Abstract
The sense of touch is fundamental for daily behavior. The aim of this work is to understand the neural network responsible for touch processing during a prolonged tactile stimulation, delivered by means of a mechatronic platform by passively sliding a ridged surface under the subject’s fingertip while recording the electroencephalogram (EEG). We then analyzed: (i) the temporal features of the Somatosensory Evoked Potentials and their topographical distribution bilaterally across the cortex; (ii) the associated temporal modulation of the EEG frequency bands. Long-latency SEP were identified with the following physiological sequence P100—N140—P240. P100 and N140 were bilateral potentials with higher amplitude in the contralateral hemisphere and with delayed latency in the ipsilateral side. Moreover, we found a late potential elicited around 200 ms after the stimulation was stopped, which likely encoded the end of tactile input. The analysis of cortical oscillations indicated an initial increase in the power of theta band (4–7 Hz) for 500 ms after the stimulus onset followed a decrease in the power of the alpha band (8–15 Hz) that lasted for the remainder of stimulation. This decrease was prominent in the somatosensory cortex and equally distributed in both contralateral and ipsilateral hemispheres. This study shows that prolonged stimulation of the human fingertip engages the cortex in widespread bilateral processing of tactile information, with different modulations of the theta and alpha bands across time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sense of touch is fundamental in performing common actions such as manipulation and recognition of objects (Johansson and Flanagan 2009; Klatzky et al. 1985). It is well known that the somatosensory cortex represents and processes tactile information originating from the contralateral side of the body (e.g., sensory signals sent from the right side of the body are processed in the left cortex and vice versa) (Nieuwenhuys et al. 2007). However, several studies conducted both with animals and humans demonstrated that the somatosensory cortex also receives inputs from the ipsilateral side of the body (Hansson and Brismar 1999; Iwamura et al. 1994; Jiang et al. 1997; Tamura 1972), though the afferent sensory pathway responsible for this is still uncertain (Tamè et al. 2016). In particular, three possible anatomical pathways have been proposed to explain tactile processing, which are not mutually exclusive: (i) direct projection of the sensory signals from the contralateral to ipsilateral Somatosensory Cortex (SC) passing through the corpus callosum (Allison et al. 1992); (ii) direct uncrossed afferent projection, by which the sensory information runs in parallel to the contralateral pathway (Kanno et al. 2003); (iii) higher-level processing of the secondary somatosensory cortex, in which bilateral receptive fields of the hand are present (Shanks et al. 1985; Tommerdahl et al. 2006).
A fundamental aspect to generate and thus investigate the bilateral cortical response concerns the tactile stimulation. Electrical stimuli applied to the median nerve or to fingers are widely used in touch studies (Allison et al. 1991; Palva et al. 2005), but this approach can be suboptimal since single electrical pulses may not mimic the complex interactions between different mechanoreceptors (Gordon and Ghex 1991; Kandel et al. 2000). On the other hand, many other approaches based on artificial stimuli such as static skin indentation or vibrations have been used to investigate the cortical processing of touch (Burton et al. 1993; McGlone et al. 2002; Tamè et al. 2015) and in this setup the tactile information elaborated by the brain would not come from the activation of the full range of mechanoreceptors that would occur during a dynamic action (Greenspan and Bolanowski 1996).
The goal of this study is therefore to investigate the neural network of touch processing in a passive dynamic condition that replicates a controlled sliding action of the human fingertip. To this end, we used high density electroencephalography (EEG) as neuroimaging technique and a mechatronic platform for human tactile studies to deliver the tactile stimulation using a textured mechanical stimulus, i.e., grating (Oddo et al. 2011, 2016). This device enables to deliver precise, repeatable and smooth sustained stimulus presentation without vibration.
Previous studies that used a similar experimental setup (Aviles et al. 2010) to ours either focused on roughness discrimination via oddball paradigm (Munoz et al. 2014) or limited their analysis to the frequency content of Steady-State Evoked Potentials (SS-EP) generated in the contralateral hemisphere (Moungou et al. 2016). To our knowledge no study has investigated the bilateral brain response and its spatiotemporal characteristics during a prolonged passive stimulation of the human fingertip. Specifically, such stimulation allowed us to investigate the bilateral neural processing of touch during the ongoing stimulus presentation. We monitored both the early integration of tactile information by means of Somatosensory Evoked Potentials and the late evolution of the brain responses by computing Event-Related Synchronization and Desynchronization.
In EEG recordings, the direct cortical response of the Central Nervous System to sensory stimuli can be measured by identifying Somatosensory Evoked Potentials (SEPs) (Allison et al. 1992; Salenius et al. 1997). In fact the experimental paradigm and the tactile stimulation can modulate SEP components differently by changing their latency, amplitude, and topography (Allison et al. 1989b; Garcia-Larrea et al. 1991; Kida et al. 2004a). For example, electrical stimuli can elicit short-latency SEPs within 40 ms after the stimulus onset (Allison et al. 1989a), whereas mechanical stimulation typically generates long-latency potentials such as P50, N70, P100 and N140 (Hämäläinen et al. 1990; Ishiko et al. 1980; Zopf et al. 2004). SEP temporal characterization is thus a suitable tool to understand how tactile stimulation affects the mechanisms underlying the bilateral generation of these potentials (Allison et al. 1992; Schomer and Da Silva 2012).
Time–frequency analysis of cortical oscillations such as Event Related Synchronization (ERS) and desynchronization (ERD) could also provide useful information on the processing of tactile information and bilateral activation (Adhikari et al. 2014; Bauer et al. 2006; Neuper et al. 2006; Stancak 2003). Indeed, the somatosensory ERD/ERS have previously been used to investigate how EEG frequency bands are modulated by a variety of variables such as stimulus intensity and duration of tactile stimulation (Pfurtscheller et al. 2001; Stancak 2003).
In this study we provide a comprehensive and quantitative measure of the whole brain activation and the dynamics of the cortical response during a sliding action by implementing the following steps: (i) characterization of SEPs in the time domain, i.e., amplitude, latency and scalp topography while the stimulation is ongoing; (ii) the spatiotemporal modulation of the frequency-bands in the time–frequency domain.
Materials and Methods
Participants
10 healthy subjects (4 females; mean population age: 22.4 years) were involved in the study. None of the participants had any history of neurological diseases and as far as known all of them had normally functioning somatosensory systems. Further, they were naïve to the particular purpose of this study.
Tactile Experimental Set Up
The experimental setup is depicted in Fig. 1a. The tactile stimulation was generated by means of a mechanical stimulus (i.e., grating) with medium spatial coarseness (Weber et al. 2013) fabricated with 3D printing (Project HD 3000, 3D Systems).
Tactile experimental setup. a The mechanical stimulus was a grating, i.e., a parallelepiped with alternated ridges and grooves on the top. The spatial period (SP) was defined as the sum of the ridge width (RW) and the groove width (GW). In this work, a stimulus with medium coarseness having a 650 µm SP (250 µm RW + 400 µm GW) was used (Weber et al. 2013). The subject fingertip was positioned over the tactile stimulus and maintains fixed while the stimulus slides and thus was perceived by the subject. Subjects were instructed to keep their eyes closed while concentrating on the stimulus response. b Representation of the stimulation sequence, consisting on alternating indentation, pause and sliding. Each trial is time-locked to the start of the sliding
The mechanical stimulation consisted in a passive sliding of that stimulus under the subject fingertip and it was carried out by a mechatronic platform used in previous tactile studies (Oddo et al. 2011, 2016). The device has 2 Degrees of Freedom (DoFs), which allow indentation (i.e., vertical motion of the stimulus for the direct contact between stimulus and fingertip) and sliding (i.e., horizontal motion to perform the stimulation).
Simultaneously to the passive stimulation, the EEG activity was recorded by means of a 64ch HD-EEG device (SD LTM Express, Micromed S.p.A, Italy) with a sample rate of 1024 Hz. The montage was in accordance with the 5% 10/20 system and the electrode impedance was kept below 10 kΩ (Oostenveld and Praamstra 2001).
Stimulation Procedure
During the experimental protocol, the subject sat in a comfortable chair, while the right arm laid on an ergonomic arm support and the index finger attached to a digit support. This ensured a stable contact over the mechanical stimulus throughout the experiment. The load force was set to 400 mN and the sliding velocity to 10 mm/s and these values were kept constant throughout the experimental sessions. During the stimulation, subjects were instructed to concentrate on the current tactile sensations.
An ad-hoc stimulation sequence was implemented in order to synchronize the EEG traces with the start of the stimulation. Each step is reported in Fig. 1b. From the initial position, the indentation of the grating (i.e., phase in which the stimulus is in contact with the finger) was performed smoothly in 4 s time, allowing a gentle contact with the subject fingertip. Subsequently, a pause of 2 s was introduced in order to remove any perturbation of the EEG signals due to the contact with the stimulus-finger. Then the passive stimulation started and this time was considered as the onset to which EEG trials were time-locked. After 2 s of sliding, an additional pause of 2 s was introduced in order to isolate responses due to the post stimulus. Finally, the stimulus was removed from contact with the finger and prepared for the next repetition. With this sequence, we ensured that SEPs were actually due to sliding (as opposed to indentation). Also the inter-trial interval, longer than 4 s, helped avoiding afferent adaptation (Leung et al. 2005). A sufficiently large number of repetitions (>40) was collected to ensure a robust time–frequency analysis (Graimann et al. 2002).
EEG Processing
Data Pre-Processing
Data were analyzed using Matlab scripts based on the EEGLAB toolbox (Delorme and Makeig 2004). Continuous data were re-referenced to the TP7/TP8, i.e., channels behind the ears, and high-pass filtered (0.5 Hz, 94th order, Chebyshev type II filter) before applying 50 Hz comb notch filter (Menicucci et al. 2014). Channels with prolonged prominent artifacts (identified by visual inspection) were removed (Artoni et al. 2012a; Sebastiani et al. 2015) and remaining channels were considered for further analysis. Epochs containing high-amplitude artifactual potentials, high-frequency muscle noise and other irregular artifacts as per careful visual inspection were removed and remaining data were processed via Independent Component Analysis (ICA) filtering to remove non-neural sources and artifacts (Artoni et al. 2012b; Artoni et al. 2014). Epochs ranging from −2 s to 4 s time locked to the onset of the stimulation (i.e., trials) were then extracted (see Fig. 1b) and visually inspected for residual artifacts. The baseline value (i.e., mean in −2 0 s) was subtracted from each trial. Each trial was converted to Z-score values by dividing the EEG signals during stimulation (2–4 s) by the baseline standard deviation (Makeig et al. 2004).
Identification of SEPs
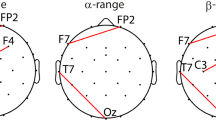
The mean for each subject was computed by averaging the time-locked Z-scored trials. Subsequently, long-latency SEPs were identified by computing the Grand Average (GA), i.e., the average of all subject means. SEPs consisted of P100, N140 and P240 components. Scalp topographies were generated by computing for each channel its GA maximum (or minimum for negative peaks) in a specific time range that included the identified component, namely: (i) maximum in 50–120 ms for the P100, (ii) minimum in 120–140 ms for the N140 and (iii) maximum in 220–260 ms for the P240. Moreover, in our experimental setting, an additional evoked potential (here named PS) was identified in the post-stimulus (i.e., segment POST in Fig. 1b) and the relative scalp distribution was computed as maximum in 2150–2250 ms. The significance of the identified potentials was assessed by evaluating, for each channel, whether each time sample had amplitude statistically different from zero (t-test, p-value < 0.05). In order to reduce false positives we computed the mean duration of the interval of consecutive significant samples in the baseline (i.e., segment PRE in Fig. 1b). This value was used as the threshold above which the duration of significant intervals, after the stimulus onset, could be considered sufficiently long for the signal to qualify as an evoked potential. For each selected temporal window, specific for P100, N140, P240, PS (as described above), electrodes that contained a significant interval within that window, formed well defined clusters that identified the specific location of the component on the scalp (bold black dots in Fig. 2).
Temporal shape and topography of SEPs. The panels show the Grand Average temporal evolution with the standard deviation across subjects (i.e., shadowed area) and scalp distribution of P100 in CP3 (a), N140 in C3 (b), P240 in Fz (c) e PS in Cz (d). The scalp distribution P100 (a) and N140 (b) showed higher activation in the contralateral hemisphere and also a slight amplitude increment in the ipsilateral side. P240 topography depicted activation mainly in the mid-frontal-central area (c), whereas PS was located in the mid-central area of the scalp (d). The shaded vertical in a–d indicates the time interval of significant amplitude (* stays for p < 0.05), whereas the black dots in a–d represent the cluster of statistically significant electrodes. (Color figure online)
SEP Latency Symmetry Study
After identifying significant SEPs as described in the section above, we focused on quantifying the symmetry of their distribution across the scalp. In particular, the P100, N140, P240 and PS latencies were respectively estimated as temporal occurrence of maximum (or minimum in case of negative peak) in the representative time-window of the potential (see “Identification of SEPs” section). This type of analysis allowed us to observe latency shifts across channels.
Event-Related Synchronization/Desynchronization Analysis
Time–frequency (TF) analysis was performed by computing Event-Related Synchronization (ERS) and Desynchronization (ERD) as reported in (Pfurtscheller and Lopes da Silva 1999). Briefly, standard ERD/ERS calculation included a bandpass filtering of each trial (5th order, Butterworth filter), squaring and subsequent averaging over trials and over samples to smooth the data. For the band-pass filtering, we first inspected the overall frequency axis (ranging from 0 Hz to 30 Hz) in order to select which frequency bands were reactive to tactile stimulation. Then we selected two frequency ranges (Michail et al. 2016): (i) theta-frequency band (4–7 Hz) to quantify the contribution of slow potentials and (ii) alpha-frequency band (8–15 Hz) to evaluate the modulation of mu rhythms. Significance was determined via bootstrap analysis with significance set at 5% for each time–frequency map (Graimann et al. 2002). The Grand-Average was then computed by averaging significant time–frequency maps across subjects.
This analysis revealed a band-specific significant ERD and ERS, respectively in alpha and theta bands that in the following text we will call θERS and αERD.
θERS and αERD Symmetry Study
To quantify the specific contribution of θERS and αERD in the contralateral hemisphere (here named CLH) and the ipsilateral one (here named ILH), two specular regions of interest (ROI) located in the somatosensory cortex were considered by selecting representative channels as following: (i) ROI-CLH: FC5, FC3, FC1, C5, C3, C1, CP5, CP3, P5, P3; (ii) ROI-ILH: FC6, FC4, FC2, C6, C4, C2, CP6, CP4, P4, P6 (see Fig. 5a). For each of these channels, θERS and αERD were quantified both in PRE segment (integral over time of −2 to 0 s interval) and in the STIM one (integral over time of 0–0.5 s interval for θERS and 0.5–2 s interval for αERD). θERS and αERD were then averaged over the channels within ROI-CLH and ROI-ILH respectively. Significant differences in θERS and αERD (stimulation phase vs. baseline, ipsilateral vs contralateral hemisphere) were tested via paired t-tests.
Results
Long-Latency SEPs
All subjects were able to perform the experimental protocol easily. The passive stimulation generated long-latency evoked potentials and they are reported in Fig. 2. P100, N140, P240 can be identified in their representative channels, respectively CP3, C3 and Fz (see Fig. 2a–c). P100 was a positive peak, showing a prominent contralateral distribution located mainly in the central and central-parietal areas and a slightly appreciable activation in the ipsilateral hemisphere (see Fig. 2a). N140 (negative peak) had the highest amplitude among the identified SEPs (see Fig. 2b).
Its topography showed mainly a contralateral negativity located in the central areas with activation also in the ipsilateral hemisphere. A frontal-central positivity, P240, appeared around 240 ms (see Fig. 2c). Finally, a positive peak (PS) component was identified in the interval 2.15, 2.25 s, i.e. at the time point where the movement of the grating stopped, and it showed a strictly central distribution (see Fig. 2d). All above described distributions corresponded to significant electrode clusters (black dots of the scalp distribution in Fig. 2).
Comparison Between CLH and ILH SEPs
The bilateral representation of somatosensory potentials in the time domain is shown in Fig. 3. In particular, P100 in C2 occurred later and with smaller amplitude with respect to C1 (see blue shadow in Fig. 3). Instead P240 showed the same amplitude and time of occurrence both in C1 and C2, as well as in the frontal area of both contralateral and ipsilateral sides (red shadow in Fig. 3). Similar effects were observed also for the N140 component. This potential reached the highest amplitude in C3 around 140 ms and appeared with smaller amplitude and later latency (around 200 ms) in C4 (green shadow in Fig. 3). N140 had a bilateral distribution and in particular this negative deflection was absent both in Cz and CPz. The later PS potential showed central distribution and thus amplitude and latency were comparable between contralateral and ipsilateral hemispheres (black shadow in Fig. 3).
Amplitude and latency evolution of SEPs across different scalp channels. Blue, green, red and black shadows locate the Grand Average temporal occurrence of P100, N140, P240 and PS, respectively. Only P100 and N140 were generated bilaterally. In the ipsilateral side, both potentials showed a smaller amplitude and longer latency with respect to the contralateral hemisphere. Since P240 and PS were not bilateral potentials, their amplitude and latency were comparable across ipsilateral and contralateral channels. (Color figure online)
Scalp Representation of θERS and αERD
Figure 4 shows the GA of the time–frequency maps of θERS and αERD in select channels.
Scalp distribution of θERS and αERD. a θERS, i.e., the power increment of the Grand Average with respect to the baseline in the theta band, lasted 500 ms after the stimulus onset and it showed a prevalent contralateral distribution from the frontal to the central parietal side, reaching the highest value in C3. b αERD, i.e., the power decrement of the Grand Average with respect to the baseline in alpha band, was delayed and lasted throughout the stimulation. After the end of stimulation, the ERD was sustained within 500 ms before reverting to baseline values. ERD showed also bilateral activation (see C3, CP3, C4 and C6) without any contributions in the midline (e.g., see Cz). (Color figure online)
θERS, which represents an increment of the power with respect to the baseline, started after the beginning of the stimulation and lasted around 500 ms (see Fig. 4a). Furthermore, θERS showed a contralateral distribution reaching the highest amplitude in C3 and it was also distributed around the frontal electrodes (see AF3, AF4, FC1, Fz in Fig. 4a).
On the other hand, significant αERD, which represented a decrement of the power with respect to the baseline, occurred after θERS started to decrease in amplitude and lasted throughout the whole stimulation phase before reverting to baseline values after 500 ms. The αERD signal also showed that there was a bilateral change in synchronization especially in C3, CP3 of the contralateral hemisphere and in C4, C6 in the ipsilateral one (see Fig. 4b).
Quantification of Bilateral Activation of θERS and αERD
The frequency modulation in each hemisphere is reported in Fig. 5 for both θERS and αERD.
Quantification of bilateral activation of θERS and αERD. a EEG channels of the contralateral hemisphere ROI-CLH: FC5, FC3, FC1, C5, C3, C1, CP5, CP3, P5 and P3; EEG channels of the ipsilateral hemisphere ROI-ILH: FC6, FC4, FC2, C6, C4, C2, CP6, CP4, P6 and P4. b θERS showed contralateral distribution under stimulation and thus the CLH activity was significantly higher than both baseline (p < 0.01,**) and ILH θERS (p < 0.05,*), which instead was comparable to the baseline activation. c αERD showed bilateral activation under stimulation. The CLH and ILH αERD had comparable amplitude and were both significantly higher than the baseline (p < 0.01,**)
θERS under stimulation showed a prominent increment of the power in the contralateral hemisphere, spread also in the frontal area, but with the highest value in C3 (see stimulation in Fig. 5b). Indeed, θERS indicated a significant change contralaterally with respect to the baseline (p < 0.01, see CLH bars in Fig. 5b), whereas the ipsilateral level under stimulation was similar to that of the baseline (i.e., no statistical difference) (see ILH bars in Fig. 5b). Accordingly, the CLH-θERS under stimulation was significantly different from the ILH-θERS (p < 0.05, see CLH and ILH bars under stimulation in Fig. 5b).
On the other hand, αERD indicated a clear bilateral change in the frequency composition of the EEG signal under stimulation (see stimulation in Fig. 5c). On the CLH side, the αERD signal was distributed in the frontal - parietal electrodes, whereas on the ILH side it was distributed in frontal and central-parietal electrodes. Indeed, tactile stimulation induced a significant increment in the αERD signal on both the CLH and ILH sides with respect to the baseline (p < 0.01, see CLH bars and ILH bars in Fig. 5c). Finally, the level of the αERD signal in the CLH and ILH sides under stimulation was comparable, i.e., not statistically different (see CLH and ILH bars under stimulation in Fig. 5c).
Discussion
The goal of this work was to provide a time and frequency domains characterization of the whole brain activation during prolonged tactile stimulation of the human fingertip.
The first analysis concerned the identification and thus the characterization of evoked responses during the ongoing stimulation (see Fig. 1b). The following temporal sequence of evoked potentials was clearly identified: P100-N140-P240-PS (see Fig. 2).
P100, N140 and P240 are long-latency SEPs and their longer latency of the responses in this study as compared to (Zopf et al. 2004), for example, or other studies using electrical nerve stimulation (Allison et al. 1992) is most easily explained by that the tactile input provided by the mechanical stimulus generated less intense and less synchronized activation of the primary afferents.
Both P100 and N140 showed a contralateral distribution (see Fig. 2a, b) in the central-parietal and central area, respectively. The localization in the somatosensory cortex (SC) of N140 is in agreement with previous studies. (Garcia-Larrea et al. 1995; Hari et al. 1993) have demonstrated that N140 can be generated in multiple cortical regions other than prefrontal area or in the anterior cingulate cortex (ACC) as also reported by (Tanaka et al. 2008). N140 localization may be related to the functional meaning of these potentials. The origin of N140 in the somatosensory cortex is usually dependent on the tactile stimulation modality (Ku et al. 2007), whereas the N140 in prefrontal or ACC usually reflects sensory non-specific events (Legrain et al. 2003; Lorenz and Garcia-Larrea 2003) which can actually elicit either negative or positive scalp potentials (Allison et al. 1992). Our results suggest that N140 in SC reflected the late sensory processing of the tactile stimulation, whereas P240 in prefrontal/ACC may have synthetized the activation due to a non-specific function.
The localization in SC of N140 is justified as part of the P100-N140 sequence, which modulates neural changes in the somatosensory cortex where bilateral generators are present (Hämäläinen et al. 1990; Ku et al. 2007). Indeed, both P100 and N140 showed a slight amplitude increment also in the ipsilateral hemisphere (see Fig. 2a, b) and this activation did not consist in a transition phase from the contralateral to the ipsilateral side, since no potentials were found in the midline (see Cz and CPz in Fig. 3). Furthermore, their amplitude was higher and the latency times shorter in the contralateral than in the ipsilateral hemisphere (see Fig. 3). This result is in accordance with (Garcia-Larrea et al. 1995) concerning the N140. However, in our work we observed a delayed response of the ipsilateral hemisphere for both somatosensory components with a largest value for N140 (see dotted line in Fig. 3).
Previous studies indicated that the detection of evoked ipsilateral response was highly variable and not detectable in all subjects (Cheyne et al. 2003; Kanno et al. 2003). On the other hand, other studies reported that a robust and consistent ipsilateral response was measured using unilateral medial nerve stimulation (Sutherland and Tang 2006), whereas the early fusion of the tactile information between hemispheres was observed by stimulating the fingertip with electrical pulse (Palva et al. 2005) and short mechanical pulse (Tamè et al. 2015). In this study, we reported that sliding a finger over a mechanical stimulus generated a consistent ipsilateral response across 10 subjects with earlier engagement of the contralateral hemisphere. Moreover, the delayed response of ipsilateral hemisphere for both P100 and N140 in Fig. 3 showed the early stage of somatosensory information processing, confirming the hypothesis cited above but here reported for the first time during a passive sliding action. The monitored early engagement can be due to the fact that sensory information is processed first in the contralateral somatosensory cortex and then projected to the ipsilateral one passing through the corpus callosum (Tamè et al. 2016). Furthermore, the interhemispheric transfer of tactile information observed in this study confirmed the hypothesis of the existence of a neurophysiological substrate for efficient sensory fusion between hands (Dupin et al. 2015; Iwamura et al. 1994; Schnitzler et al. 1995).
An additional frontal positivity was found around 240 ms (see Figs. 2c, 3). This potential has been recorded previously as an endogenous component occurring after the N140 and still associable to the tactile processing (Hämäläinen et al. 1990). Furthermore, the P240 distribution over the frontal area represented the prefrontal/ACC activation (see previous comments on N140 localization) that may suggest engagement in non-specific functions such as spatial attention at one body side (i.e., right finger) (Klimesch 2012; Ku et al. 2007) or a shift in concentration due to the alternation of the sliding phase and waiting for the next stimulus (Polich 2007).
Finally, a central positivity PS was identified around 200 ms after the end of the stimulation (see Figs. 2d, 3). PS is classified as “off-response”, i.e. a component which encodes the cognitive processing of the end of the tactile input, since it is still located in the sensory functional area (Schomer and Da Silva 2012). Its late latency might be the result of overlapping cortical activities generated during the repeated single-ridge stimulation (i.e., prolonged stimulation as a collection of ridges). In fact, we did not observe any similar somatosensory on/off responses or earlier potentials other than PS after the offset. Possibly, the strong on-response activation in the somatosensory cortex (i.e., sequence P100-N140) may have restricted the generation of early somatosensory potentials to the onset of the stimulation (Yamashiro et al. 2009).
The analysis of cortical oscillation identified the components of synchronization/desynchronization related respectively to the theta and alpha bands (see Figs. 4, 5). The θERS in theta band within 500 ms was associated with the slow potentials P100, N140 and P240. Furthermore, the scalp topography showed widespread activation with the highest θERS signal in C3, i.e., in the contralateral side, and some activation in the ipsilateral hemisphere (see Figs. 4a, 5b), confirming that the ipsilateral side processed tactile information even when the intensity was lower with respect to the baseline (Schnitzler et al. 1995). A recent study reported that the theta band could encode the ability of the subject to discriminate features such as surface roughness (Michail et al. 2016). Therefore, the widespread activation could be related to the complex somatosensory processing located mainly in the somatosensory cortex of both contralateral and ipsilateral hemispheres, but involving also other functional area of the brain.
On the other hand, the αERD signal lasted throughout the entire stimulation (see Fig. 4b) and it was present equally in both hemispheres in the somatosensory cortex (see Fig. 5c). The αERD topography confirmed the hypothesis that both ipsilateral and contralateral hemispheres were engaged during tactile stimulation (Hlushchuk and Hari 2006; Schnitzler et al. 1995). The presence of αERD mu rhythms for the duration of the stimulation could indicate an active cortical processing of tactile information, which emphasizes the direct involvement of activity in the alpha-frequency band in neuronal processes supporting consciousness and attention (Cheyne et al. 2003; Klimesch 2012; Palva et al. 2005).
In this work, SEPs analysis together with somatosensory ERD/ERS provided information about the cortical circuit participating in the processing of tactile stimuli. Unlike previous studies focused mainly on SEPs (Eimer et al. 2002; Kida et al. 2004b), here we described the neural processing of tactile inputs during the ongoing prolonged stimulation and we identified two consecutive stages. Within 500 ms, the tactile input elicited the physiological sequence of SEPs (P100-N140-P240) that may encode the stimulus properties. This sequence could be associated to the active stimulus-dependent processing that also generated the transfer of tactile information between hemispheres. After 500 ms, the encoded tactile information was maintained during the ongoing stimulation by modulating the alpha band (i.e., spatial attention at one specific body part). Most likely, this second process can be related to feeling adaptation phenomena that involved both hemispheres. In this regard, future works could analyze brain responses elicited by a tactile stimulus with a surface discontinuity to determine whether these two phenomena are systematically activated every time a new tactile input occurs, regardless of the time interval between them.
Additional future works may focus on the evaluation of the brain responses adding a cognitive task such as discrimination of surfaces. The cognitive task may not affect the theta and alpha modulation but could change some features of identified SEPs such as latency or amplitude. Moreover, a discrimination task may also be useful to investigate which of the identified SEPs (i.e., P100-N140-P240) have a functional role during tactile perception. Regarding the bilateral representation of touch, adding a cognitive task should not change the bilateral nature of the activation, but may shed light on the role of the ipsilateral activation in processing tactile stimuli.
Conclusions
Our study aimed at describing the brain response responsible for touch processing by delivering a prolonged tactile stimulation of the human fingertip. The passive sliding over a mechanical stimulus elicited a physiological temporal sequence of long-latency somatosensory potentials, i.e., bilateral P100, bilateral N140 and frontal P240. Moreover, the tactile stimulation generated a final evoked potential, which encoded the end of the stimulus sliding and thus the end of the tactile input.
The somatosensory ERD/ERS analysis showed that the finger sliding over a surface generated two consecutive cortical phenomena.
The first one occurred during the initial phase of the sliding and it concerned the increasing of oscillation amplitude (i.e., ERS) in the theta band and lasted for 500 ms after the stimulus onset. This change was distributed globally in the cortex, although there was a focus in the contralateral somatosensory area. Moreover, ERS were associated to the identified SEP potentials P100, N140 and P240 due to the overlapping of temporal windows.
The second phenomenon occurred while the initial response faded away and it appeared as a decrease of the oscillation amplitude (i.e., ERD) in the alpha band. This decrease lasted for the remainder of stimulation and its activity was bilaterally distributed in the somatosensory cortex.
The results presented in this work show for the first time that in response to a prolonged passive stimulation of the human fingertip the cortex is engaged in a widespread bilateral processing of tactile information by differential spatiotemporal intensity modulation in both theta and alpha bands.
References
Adhikari BM, Sathian K, Epstein CM, Lamichhane B, Dhamala M (2014) Oscillatory activity in neocortical networks during tactile discrimination near the limit of spatial acuity. Neuroimage 91:300–310. doi:10.1016/j.neuroimage.2014.01.007
Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD (1989a) Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol 62:694–710
Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD (1989b) Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol 62:711–722
Allison T, McCarthy G, Wood CC, Jones SJ (1991) Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114:2465–2503. doi:10.1093/brain/114.6.2465
Allison T, McCarthy G, Wood CC (1992) The relationship between human long-latency somatosensory evoked potentia ls recorded from the cortical surface and from the scalp. Electroencephalogr Clin Neurophysiol 84:301–314. doi:10.1016/0168-5597(92)90082-M
Artoni F, Chisari C, Menicucci D, Fanciullacci C, Micera S (2012a) REMOV: EEG artifacts removal methods during Lokomat lower-limb rehabilitation. In: 2012 IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics. IEEE, New York, pp 992–997
Artoni F, Gemignani A, Sebastiani L, Bedini R, Landi A, Menicucci D (2012b) ErpICASSO: a tool for reliability estimates of independent components in EEG event-related analysis. In: 2012 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, New York, pp 368–371
Artoni F, Menicucci D, Delorme A, Makeig S, Micera S (2014) RELICA: a method for estimating the reliability of independent components. Neuroimage 103:391–400. doi:10.1016/j.neuroimage.2014.09.010
Aviles JM, Munoz FM, Kleinbohl D, Sebastian M, Jimenez SB (2010) A new device to present textured stimuli to touch with simultaneous EEG recording. Behav Res Methods 42:547–555. doi:10.3758/BRM.42.2.547
Bauer M, Oostenveld R, Peeters M, Fries P (2006) Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26:490–501. doi:10.1523/JNEUROSCI.5228-04.2006
Burton H, Videen T, Raichle M (1993) Tactile-vibration-activated foci in insular and parietal-opercular cortex studied with positron emission tomography: mapping the second somatosensory area in humans. Somatosens Mot Res 10:297–308
Cheyne D, Gaetz W, Garnero L, Lachaux J-P, Ducorps A, Schwartz D, Varela FJ (2003) Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Cogn Brain Res 17:599–611. doi:10.1016/s0926-6410(03)00173-3
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. doi:10.1016/j.jneumeth.2003.10.009
Dupin L, Hayward V, Wexler M (2015) Direct coupling of haptic signals between hands. Proc Natl Acad Sci USA 112:619–624. doi:10.1073/pnas.1419539112
Eimer M, van Velzen J, Driver J (2002) Cross-modal interactions between audition, touch, and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J Cogn Neurosci 14:254–271. doi:10.1162/089892902317236885
Garcia-Larrea L, Bastuji H, Mauguiere F (1991) Mapping study of somatosensory evoked potentials during selective spatial attention. Electroencephalogr Clin Neurophysiol 80:201–214. doi:10.1016/0168-5597(91)90122-E
Garcia-Larrea L, Lukaszewicz A-C, Mauguiere F (1995) Somatosensory responses during selective spatial attention: the N120-to-N140 trasition. Psychophysiology 32:526–537. doi:10.1111/j.1469-8986.1995.tb01229.x
Gordon J, Ghex C (1991) Modality coding in the somatic sensory system. Principles of Neural Science. 3rd edn. Elsevier, New York
Graimann B, Huggins JE, Levine SP, Pfurtscheller G (2002) Visualization of significant ERD/ERS patterns in multichannel EEG and ECoG data. Clin Neurophysiol 113:43–47. doi:10.1016/S1388-2457(01)00697-6
Greenspan JD, Bolanowski SJ (1996) The psychophysics of tactile perception and its peripheral physiological basis. In: Pain and touch, vol 2. Academic Press, San Diego, pp 25–103
Hämäläinen H, Kekoni J, Sams M, Reinikainen K, Näätänen R (1990) Human somatosensory evoked potentials to mechanical pulses and vibration: contributions of SI and SII somatosensory cortices to P50 and P100 components. Electroencephalogr Clin Neurophysiol 75:13–21. doi:10.1016/0013-4694(90)90148-D
Hansson T, Brismar T (1999) Tactile stimulation of the hand causes bilateral cortical activation: a functional magnetic resonance study in humans. Neurosci Lett 271:29–32. doi:10.1016/S0304-3940(99)00508-X
Hari R, Karhu J, Hämäläinen M, Knuutila J, Salonen O, Sams M, Vilkman V (1993) Functional organization of the human first and second somatosensory cortices: a neuromagnetic study. Eur J Neurosci 5:724–734
Hlushchuk Y, Hari R (2006) Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci 26:5819–5824. doi:10.1523/JNEUROSCI.5536-05.2006
Ishiko N, Hanamori T, Murayama N (1980) Spatial distribution of somatosensory responses evoked by tapping the tongue and finger in man. Electroencephalogr Clin Neurophysiol 50:1–10. doi:10.1016/0013-4694(80)90317-X
Iwamura Y, Iriki A, Tanaka M (1994) Bilateral hand representation in the postcentral somatosensory cortex. Nature 369:554–556. doi:10.1038/369554a0
Jiang W, Tremblay F, Chapman CE (1997) Neuronal encoding of texture changes in the primary and the secondary somatosensory cortical areas of monkeys during passive texture discrimination. J Neurophysiol 77:1656–1662
Johansson RS, Flanagan JR (2009) Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 10:345–359. doi:10.1038/nrn2621
Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth A (2000) Principles of neural science, vol 4. McGraw-Hill, New York
Kanno A, Nakasato N, Hatanaka K, Yoshimoto T (2003) Ipsilateral area 3b responses to median nerve somatosensory stimulation. Neuroimage 18:169–177. doi:10.1006/nimg.2002.1283
Kida T, Nishihira Y, Wasaka T, Nakata H, Sakamoto M (2004a) Differential modulation of temporal and frontal components of the somatosensory N140 and the effect of interstimulus interval in a selective attention task. Brain Res Cogn Brain Res 19:33–39. doi:10.1016/j.cogbrainres.2003.10.016
Kida T, Nishihira Y, Wasaka T, Nakata H, Sakamoto M (2004b) Passive enhancement of the somatosensory P100 and N140 in an active attention task using deviant alone condition. Clin Neurophysiol 115:871–879
Klatzky RL, Lederman SJ, Metzger VA (1985) Identifying objects by touch: an “expert system”. Percept Psychophys 37:299–302. doi:10.3758/bf03211351
Klimesch W (2012) Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16:606–617. doi:10.1016/j.tics.2012.10.007
Ku Y et al. (2007) Prefrontal cortex and somatosensory cortex in tactile crossmodal association: an independent component analysis of ERP recordings. PLoS ONE 2:e771. doi:10.1371/journal.pone.0000771
Legrain V, Bruyer R, Guérit J-M, Plaghki L (2003) Nociceptive processing in the human brain of infrequent task-relevant and task-irrelevant noxious stimuli. A study with event-related potentials evoked by CO2 laser radiant heat stimuli. Pain 103:237–248
Leung YY, Bensmaia SJ, Hsiao SS, Johnson KO (2005) Time-course of vibratory adaptation and recovery in cutaneous mechanoreceptive afferents. J Neurophysiol 94:3037–3045. doi:10.1152/jn.00001.2005
Lorenz J, Garcia-Larrea L (2003) Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophys Clin/Clin Neurophysiol 33:293–301.
Makeig S, Delorme A, Westerfield M, Jung TP, Townsend J, Courchesne E, Sejnowski TJ (2004) Electroencephalographic brain dynamics following manually responded visual targets. PLoS Biol 2:e176. doi:10.1371/journal.pbio.0020176
McGlone F, Kelly EF, Trulsson M, Francis ST, Westling G, Bowtell R (2002) Functional neuroimaging studies of human somatosensory cortex. Behav Brain Res 135:147–158. doi:10.1016/S0166-4328(02)00144-4
Menicucci D et al (2014) Brain responses to emotional stimuli during breath holding and hypoxia: an approach based on the independent component analysis. Brain Topogr 27:771–785. doi:10.1007/s10548-013-0349-z
Michail G, Dresel C, Witkovsky V, Stankewitz A, Schulz E (2016) Neuronal oscillations in various frequency bands differ between pain and touch. Front Hum Neurosci 10:182. doi:10.3389/fnhum.2016.00182
Moungou A, Thonnard JL, Mouraux A (2016) EEG frequency tagging to explore the cortical activity related to the tactile exploration of natural textures. Sci Rep 6:20738. doi:10.1038/srep20738
Munoz F, Reales JM, Sebastian MA, Ballesteros S (2014) An electrophysiological study of haptic roughness: effects of levels of texture and stimulus uncertainty in the P300. Brain Res 1562:59–68. doi:10.1016/j.brainres.2014.03.013
Neuper C, Wörtz M, Pfurtscheller G (2006) ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 159:211–222
Nieuwenhuys R, Voogd J, Van Huijzen C (2007) The human central nervous system: a synopsis and atlas. Springer, Berlin
Oddo CM, Beccai L, Vitiello N, Wasling HB, Wessberg J, Carrozza MC (2011) A mechatronic platform for human touch studies. Mechatronics 21:604–613. doi:10.1016/j.mechatronics.2011.02.012
Oddo CM et al. (2016) Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. eLife 5:e09148. doi:10.7554/eLife.09148
Oostenveld R, Praamstra P (2001) The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol 112:713–719. doi:10.1016/S1388-2457(00)00527-7
Palva S, Linkenkaer-Hansen K, Naatanen R, Palva JM (2005) Early neural correlates of conscious somatosensory perception. J Neurosci 25:5248–5258. doi:10.1523/JNEUROSCI.0141-05.2005
Pfurtscheller G, Lopes da Silva FH (1999) Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110:1842–1857. doi:10.1016/S1388-2457(99)00141-8
Pfurtscheller G, Krausz G, Neuper C (2001) Mechanical stimulation of the fingertip can induce bursts of beta oscillations in sensorimotor areas. J Clin Neurophysiol 18:559–564
Polich J (2007) Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118:2128–2148. 10.1016/j.clinph.2007.04.019
Salenius S, Schnitzler A, Salmelin R, Jousmaki V, Hari R (1997) Modulation of human cortical rolandic rhythms during natural sensorimotor tasks. Neuroimage 5:221–228. doi:10.1006/nimg.1997.0261
Schnitzler A, Salmelin R, Salenius S, Jousmaki V, Hari R (1995) Tactile information from the human hand reaches the ipsilateral primary somatosensory cortex. Neurosci Lett 200:25–28. doi:10.1016/0304-3940(95)12065-C
Schomer DL, Da Silva FL (2012) Niedermeyer’s electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins, Philadelphia
Sebastiani L, Castellani E, Gemignani A, Artoni F, Menicucci D (2015) Inefficient stimulus processing at encoding affects formation of high-order general representation: a study on cross-modal word-stem completion task. Brain Res 1622:386–396. doi:10.1016/j.brainres.2015.06.042
Shanks M, Pearson R, Powell T (1985) The ipsilateral cortico-cortical connexions between the cytoarchitectonic subdivisions of the primary somatic sensory cortex in the monkey. Brain Res Rev 9:67–88
Stancak A (2003) Desynchronization of cortical rhythms following cutaneous stimulation: effects of stimulus repetition and intensity, and of the size of corpus callosum. Clin Neurophysiol 114:1936–1947. doi:10.1016/s1388-2457(03)00201-3
Sutherland MT, Tang AC (2006) Reliable detection of bilateral activation in human primary somatosensory cortex by unilateral median nerve stimulation. Neuroimage 33:1042–1054. doi:10.1016/j.neuroimage.2006.08.015
Tamè L, Pavani F, Papadelis C, Farne A, Braun C (2015) Early integration of bilateral touch in the primary somatosensory cortex. Hum Brain Mapp 36:1506–1523. doi:10.1002/hbm.22719
Tamè L, Braun C, Holmes NP, Farne A, Pavani F (2016) Bilateral representations of touch in the primary somatosensory cortex. Cogn Neuropsychol 33:48–66. doi:10.1080/02643294.2016.1159547
Tamura K (1972) Ipsilateral somatosensory evoked responses in man. Psychiatr Neurol J 26:83–94. doi:10.1111/j.1440-1819.1972.tb01115.x
Tanaka E, Inui K, Kida T, Miyazaki T, Takeshima Y, Kakigi R (2008) A transition from unimodal to multimodal activations in four sensory modalities in humans: an electrophysiological study. BMC Neurosci 9:116. doi:10.1186/1471-2202-9-116
Tommerdahl M, Simons SB, Chiu JS, Favorov O, Whitsel BL (2006) Ipsilateral input modifies the primary somatosensory cortex response to contralateral skin flutter. J Neurosci 26:5970–5977
Weber AI, Saal HP, Lieber JD, Cheng JW, Manfredi LR, Dammann JF, Bensmaia SJ (2013) Spatial and temporal codes mediate the tactile perception of natural textures. Proc Natl Acad Sci USA 110:17107–17112. doi:10.1073/pnas.1305509110
Yamashiro K, Inui K, Otsuru N, Kida T, Kakigi R (2009) Somatosensory off-response in humans: an MEG study. Neuroimage 44:1363–1368
Zopf R, Giabbiconi CM, Gruber T, Müller MM (2004) Attentional modulation of the human somatosensory evoked potential in a trial-by-trial spatial cueing and sustained spatial attention task measured with high density 128 channels EEG. Cogn. Brain Res 20:491–509. doi:10.1016/j.cogbrainres.2004.02.014
Acknowledgements
We thank Alessio Tommasetti for the graphic design of Fig. 1. This work was supported by the Ministry of Education, Universities and Research (Italy) and the Swedish Research Council via the Italy-Sweden bilateral research project J52I15000030005 SensBrain (Brain network mechanisms for integration of natural tactile input patterns), by the EU Grant FET 611687 NEBIAS Project (NEurocontrolled BIdirectional Artificial upper limb and hand prosthesiS) and by the Ronda project, Robotica indossabile personalizzata per la riabilitazione motoria dell’arto superiore in pazienti neurologici. Regione Toscana, Bando FAS Salute 2014 PAR FAS 2007-2013.
Author Contributions
CG designed the study, developed the experimental set up, performed the experiments, analyzed the data, discussed the results and wrote the paper. FA designed the study, co-supervised the experiments, analyzed the data, discussed the results and wrote the paper. CMO co-designed the study, developed the tactile stimulation platform and supervised its experimentation, discussed the results and wrote the paper. CF performed the experiments. CC and HJ provided neurophysiological background and discussed the results. SM co-supervised the experiments, co-designed the study, discussed the results and wrote the paper. All authors reviewed and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in this study involving healthy subjects were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Fiorenzo Artoni and Silvestro Micera have contributed equally.
Rights and permissions
About this article
Cite this article
Genna, C., Oddo, C.M., Fanciullacci, C. et al. Spatiotemporal Dynamics of the Cortical Responses Induced by a Prolonged Tactile Stimulation of the Human Fingertips. Brain Topogr 30, 473–485 (2017). https://doi.org/10.1007/s10548-017-0569-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-017-0569-8