive data synthesis
Table 1 presents the study characteristics of the 15 RCTs included for review, which were published between 2006 and 2013 [26–40]. Ten studies were conducted in the USA [26, 27, 29–33, 35, 36, 38] with others conducted in Canada [28, 40], Australia [37, 39], and Korea [34]. Major inclusion criteria typically were the completion of all breast cancer-related treatments (except hormonal therapy) [26, 27, 30–33, 35, 36, 38] or the initiation of chemotherapy treatment for breast cancer [28, 40]. Lymphedema-related inclusion criteria were lymph node dissection (or sentinel node biopsy) [26, 31, 32, 37] and/or clinical diagnosis of lymphedema by clinician [31, 34, 39]. Major exclusion criteria primarily emphasized uncontrolled cardiovascular diseases and other chronic illnesses that would contraindicate PRT. Lymphedema-related exclusion criteria included bilateral lymph node dissection [31–33], bilateral lymphedema [34], history of lymphedema [37], unstable lymphedema [39], and incomplete axillary surgery [28, 40]. Analyzed sample sizes ranged from 21 to 232, resulting in a total of 1,652 participants across studies. Mean age of the samples ranged from 46 to 62 years.
PRT interventions were prescribed two to three times per week in 12 studies (Table 1). Other studies prescribed a split-routine four sessions per week [29] or lower intensity training for five [34] or seven sessions per week [37]. Upper body training was prescribed in all studies (Table 1), and only two studies did not target lower body musculature [34, 37]. PRT was typically prescribed using machine and/or free weights, while two studies used resistance bands only [29, 36], and three studies incorporated a combination thereof [35, 37, 38]. Training sessions were fully supervised in only three studies [28, 39, 40], while ten studies involved partial supervision [26, 27, 30–35, 37, 38], and two studies did not provide supervision [29, 36]. In general, lower body PRT was prescribed according to standard training principles for healthy adults. Upper body exercises were initiated at low intensities and progressed according to tolerance in most studies, while four studies prescribed upper body PRT at approximately 65–75 % of one repetition maximum (RM) [28, 35, 38, 40], and one study prescribed upper body PRT at 6–10 RM intensity. All studies indicated that training loads were progressively increased with strength adaptation.
Nine studies compared PRT intervention to usual care (no exercise) [26–29, 31–33, 37, 39], while three studies incorporated flexibility training as a sham condition [35, 36, 38]. The other three studies compared PRT plus an additional intervention (i.e., calcium and vitamin D supplement [30], complete decongestive physiotherapy [34], and aerobic training [40]) compared to the latter intervention only. Trial durations ranged from 8 to 104 weeks in duration; six studies were ≥52 weeks, three studies were 26 weeks, and six studies ranged from 8 to 17 weeks.
Primary outcomes were (1) cases of lymphedema incidence or exacerbation evaluated via clinician-defined assessment based on multiple objective tests [31, 32], the interlimb volume [28, 35, 37, 38, 39], or circumference difference [26, 34]; (2) extent of arm swelling outcomes evaluated via the interlimb volume difference [28, 31, 39] or volume of the ipsilateral extremity [34]; and (3) lymphedema symptom severity outcomes evaluated via validated [54] questionnaire [31, 32] or the arm symptoms subscale of the QLQ-BR23 [37, 39]. Secondary outcomes were upper body muscular strength evaluated via bench press [28, 31, 32, 35, 36, 38–40], shoulder press [29], arm flexion [37], and wrist flexion [30], lower body muscular strength evaluated via leg press [31, 32, 35, 38–40] or knee extension [28–30], and HRQoL evaluated via the physical global score on the Cancer Rehabilitation and Evaluation System short form [27], the Functional Assessment of Cancer Therapy–Anemia scale [28], and the physical functioning domain score [34, 35, 39, 40] and physical component summary scale [33] of the Medical Outcomes Trust Short Form-36 (SF-36). Quality scores ranged from 5.0 to 9.5, and 13 studies received a score of 8.0 or higher (Electronic Supplementary Material, Table S1).
Quantitative data synthesis
Primary outcomes
Figure 2 presents the OR for the incidence and/or exacerbation of BCRL after PRT intervention between the treatment and control groups for five studies in 647 participants [26, 28, 31, 32, 37]. Four studies [34, 35, 38, 39] were excluded from the analysis given that no cases of BCRL were observed in either group. PRT resulted in significantly lower risk of BCRL incidence/exacerbation compared with control conditions [OR = 0.53 (95 % CI 0.31–0.90)]. There was no statistical heterogeneity between studies (I 2 = 0 %, P = 0.80).
Figure 3 presents the SMD for arm volume (4 studies in 384 participants [28, 31, 34, 39]) and patient-reported severity of BCRL (4 studies in 479 participants [31, 32, 37, 39]) after PRT between the treatment and control groups. PRT did not change arm volume [SMD = −0.07 (95 % CI −0.28 to 0.14)] or patient-reported severity of BCRL [SMD = −0.07 (95 % CI −0.25 to 0.11)] compared with control conditions. There was no evidence of statistical heterogeneity in either of these analyses (both I 2 = 0 %, Fig. 3). Funnel plots showed no evidence of publication bias for either outcome (Electronic Supplementary Material, Figs. S1 and S2).
Descriptive synthesis of additional PRT-related adverse events
Five studies reported that no adverse events occurred as a consequence of exercise training [31, 34, 35, 39, 40]. Other studies generally reported temporary muscle soreness [30] or musculoskeletal injuries. Winters-Stone et al. [38] reported episodes of back (N = 2) and knee pain (N = 1) which resulted in one participant discontinuing with lower body training. Adverse events in the study by Ohira et al. [27] have been documented in a separate article [55] which noted back injuries (N = 4) in the experimental group; however, none of these participants became unable to exercise. Musanti [36] noted two cases of tendinitis (shoulder and foot) during their study but did not specify the group allocation of the participants affected. Brown et al. [56] have summarized the adverse events encountered in three trials included in our review [31–33]. Nine women randomized to the PRT group reported 10 musculoskeletal injuries related to training that impaired activities of daily living for ≥1 week [56]. Of these, there were a greater number of incidents in women with BCRL (8 injuries) as compared to those at risk for lymphedema (N = 2) [56]. Courneya et al. [28] reported on two adverse events unrelated to PRT. Three studies did not report on adverse events beyond lymphedema [26, 29, 37].
Secondary outcomes
Figure 4 presents the SMD for upper body muscular strength (11 studies in 1,252 participants [28–32, 35–40]), lower body muscular strength (9 studies in 1,079 participants [28–32, 35, 38–40]), and HRQoL outcomes (7 studies in 823 participants [27, 28, 33–35, 39, 40]) after PRT between the treatment and control groups. PRT significantly improved standardized upper body [SMD = 0.57 (95 % CI 0.37–0.76)] and lower body [SMD = 0.48 (95 % CI 0.30–0.67)] muscular strength outcomes compared with control conditions. There was evidence of moderate heterogeneity between studies in each of these analyses. The sensitivity analyses (Electronic Supplementary Material, Tables S2 and S3) showed that the pooled SMD was similarly medium to large in the fixed effect model and after each of the various studies was excluded (SMD = 0.49–0.68 and 0.40–0.59 for upper and lower body muscular strength outcomes). Heterogeneity in upper body strength outcomes (I 2 = 58.4 %) could not be explained by our sensitivity analysis, whereas heterogeneity in lower body strength outcomes (I 2 = 46.7 %) was reduced with the exclusion of one study [31] that noted a significant difference between groups at baseline. Funnel plots were produced and showed little evidence of publication bias (Electronic Supplementary Material, Figs. S3 and S4).
Our primary analysis revealed that PRT induced a small improvement in HRQoL [SMD = 0.17 (95 % CI −0.03 to 0.38)] compared with control conditions, but this effect was not statistically significant, and there was evidence of moderate heterogeneity (I 2 = 47.0 %). The sensitivity analyses presented in Table S4 showed that the pooled SMD was similarly small in the fixed effect model and after each of the various studies was excluded (SMD = 0.11–0.24). Notably, the findings became significant, and heterogeneity was reduced, when studies conducted during adjuvant chemotherapy [SMD = 0.30 (95 % CI 0.04–0.55), I 2 = 37.0 %] and studies that did not include a no-treatment control group [SMD = 0.24 (95 % CI 0.01–0.46), I 2 = 29.2 %] were excluded. The corresponding funnel plot showed little evidence of publication bias (Electronic Supplementary Material, Fig. S5).
Discussion
Summary of the evidence
Based on RCT evidence in women surgically treated for breast cancer, our results for safety outcomes were consistent. PRT reduced the risk of BCRL and did not exacerbate arm volume or patient-reported severity of BCRL versus control conditions (Figs. 2, 3). Our finding that PRT nearly halves the odds of BCRL incidence/exacerbation is clinically relevant given that studies have consistently shown that women with a diagnosis of BCRL suffer greater impairments of upper body functioning [57, 58] and HRQoL [59, 60] compared to their non-affected peers. Further, the null effect of PRT on measures of arm volume and BCRL symptom severity indicates that PRT does not worsen lymphedema symptoms, in contrast to prior assertions [25].
For efficacy data, our results indicate that PRT significantly improved upper and lower body muscular strength and induced a small improvement of HRQoL (Fig. 4). The mean improvement in upper body muscular strength was more than half a standard deviation in our primary and sensitivity analysis (SMD = 0.49–0.68) and is clinically relevant. A recent prospective study [61] showed that mean upper body strength of both the affected and unaffected extremity is significantly reduced from pre-surgery to 2.5 years post-treatment in women who received axillary lymph node dissection (both SMD = −0.42). The mean PRT-induced improvement of upper body muscular strength documented in our study (SMD = 0.57) is therefore greater than the expected long-term (2.5 year) decline [61] indicating that PRT can, on average, counteract treatment-induced upper body morbidity [57, 58].
The mean improvement in lower body muscular strength approached half a standard deviation in our primary and sensitivity analysis (SMD = 0.40–0.59) and is also clinically relevant. Breast cancer survivors suffer from significantly reduced leg strength compared to healthy controls (SMD = −1.16) [62]. Moreover, prospective studies have shown that mean lower body strength declines rapidly with age (2.6–3.0 % per year) [63], and the loss of lower body strength (SMD = −0.44) is a powerful predictor of all-cause mortality [64, 65]. Poor leg strength is therefore an important target for rehabilitation in the breast cancer population.
The small effect of PRT on mean HRQoL noted in our primary and sensitivity analysis is also clinically relevant. HRQoL is reduced in women with breast cancer, both at diagnosis and post-treatment, compared to the general population [66, 67]. However, higher levels of physical activity pre- or post-breast cancer treatment can contribute to higher HRQoL, particularly in the physical domains of HRQoL [68, 69]. For example, in a cancer registry study [67] that identified and recruited women at 5, 10, and 15 years post-breast cancer diagnosis, the mean score of the physical functioning domain of HRQoL was reduced at the 5-year (SMD = −0.27) and 10-year timepoint (SMD = −0.18) compared to healthy controls. The magnitude of change of HRQoL in our study was SMD = 0.30 exclusive of two studies conducted during adjuvant chemotherapy [28, 40], suggesting that women engaging in PRT post-chemotherapy can experience an improvement of HRQoL beyond the levels expected in healthy peers.
The effect of PRT on upper and lower body muscular strength remained robust in fixed effect models and after exclusion of studies that combined PRT with other exercise modalities (or therapies), studies without a no-treatment control group, studies prescribed PRT during chemotherapy treatment, studies conducted outside the US, studies of shorter duration, studies in older cohorts, studies in which BCRL was an entry criterion, and studies of lower quality.
In summary, our primary results indicate that that PRT does not exacerbate measures of BCRL and may lower risk. PRT also improves upper and lower body muscular strength, and elicits a small improvement in HRQoL. No serious PRT-induced adverse events were reported in the studies reviewed. These findings are clinically relevant. Therefore, clinical practice guidelines should be updated to inform clinicians on the benefits of PRT in this patient group.
Limitations
Several limitations require careful consideration. First, our analysis of arm volume and patient-reported severity of BCRL outcomes was based on a limited number of studies (Fig. 3), and only three of these studies included a clinical diagnosis of BCRL as a participant entry criterion. Women without BCRL are unlikely to improve these outcomes, and therefore additional studies limited to women with BCRL are warranted. Second, we did not distinguish the affected and non-affected extremity in the assessment of upper body muscular strength outcomes. There is evidence that bilateral strength deficits may be incurred by breast cancer treatment [70], and future research is required to distinguish the effect of PRT on both the surgically treated and non-treated side. Third, there was heterogeneity with respect to the exercise prescriptions, including the level of supervision, training equipment, and training frequency and intensity (Table 1); training intensity was also not quantitatively defined in many studies. We did not investigate any dose–response effects in the present review; accordingly, the optimal dosages of PRT to adapt the specific outcomes in this patient group remain unknown and require further research.
Conclusion
Our meta-analytic results are sufficiently reliable to recommend that clinicians consider PRT for reducing the risk of BCRL and improving upper and lower body muscular strength and HRQoL outcomes in women treated for breast cancer.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
GLOBOCAN: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J et al (2011) Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 377(9760):127–138
Bray F, Ren J-S, Masuyer E, Ferlay J (2013) Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 132(5):1133–1145
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA (2013) Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 119(7):1402–1411
Dominick SA, Madlensky L, Natarajan L, Pierce JP (2013) Risk factors associated with breast cancer-related lymphedema in the WHEL Study. J Cancer Surv 7(1):115–123
Harrington S, Padua D, Battaglini C, Michener LA (2013) Upper extremity strength and range of motion and their relationship to function in breast cancer survivors. Physiother Theory Pract 29(7):513–520
Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD (2010) Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surv 4(4):405–414
Vance V, Mourtzakis M, McCargar L, Hanning R (2011) Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev 12(4):282–294
Villasenor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, Neuhouser ML (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surv 6(4):398–406
Chen Z, Maricic M, Bassford TL et al (2005) Fracture risk among breast cancer survivors: results from the women’s health initiative observational study. Arch Intern Med 165(5):552–558
Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A et al (2009) Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 27(21):3437–3444
Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I et al (2009) Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 69(13):5383–5391
Speck RM, DeMichele A, Farrar JT, Hennessy S, Mao JJ, Stineman MG, Barg FK (2012) Scope of symptoms and self-management strategies for chemotherapy-induced peripheral neuropathy in breast cancer patients. Support Care Cancer 20(10):2433–2439
Galiano-Castillo N, Ariza-Garcia A, Cantarero-Villanueva I, Fernandez-Lao C, Diaz-Rodriguez L, Arroyo-Morales M (2013) Depressed mood in breast cancer survivors: associations with physical activity, cancer-related fatigue, quality of life, and fitness level. Eur J Oncol Nurs 18(2):206–210
Kwan ML, Sternfeld B, Ergas IJ, Timperi AW, Roh JM, Hong CC, Quesenberry CP, Kushi LH (2012) Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat 131(2):679–690
Smoot B, Johnson M, Duda JJ, Krasnoff J, Dodd M (2012) Cardiorespiratory fitness in women with and without lymphedema following breast cancer treatment. Cancer Clin Oncol 1(1):21–31
Pinto M, Gimigliano F, Tatangelo F, Megna M, Izzo F, Gimigliano R, Iolascon G (2013) Upper limb function and quality of life in breast cancer related lymphedema: a cross-sectional study. Eur J Phys Rehabil Med 49(5):665–673
Howard-Anderson J, Ganz PA, Bower JE, Stanton AL (2012) Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst 104(5):386–405
Sehl M, Lu X, Silliman R, Ganz PA (2013) Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surv 7(1):20–31
Epplein M, Zheng Y, Zheng W, Chen Z, Gu K, Penson D, Lu W, Shu XO (2011) Quality of life after breast cancer diagnosis and survival. J Clin Oncol 29(4):406–412
Cheema B, Gaul C, Lane K, Fiatarone Singh MA (2008) Progressive resistance training in breast cancer: a systematic review of clinical trials. Breast Cancer Res Treat 109:9–26
Demark-Wahnefried W (2009) A weighty matter—lifting after breast cancer. N Engl J Med 361(7):710–711
Ahmed R, Thomas W, Yee D, Schmitz K (2006) Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol 24(18):2765–2772
Ohira T, Schmitz K, Ahmed R, Yee D (2006) Effects of weight training on quality of life in recent breast cancer survivors. Cancer 106:2076–2083
Courneya K, Segal R, Mackey J, Gelmon K, Reid R, Friedenreich C, Ladha A, Proulx C, Vallance J, Lane K et al (2007) Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 25(28):4396–4404
Schwartz AL, Winters-Stone K, Gallucci B (2007) Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum 34(3):627–633
Twiss JJ, Waltman NL, Berg K, Ott CD, Gross GJ, Lindsey AM (2009) An exercise intervention for breast cancer survivors with bone loss. J Nurs Scholarsh 41(1):20–27
Schmitz K, Ahmed R, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan C, Williams-Smith C, Greene Q (2009) Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med 361:664–673
Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, Bryan CJ, Williams-Smith CT, Chittams J (2010) Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA 304(24):2699–2705
Speck RM, Gross CR, Hormes JM, Ahmed RL, Lytle LA, Hwang WT, Schmitz KH (2010) Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat 121(2):421–430
Kim do S, Sim YJ, Jeong HJ, Kim GC (2010) Effect of active resistive exercise on breast cancer-related lymphedema: a randomized controlled trial. Arch Phys Med Rehabil 91(12):1844–1848
Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A (2012) The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surv 6(2):189–199
Musanti R (2012) A study of exercise modality and physical self-esteem in breast cancer survivors. Med Sci Sports Exerc 44(2):352–361
Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Lee M, Simpson JM, Hansen R (2012) Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: a randomized controlled trial. Breast Cancer Res Treat 133(2):667–676
Winters-Stone KM, Dobek J, Nail LM, Bennett JA, Leo MC, Torgrimson-Ojerio B, Luoh SW, Schwartz A (2013) Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int 24(5):1637–1646
Cormie P, Pumpa K, Galvao DA, Turner E, Spry N, Saunders C, Zissiadis Y, Newton RU (2013) Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomised controlled trial. J Cancer Surv 7(3):413–424
Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Cook D, Jespersen D, Proulx C et al (2013) Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst 105(23):1821–1832
Aebi S, Davidson T, Gruber G, Cardoso F (2011) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 22(Suppl 6):vi12–vi24
Yarnold J (2009) Early and locally advanced breast cancer: diagnosis and treatment National Institute for Health and Clinical Excellence guideline 2009. Clin Oncol 21(3):159–160
Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ, Gradishar WJ (2009) Breast cancer. J Natl Compr Canc Netw 7(2):122–192
Foldi E, Foldi M, Clodius L (1989) The lymphedema chaos: a lancet. Ann Plast Surg 22:505–515
Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, Franzini L, Williams A, de Haes HC, Hopwood P et al (1996) The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 14(10):2756–2768
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 11:32
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions, version 5.1.0
Haldane JB (1956) The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet 20(4):309–311
Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23(9):1351–1375
Higgins J, Thompson S, Deeks J, Altman D (2003) Measuring inconsistency in meta-analysis. Br Med J 327(7414):557–560
Higgins J, Thompson S (2002) Quantifying heterogeneity in meta-analysis. Stat Med 21(11):1539–1558
Eggers M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Br Med J 315(7109):629–634
Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R (2001) Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther 81(6):1192–1205
Schmitz K, Ahmed R, Hannan P, Yee D (2005) Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin and insulin-like growth factor axis proteins. Cancer Epidemiol Biomark Prev 14(7):1672–1680
Brown JC, Troxel AB, Schmitz KH (2012) Safety of weightlifting among women with or at risk for breast cancer-related lymphedema: musculoskeletal injuries and health care use in a weightlifting rehabilitation trial. Oncologist 17(8):1120–1128
Hayes SC, Janda M, Cornish B, Battistutta D, Newman B (2008) Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol 26(21):3536–3542
Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, Schmitz KH (2012) Upper-body morbidity after breast cancer. Cancer 118(S8):2237–2249
Beaulac S, McNair L, Scott T, LaMorte W, Kavanah M (2002) Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg 137(11):1253–1257
Ahmed RL, Schmitz KH, Prizment AE, Folsom AR (2011) Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat 130(3):981–991
Sagen A, Kaaresen R, Sandvik L, Thune I, Risberg MA (2014) Upper limb physical function and adverse effects after breast cancer surgery: a prospective 2.5-year follow-up study and preoperative measures. Arch Phys Med Rehabil 95(5):875–881
Simonavice E, Liu P-Y, Ilich JZ, Kim J-S, Panton LB (2011) Body composition, muscular strength, and physical function in breast cancer survivors. Int J Body Compos Res 9(2):57–64
Goodpaster B, Park S, Harris T, Kritchevsky S, Nevitt M, Schwartz A, Simonsick E, Tylavsky F, Visser M, Newman A (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol 61A(10):1059–1064
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol Ser A 61(1):72–77
Takata Y, Shimada M, Ansai T, Yoshitake Y, Nishimuta M, Nakagawa N, Ohashi M, Yoshihara A, Miyazaki H (2012) Physical performance and 10-year mortality in a 70-year-old community-dwelling population. Aging Clin Exp Res 24(3):257–264
Lee ES, Lee MK, Kim SH, Ro JS, Kang HS, Kim SW, Lee KS, Yun YH (2011) Health-related quality of life in survivors with breast cancer 1 year after diagnosis compared with the general population: a prospective cohort study. Ann Surg 253(1):101–108
Klein D, Mercier M, Abeilard E, Puyraveau M, Danzon A, Dalstein V, Pozet A, Guizard A-V, Henry-Amar M, Velten M (2011) Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Res Treat 129(1):125–134
Alfano CM, Smith AW, Irwin ML, Bowen DJ, Sorensen B, Reeve BB, Meeske KA, Bernstein L, Baumgartner KB, Ballard-Barbash R et al (2007) Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: a prospective analysis. J Cancer Surv 1(2):116–128
Blanchard CM, Courneya KS, Stein K (2008) Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol 26(13):2198–2204
Merchant CR, Chapman T, Kilbreath SL, Refshauge KM, Krupa K (2008) Decreased muscle strength following management of breast cancer. Disabil Rehabil 30(15):1098–1105
Acknowledgments
We sincerely thank Ms Katrina Chaudhary for her work in developing the database searches.
Funding
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheema, B.S., Kilbreath, S.L., Fahey, P.P. et al. Safety and efficacy of progressive resistance training in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 148, 249–268 (2014). https://doi.org/10.1007/s10549-014-3162-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3162-9