Abstract
Millions of people are subject to infertility worldwide and one in every six people, regardless of gender, experiences infertility at some period in their life, according to the World Health Organization. Assisted reproductive technologies are defined as a set of procedures that can address the infertility issue among couples, culminating in the alleviation of the condition. However, the costly conventional procedures of assisted reproduction and the inherent vagaries of the processes involved represent a setback for its successful implementation. Microfluidics, an emerging tool for processing low-volume samples, have recently started to play a role in infertility diagnosis and treatment. Given its host of benefits, including manipulating cells at the microscale, repeatability, automation, and superior biocompatibility, microfluidics have been adopted for various procedures in assisted reproduction, ranging from sperm sorting and analysis to more advanced processes such as IVF-on-a-chip. In this review, we try to adopt a more holistic approach and cover different uses of microfluidics for a variety of applications, specifically aimed at sperm separation and analysis. We present various sperm separation microfluidic techniques, categorized as natural and non-natural methods. A few of the recent developments in on-chip fertilization are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Infertility is counted as one of the most important public health concerns, as it affects one in every six people worldwide, according to the World Health Organization (WHO 2023). Given its indiscriminate prevalence in both developed and developing countries and the significant number of cases worldwide, it has garnered much attention in recent decades (Ombelet 1988). Both male and female infertility factors can lead to infertility in couples, and approximately half of the reported cases are attributed to male infertility (Agarwal et al. 2021). Ovulatory disorders, polycystic ovary syndrome, and blocked Fallopian tubes are important contributing factors in female infertility. On the other hand, erectile dysfunction, low semen volume, low sperm count, low motility, and a higher percentage of abnormal and defective sperm are deemed the major causes of male infertility (Agarwal et al. 2021; Carson and Kallen 2021).
Over the past decades, there has been some development in assisted reproductive technologies (ART), offering promising solutions to couples experiencing infertility (Wang and Sauer 2006). Intrauterine insemination (IUI), in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI) are widely recognized procedures and have been proven effective by years of experience (Thouas et al. 2010; Aboulghar et al. 2009). These procedures heavily rely on sperm quality to be effective, and as a result, there have been some techniques to improve the quality of the sperm sample (Oseguera-López et al. 2019). Routinely used methods for sperm selection include manual examination of sperm under a microscope, swim-up, and density gradient centrifugation (DGC). Swim-up and DGC sort motile and viable sperm based on their motility and density, respectively (Henkel and Schill 2003). These methods, while demonstrating some success, still suffer from being subjective, time-consuming, having costly procedures, and they usually depend on well-trained clinicians to operate (Sun and Sethu 2017; Nosrati et al. 2017). In addition, DGC, as one of the most well-established techniques, exerts significant force on sperm during the process and, thus, introduces reactive oxygen species (ROS), which leads to inevitable damage to the sperm DNA (Leisinger et al. 2021). All these factors can lead to inefficient treatment procedures, thereby requiring multiple attempts to reach a successful pregnancy. The current success rate of ART procedures is capped at around 33% owing to this very reason (Gnoth et al. 2011). Given the fact that a single ART cycle in the United States costs anywhere from $15,000 to $30,000, multiple treatment cycles translate to incurring huge financial burdens on both families and the healthcare sector (Conrad 2023). There have also been some attempts to use magnetic and fluorescent-activated cell sorting (MACS and FACS) for selecting non-apoptotic sperm and sperm with intact chromatin content, respectively (Stimpfel et al. 2018; Pacheco et al. 2020; Funaro et al. 2013). Despite being effective in selecting sperm with desired traits, the complexity of these methods, coupled with the associated secondary damage, render these procedures ineffective in the treatment cycle (Vasilescu et al. 2023). In addition to the aforementioned methods, physiological intracytoplasmic sperm injection (PICSI), as a modified version of ICSI, has also been utilized as part of ART procedures for improving treatment outcomes. In this technique, sperm are selected based on their ability to bind to hyaluronic acid, a naturally occurring substance in the human body. Mature sperm possess specific receptors that make them capable of binding to these molecules. As a result, this technique selects mature sperm that are shown to have a significantly lower DNA fragmentation compared with the immature population (Ní Dhuifin et al. 2022). Notwithstanding the promising results for the selection of mature sperm, the clinical outcomes have not yet conclusively suggested significant improvements over the more conventional ART treatments (Ní Dhuifin et al. 2022; Lepine et al. 2019).
Apart from the quality and efficacy, the availability and accessibility of the treatment procedures are still in question. According to recent studies conducted in Africa, the people’s access to these procedures remains low compared with other regions of the world (Ombelet and Onofre 2019). This highlights the necessity of developing low-cost, easy-to-use, accessible, and effective procedures that can be easily incorporated into the current treatment methods without requiring capital investment or significant changes in infrastructure. Microfluidics has been in the spotlight during the past decade for its remarkable potential in a wide variety of applications including but not limited to cell sorting (Autebert et al. 2012), drug delivery and screening (Damiati et al. 2018), single-cell analysis (Yin and Marshall 2012), cancer research (Ayuso et al. 2022; Žvirblytė et al. 2022), and biological assays (Pihl et al. 2005; Guo et al. 2012). Similarly, microfluidics have gained attraction in ART given the myriad of benefits it can offer, such as the ability to simulate the human body and manipulate sperms at a micron-scale level, superior biocompatibility, and the non-invasive nature of the devices (Alias et al. 2021; Kashaninejad et al. 2018; Bouloorchi Tabalvandani et al. 2024). Microfluidics also possesses the capability of reducing processing time considerably, thereby reducing the generation of ROS (Nosrati et al. 2014). This is crucial in the procedures of assisted reproduction as ROS can cause DNA fragmentation, which is believed to have a strong correlation with diminished chance of fertility, poor embryo development, and an increase in miscarriage rate (Agarwal et al. 2003; Borges et al. 2019). Furthermore, the possibility of integrating multiple stages of ART procedures such as sperm separation, vitrification, oocyte denudation, insemination, and so forth in one microfluidic device can have far-reaching effects, as it smooths the workflow and can eventually decrease the overall cost of treatments (Weng 2019).
In recent years, a multitude of microfluidic devices have been proposed for different purposes, ranging from motile and high-quality sperm sorting to sperm screening and to on-chip fertilization, all of which can become increasingly helpful in elevating the success rate of an ART procedure (Leung et al. 2022; Samuel et al. 2018; Kashaninejad et al. 2018; Wu et al. 2024). ZyMōt Fertility Inc. is one of the leading companies in commercializing the first-ever microfluidic devices specifically designed to separate motile and healthy sperms from semen samples. ZyMōt offers two different devices, namely, ZyMōt multi and ZyMōt ICSI, that utilize microchannels and micropores, respectively, to separate sperm based on their motility. The former device can process samples up to 3 mL and can yield 1–1.5 mL of enriched sperm sample in the output, rendering it suitable for even an IUI procedure. The latter device, on the other hand, is tailored to the ICSI procedure where it can process low-volume samples and separate motile sperm (ZyMōt fertility Inc 2023). There have been some ongoing clinical trials and studies that benchmarked these devices against common procedures such as DGC in assisted reproduction that suggested these microfluidic devices improved sample motility and DNA integrity, resulting in increased pregnancy and decreased miscarriage rates in patients (Morishita et al. 2022; Gode et al. 2019). In one such study, a population of couples with an exceptionally high incidence rate of embryo aneuploidy was treated with the ICSI procedure with both DGC and ZyMōt multi-yielded sperm. The obtained embryo euploidy rate was significantly higher in patients treated with ZyMōt multi. The clinical pregnancy rate also experienced a major boost, reaching almost 65% in ZyMōt-treated patients compared with 10.5% in the DGC-treated group. ZyMōt is just one clear example of how game-changing microfluidics can be in ART, and how many lives it can touch in years to come (Kocur et al. 2023).
In this review, we aim to review the recent advances in emerging microfluidic technologies for sperm sorting and screening and devices specifically tailored to IVF-on-a-chip. We first briefly touch upon sperm structure and the relevant morphological abnormalities. Subsequently, we also mention female reproductive tract (FRT) traits and the natural selection mechanisms it harnesses for the selection of desired sperm. Lastly, the devised microfluidic approaches for these purposes are presented under two categories of naturally-inspired and non-natural mechanisms. In the latter, we cover rather overlooked methods, such as electrical and optical methods, that were developed in recent years for a variety of applications in the assisted reproduction.
2 Sperm morphology
A spermatozoon consists of three major parts: head, midpiece, and tail. The head of the sperm is the anterior portion and surrounds a dense nucleus that houses the genetic material in the form of tightly packed chromosomes necessary for fertilization. The head is covered by a cap-like structure called the acrosome, which is derived from the Golgi complex. The acrosome contains enzymes that help the sperm penetrate the egg during fertilization (Alberts et al. 2017; Mortimer 2018). The midpiece is the central region of the sperm, located between the head and the tail, which is characterized by a dense concentration of mitochondria. They produce ATP (adenosine triphosphate), which is the energy currency of the cell. Indeed, the midpiece is responsible for generating the energy required for sperm motility and swimming motion (Orsolini et al. 2021; Mortimer 2018). The tail, also known as the flagellum, is the long, whip-like structure that extends from the midpiece. It is responsible for propelling the sperm forward through a whip-like motion. The tail is composed of microtubules, which are cylindrical structures made of protein. These microtubules slide past each other, causing the tail to bend and move in a wave-like pattern, allowing the sperm to swim towards the egg (Fig. 1A) (Kumar and Singh 2021; Mortimer 2018).
Schematic representation of sperm travel through different parts of the FRT. A Structure of a morphologically normal sperm B The presence of microgrooves assists sperms passage through the cervix. Reproduced from Tung et al. (2015) with permission. C The labyrinth structure of the Fallopian tube guides sperm to the fertilization site, Uthman, E. Normal Fallopian Tube, Human. https://www.flickr.com/photos/euthman/2760474960 (accessed on 5 October 2023)
The description given thus far presents an idealized representation of sperm morphology. However, it is important to note that in reality, sperms with non-ideal shapes are commonly observed. Sperm morphology disorders can arise from various causes, including genetic mutations, environmental factors, and systemic diseases. Genetic abnormalities and mutations, as well as maturation disorders, affecting the Golgi apparatus or acrosome membrane formation, can lead to abnormal head development. Environmental factors such as exposure to toxins, infections, radiation, and temperature fluctuations can also result in sperm head morphology disorders. Additionally, systemic diseases such as diabetes, hypertension, and sexually transmitted infections can contribute to abnormal sperm morphology (Orsolini et al. 2021; Mortimer 2018).
Cytoplasmic droplets are considered normal morphological features in human spermatozoa that play significant physiological role in hyperactivation, capacitation and the acrosome reaction (Rengan et al. 2012). However, when present in large amounts, these features become known as excess residual cytoplasm (ERC), that due to their elevated level of cytoplasm enzymes are associated with production of excessive reactive oxygen species (ROS) (Rengan et al. 2012; Cooper 2005). The resulting oxidative stress can have severe implications such as abnormal head and midpiece morphology (Huszar and Vigue 1993; Gomez et al. 1996; Gergely et al. 1999), poor sperm motility (Zini et al. 1998), higher chance of DNA fragmentation (Fischer et al. 2003), and reduced fertilizing capacity (Keating et al. 1997). In the case of the sperm midpiece, mitochondria malfunction and genetic mutations can lead to midpiece abnormalities, affecting sperm motility. Smoking has also been reported to contribute to abnormal midpiece development. The tail is formed during spermatogenesis, where various structural components crucial for motility are assembled. Defects during the maturation process can lead to tail abnormalities, such as bending, curling, or coiling of the tail, that affect sperm motility and, ultimately, its ability to fertilize the oocyte. Genetic mutations that alter the formation of the axoneme, fibrous sheath, or energy-producing mitochondria can also cause tail defects (Mortimer 2018; Oehninger and Kruger 2021; Orsolini et al. 2021). The most commonly encountered morphological abnormalities are shown in Fig. 2. Morphology disorders in sperm can have significant implications for fertility and the health of the offspring. Abnormal sperm morphology can reduce the chances of successful fertilization and increase the risk of genetic abnormalities or birth defects in children. It is important to address and understand these disorders to optimize reproductive outcomes and ensure the well-being of future generations (Oehninger and Kruger 2021; Bertolla 2020).
3 Sperm journey through FRT
Among millions of sperm that enter the FRT after ejaculation, only a handful are able to successfully reach the fertilization site. This journey involves overcoming various challenges, such as navigating through viscous fluids, avoiding obstacles, and evading immune cells. The selected sperms that successfully complete this journey must pass through different parts of the FRT, including the vagina, cervix, uterus, utero-tubal junction, and Fallopian tube. Throughout this path, the sperm interact with the epithelium and luminal fluid, which can influence their motility and function. Therefore, understanding the complex mechanisms involved in sperm migration is crucial for developing efficient sorting procedures and devices (Miller 2018; Bertolla 2020).
Sperm transport begins in the upper vagina and ends in the ampulla of the Fallopian tube, where the sperm meets the oocyte. The composition and buffering capacity of seminal fluid immediately protects the spermatozoa from the acidity of the upper vaginal area. The acidic vaginal fluid normally serves a bactericidal function in protecting the cervical canal from pathogenic organisms. The next barriers that the sperm cells must overcome are the cervical canal and the cervical mucus. Composed of cervical mucin (a glycoprotein with a high carbohydrate composition) and soluble components, cervical mucus is not readily penetrable (Transport 2014). It acts as a physical barrier, inhibiting sperms with poor morphology and motility to progress to the uterus. Sperm transport through the cervix to the uterus can be categorized into two separate waves, one consisting of muscular contraction of FRT which causes fast transportation of sperm to the uterus. The other comprises sperm navigation through the cervical mucus within the crypts embedded in the cervix linings (Fig. 1B). The latter is considered the slow phase of sperm migration, which can last for approximately two to four days, whereas the former is much faster and can lead to sperm arrival in the uterus within 20 min of ejaculation. The rapid phase of the migration relies exclusively on the FRT contraction rather than sperm motility. Spermatozoa that have spent more time in the FRT are more capable of fertilizing an egg than those that have arrived more rapidly. The final and slowest phase of transport involves the transport of a small number of spermatozoa to the area of the reproductive tract where the egg will be fertilized (Transport 2014; Pérez-Cerezales et al. 2015).
The uterine muscle contractions can draw sperm and watery midcycle mucus from the cervix into the relatively small human uterine cavity. Sperm can traverse through the uterus in less than 10 min (Suarez and Pacey 2006). However, the female reproductive system has defensive agents that challenge spermatozoa. Fertilizing sperm can bind to uterine epithelial cells, which helps clear excess sperm, seminal debris, and bacteria. This process reduces the acquired immune response and facilitates fertilization. After semen deposition, polymorphonuclear leukocytes, which are involved in innate and adaptive immunity, infiltrate the uterus. Seminal plasma contains immune system modulators that affect immune cells in the uterus and oviduct. It has been discovered that seminal plasma can improve pre-implantation development and have long-term effects on offspring (Miller 2018). The uterus serves as a nonmotile storage place for spermatozoa and is also the site where the fertilized ovum implants (Pérez-Cerezales et al. 2015). Successful fertilizing sperm move through the uterus and into the oviduct, where sperm are activated for fertilization. The Fallopian tubes are also known as oviducts, and they are the site where fertilization takes place. When a sperm reaches a mature egg in one of the Fallopian tubes, it can penetrate the egg outer membrane and begin fertilization (Holt and Fazeli 2018).
In addition to the aforementioned barriers, the female reproductive system, in coordination with the egg, employs various guidance mechanisms to facilitate the migration of sperm and guide them towards successful fertilization. These guidance mechanisms encompass structural characteristics of the female reproductive system, environmental fluids, and the orchestration of sperm taxis, which can be short- or long-range mechanisms. The labyrinthine lumen in the Fallopian tube has been found to induce boundary-following behavior, a natural guidance mechanism utilized by sperm to reach the fertilization site (Fig. 1C). Moreover, chemotaxis is a short-range mechanism, estimated to occur within the order of a few millimeters. Long-range mechanisms for sperm guidance in mammals include thermotaxis and rheotaxis, which guide spermatozoa along the oviduct to the fertilization site (Fig. 3) (Pérez-Cerezales et al. 2015).
Spermcells are guided towards the oocyte primarily through three mechanisms including: A rheotaxis B thermotaxis C chemotaxis. Reproduced from Lottero-Leconte et al. (2017) with permission
In the following sections, we comprehensively review the natural selection mechanisms and the inspired microfluidic devices.
4 Naturally-inspired mechanisms
4.1 Chemotaxis
As its name suggests, chemotaxis is the sperm guidance towards a concentration gradient of a chemo-attractant, secreted by the oocyte. Coming into contact with the chemo-attractant, specific receptors on the sperm flagellum membrane are activated upon binding with these molecules, guiding sperm to the oocyte. The guidance process starts with a temporary increase in calcium levels within the flagellum, which is believed to control the function of dynein motor proteins. As a consequence, this regulation influences sperm movement by guiding it towards the oocyte. This directional movement is achieved through changes in the beating pattern of the sperm tail, allowing it to swim in a straighter path towards the oocyte (Friedrich and Jülicher 2007).
Microfluidics has been extensively used to capitalize on this natural guidance mechanism for sperm screening and sorting procedures (Leung et al. 2022). These devices stimulate sperm chemotaxis behavior by creating a gradient concentration using a few substances including progesterone (Berendsen et al. 2020; Zhang et al. 2015; Yan et al. 2021), CCL21, CCL19, resact (Chang et al. 2013), and acetylcholine (Ko et al. 2012, 2018), which have been found to play as chemo-attractants in the FRT. Given their ease of fabrication and use, chemotaxis-based microfluidic devices have been used to study sperm chemotaxis behavior in vitro in recent years (Berendsen et al. 2020; Chang et al. 2013; Lu et al. 2014). Building upon this idea, Ko et al. utilized multiple channels with a chemical gradient generated along each channel as a means of separating a larger volume of sperm within a given time-frame (Ko et al. 2012). The study resulted in the selective separation of progressive motile sperms using a chemical gradient of acetylcholine in an environment that mimics that of the female oviduct. Zhang et al. introduced a microfluidic chip with a hexagonal shape that allows for the easier generation of a stable and controllable concentration gradient (Zhang et al. 2015). The chemical gradient is adjusted using peripheral channels, connected to the main chamber by a series of grooves (Fig. 4A). It reduced the experimental time and facilitated precise measurements and analysis that significantly outperformed the previous devices. In a recent study, the authors fabricated an FRT-like device for the separation of sperm based on their chemotaxis and motility (Fig. 4B) (Li et al. 2018). The presented device was a life-size model of the FRT, comprising grooves along the two channels that represent the oviducts. A mixture of agar and progesterone was placed in the middle groove in one of the channels, while the middle groove on the other side was filled with only agar as the control. After injecting the sperm sample into chamber A, motile sperm started swimming to the oviduct-like channels. The experiment was left to unfold for 150 min, after which the sperm were retrieved from the nearby grooves. The biological assays revealed enhanced morphology and DNA integrity in the sperm of both grooves compared to the initial sample. Furthermore, the sample in the progesterone-treated groove demonstrated a higher percentage of morphologically normal sperms compared to the control group, while DNA integrity was not statistically different between the two groups.
Figures illustrating microfluidic systems that rely on chemotaxis and thermotaxis. A The device employed progesterone concentration gradients in peripheral channels to create independent experimental regions, allowing the assessment of the sperm chemotactic response to varying concentrations. Reproduced from Zhang et al. (2015) with permission. B Experimental procedures of the FRT-like device that capitalizes on sperm chemotaxis and motility for separation of the normal population, reproduced from Li et al. (2018) with permission. C On-chip thermotaxis evaluation of human sperm using an interfacial valve-facilitated microfluidic device, demonstrating temperature gradient control and the enrichment of temperature-responsive sperm population, reproduced from Li et al. (2014) with permission D The microchannel is responsible for creating the concentration gradient of the chemo-attractant (acetylcholine) as well as the temperature difference generated and controlled by a microheater and microsensor. The experimental findings indicate that motile mouse sperm exhibit a heightened sensitivity towards chemical and temperature gradients when both chemotaxis and thermotaxis are integrated, compared to when they are examined individually. Reproduced from Ko et al. (2018) with permission
4.2 Thermotaxis
Thermotaxis is another important guidance mechanism used in the fertilization process, which is the sperm directional movement along a temperature gradient that exists in the Fallopian tube. The temperature gradient has been measured to be as high as 2 °C in the rabbit oviduct. In contrary to chemotaxis, thermotaxis can operate at a much farther range to guide sperm to the oocyte (Eisenbach and Giojalas 2006). In addition, recent studies have shown that sperms are responsive to temperature gradients as low as 0.014 °C\(\cdot\) mm\(^{-1}\). This responsiveness, coupled with its long-range capability, makes thermotaxis an effective guidance mechanism (Bahat et al. 2012). Sperm can react to the temperature difference by virtue of their temperature-sensing proteins, called thermosensors, on the cell membrane. After detecting temperature changes, these sensors trigger a series of signals that can activate ion channels and molecules inside the cell. This leads to changes in the electrical potential of the sperm cell and triggers flagellar movement. The coordinated beating of the flagellum propels the sperm forward, allowing it to navigate and respond to temperature fluctuations. This complex process ensures that sperm can effectively respond to thermal gradients and reach the fertilization site (Xiao et al. 2022; Rodríguez-Gil 2019; Pérez-Cerezales et al. 2018; Bahat and Eisenbach 2006).
In 2014, Li et al. introduced a microfluidic chip designed for the precise separation of temperature-sensitive sperm based on their thermotactic response (Li et al. 2014). They used temperature gradients along a microfluidic channel to separate the responsive sperm. The air valve then activates, sealing the outputs by directing the airflow, further enhancing the reliability and effectiveness of the separation process (Fig. 4C). They discovered that an insignificant percentage of motile sperm (ranging from 5.7% to 10.6%) exhibited thermotactic responses across different temperature ranges, which means a considerable portion of sperm cells may need an additional stimulus for efficient separation.
One approach to address this issue is the combination of chemotaxis with thermotaxis, as proposed by Yan et al. (2021). In this study, authors created a chemical gradient by embedding multiple pools in the microfluidic device containing high and low concentrations of progesterone, connected to each other through microgrooves. To create a controllable and stable temperature gradient, indium tin oxide (ITO) microheaters were patterned on the substrate of the device. The results demonstrated that thermotaxis and chemotaxis behaviors were not mutually connected. In other words, authors found that some sperm were responsive to the temperature gradient, while others responded to the progesterone gradient in the device. In addition, some sperm were found to respond to both stimuli simultaneously. In a similar article, scientists conducted a study where they successfully enhanced the isolation of viable sperm by increasing the amount of acetylcholine added to the chip, which led to improved efficiency in the process (Fig. 4D) (Ko et al. 2018). In this design, photolithography and wet etching were used to create the ITO heater and sensor. Thin layers of chromium and gold were deposited onto ITO glass using magnetron sputtering, followed by acetone treatment and wet etching of a patterned photoresist. The results showed that when the temperature difference between the inlet and the outlet was 2 °C, a significantly higher number of sperm reached the outlet than when it was 3 °C. Furthermore, when the temperature difference was 2 °C, a higher number of sperm reached the outlet at 35–37 °C, 36–38 °C, and 37–39 °C than at 26–28 °C and 28–30 °C, which are all lower than the body temperature range. In another study, scientists utilized cumulus cells as a chemo-attractant and implemented controlled temperature gradients to effectively sort sperm cells. This approach resulted in significant improvements in the sorting process and enhanced sperm parameters and function, particularly in patients with high DNA fragmentation (Doostabadi et al. 2022).
4.3 Rheotaxis
Rheotaxis is the phenomenon whereby sperm reorient themselves against the fluid flow direction and swim upstream. In the FRT, cilia beating coupled with peristalsis of the oviduct muscles generate a gentle flow outward to the cervix, which is counted as a guidance mechanism in sperm migration. Upon entering this pathway, sperm receive a significant signal from their sensors indicating that they have found the path to reach the egg. Subsequently, they undergo a synchronized change in trajectory and start swimming against the flow with precision to achieve successful fertilization. Utilizing their flagella, they propel themselves at maximum speed against the current; however, only those with higher velocity can succeed (Zhang et al. 2016; Ishimoto and Gaffney 2015; Pérez-Cerezales et al. 2015).
The lack of special reagents for stimulating rheotaxis behavior, accompanied by the enhanced DNA integrity of sperm showing this behavior, has prompted a large amount of research for devising rheotaxis-based microfluidic devices for motile sperm separation. In one such attempt, a microfluidic device was fabricated to assess the impact of fluid flow on the sperm rheotaxis and motility parameters (Rappa et al. 2018). It was observed that the percentage of sperm that orient their head and actively swim against the flow direction increased up to a certain flow rate (4 µ L/min) and then decreased as the flow rate increased further. For studying the motility parameters, the authors analyzed sperm under the influence of fluid flow against the groups in the no-flow condition, as well as the initial sample. The flow group had notably higher motility parameters compared to the two other groups. In contrast, sperm in the flow condition did not show any significant difference compared with other groups regarding morphology and HA-binding ability. In the subsequent research conducted by Ataei et al., the previous design was improved by adding a polycarbonate membrane, separating the loading and collection chambers (Ataei et al. 2021). The device in question used differential hydrostatic pressure between the collection chamber and the peripheral inlets to generate the fluid flow. The sperm in the loading chamber oriented themselves against the flow towards the top. The coupling effects of rheotaxis and the resulting barrier of the porous membrane separated sperms with normal morphology, enhanced DNA integrity, and motility. In another study, researchers developed a sperm-sorting device for separating motile sperm using gravity-generated flow and pipetting (Kang et al. 2019). In this design, a cam-actuated pipette was inserted into a microfluidic chamber, where the outlet width of the chamber was regulated via a bolt, adjusting the fluid velocity flowing through the device. The sperm were automatically injected into the device using the cam-controlled pipette. Following the injection, the viable and motile sperm reoriented and swam upward against the fluid flow. After 40–100 s, as enough sperm populated the area in the vicinity of the pipette tip, the sperm were sucked into the pipette. This process can be repeated in order to augment the number of collected sperm.
Tung et al. developed a microfluidic device consisting of parallel microgrooves similar to the grooves found in the bovine cervix (Fig. 5A) (Tung et al. 2015). The authors observed that these microgrooves favor the migration of sperm over the pathogen Tritrichomonas foetus. In another study conducted by the same authors, the structure of microgrooves was studied for sperm rheotaxis, and it was found that these microgrooves assist the sperm passage against the fluid flow in vitro (Tung et al. 2014). These findings suggest the importance of the special microenvironment of FRT that facilitates sperm migration. Zaferani et al. proposed a microfluidic device containing corral-shaped structures embedded within the channel (Zaferani et al. 2018). Sperm medium was injected into the channel with varying flow rates. The corral structures served as the rheotaxis zone by creating areas of low shear rate and velocity field in the channel. Incoming sperm by the flow were observed to reorient against the flow and migrate towards and into the corrals. The device demonstrated its effectiveness by capturing viable and motile sperm from the initial sample. In another similar study, authors used a series of micro pockets of low shear rate and velocity field to create rheotaxis zones along the fluid direction (Sarbandi et al. 2021). The isolated sperms in this device were reported to have enhanced motility compared to the raw semen. In addition, the devised micro pockets differed in terms of shear rate and, as a result, isolated populations with different average velocities. In similar approaches, two devices were proposed that combined sperm rheotaxis and boundary-following behaviors to isolate motile sperm (Heydari et al. 2023; Zeaei et al. 2023). In these devices, a series of rheotaxis zones were connected to collecting zones, capturing sperm showing rheotaxis and boundary-following behavior. The captured sperms showed significant enhancement in motility compared to the initial sample (Fig. 5B). In a recent study, the researchers presented a simpler approach for creating a rheotaxis zone (Mane et al. 2022). The separation of motile sperm was carried out using a simple T-shaped microchannel, connected to three reservoirs (Fig. 5C). One of the reservoirs is sealed during the experiment, creating a no-flow condition in one of the channels connected to the T-junction. Sperm, as previously discussed, reoriented towards this region and were collected in the reservoir connected to this channel.
Rheotaxis-based microfluidic devices. A This model was developed to simulate fluid flows and microgrooves in the cervix and utero-tubal junction. The swimming behavior of sperm cells was observed as they encountered the grooved and smooth channels. Reproduced from Tung et al. (2015) with permission. B The displayed device is for isolating motile sperm based on rheotactic and boundary-following behavior. The device’s symmetric geometry allows for a four-fold increase in the volume of the processed sample and injected flow rate compared to previous devices. The experimental results demonstrate 100% motility of the isolated sperms and the ability to sort them based on swimming velocity. Reproduced from Heydari et al. (2023) with permission. C The T-shaped microchannel, which is primed with PBS (phosphate buffered saline) and sealed using adhesive tape, is used to separate and isolate progressive motile sperm. The forces acting on the sperm cells during separation, including drag and rotational torque, are depicted. Reproduced from Mane et al. (2022) with permission. D Schematic representation of sperm migration through the selection channel, where planar confinement induces planar swimming. Sperm navigate from the inlet channel to the outlet channel in the selection channel. Reproduced from Riordon et al. (2019) with permission. E The microfluidic device utilizes regulated flow rates and the sperm’s rheotactic behavior to sort highly motile sperm. The device features three functional zones, including a branch zone for separating original sperm, a collecting zone with a sperm collector for retaining motile sperm, and a waste zone for removing dead or low motile sperm. Reproduced from Huang et al. (2023) with permission
In another article, an array of cone-shaped structures was uniformly placed within the microfluidic channel to trap motile sperm swimming upstream (You et al. 2019). The underlying mechanism of trapping sperm used in this chip is identical to the one proposed by Zaferani, yet the traps in this research were fabricated to only accommodate one sperm at a time, tailoring the device for single sperm analysis. The cone geometry utilized here also prevented the sperm from exiting the trap while enabling the free tail beating of the sperm. Yaghoobi et al. proposed a microfluidic device for parallelizing motile sperm separation (Yaghoobi et al. 2021). The device consisted of parallel microchambers connected to a series of main channels. By injecting a sperm sample into the device, while keeping the outlet closed, each chamber was filled with the sample. Upon removing the seal from the outlet and injecting the wash medium into the device, the motile sperm were able to swim out of the chamber and to the outlet, where they were collected. However, debris, non-viable, and immotile sperm were trapped in the chambers.
Restriction in a microchannel has been observed to act as a gate, barring the rheotaxis-stimulated sperms from swimming through (Zaferani et al. 2019). Based upon this idea, Huang et al. introduced a tree-like configuration by parallelizing these gate-like structures at different levels that can select high-quality sperm with reduced DNA fragmentation and without abnormal phenotypes (Fig. 5E) (Huang et al. 2023). In the research conducted by Riordon et al., it was revealed that the sperms capable of planar swimming show higher DNA integrity compared to the sperm swimming in a corkscrew-style motion (Riordon et al. 2019). A series of 2 µm deep channels were designed to bridge between the inlet and outlet channels (Fig. 5D). The bridges served as a way to confine sperm to a two-dimensional plane while preventing rotation of the sperm head. Only sperm that were capable of planar motion could swim through these bridges, reaching the outlet channel. The result of the DNA integrity assay highlighted a significant improvement in the DNA integrity of the collected sperms compared to both raw sample and motile sperm.
4.4 Thigmotaxis
The FRT presents a challenging landscape with narrow passages, intricate pathways, and viscous fluids, all of which pose obstacles to sperm cells on their journey to fertilization. To overcome these challenges, sperm cells have evolved the ability to exhibit thigmotaxis, also known as boundary-following behavior. This behavior involves sperm cells actively seeking and adhering to surfaces or boundaries within the reproductive tract. Boundary-following behavior is facilitated by several factors such as the viscous environment, hydrodynamic forces, surface interactions, and chemical cues. Sperm cells stick to boundaries to reduce resistance, utilize hydrodynamic forces to change direction, gain traction through surface interactions, and stay close to chemical signals guiding them towards the egg. This behavior enhances their efficiency in navigating the reproductive tract and increases the likelihood of successful fertilization (Romero-Aguirregomezcorta et al. 2018; Denissenko et al. 2012). It is important to know that sperm movement is characterized by a completely random motion similar to Brownian motion in the absence of external stimuli. This random movement is a result of continuous interactions and collisions between sperm and molecules, as well as fluid structures within the reproductive environment. The behavior of the sperm can also be influenced by factors such as the surface of the sperm itself and the inherent noise in its motor activity. Furthermore, environmental conditions such as thermodynamic noise can affect sperm trajectories. Indeed, in the absence of specific guiding stimuli, environmental conditions become the primary cause of sperm movement (Diemer et al. 2021; Tian and Wang 2021).
One of the significant environmental factors in the selection of more desirable sperm during their journey is the viscosity of the environment. The female body employs this viscous environment to impede the passage of weaker sperms, while faster and more persistent sperm possess the capability of migrating through this viscous medium. In addition, sperm approach the walls embedded in the reproductive tract linings to minimize the resistance encountered during their movement (Romero-Aguirregomezcorta et al. 2018; Denissenko et al. 2012). In a recent study, a microfluidic device was proposed that mimicked the viscosity of cervical mucus to select for highly motile sperms (Lee et al. 2021). In this device, a viscous polyvinylpyrrolidone (PVP)-based solution was employed as a medium with a viscosity resembling that of the cervical mucus. As a result, the use of the sperm-sorting chip led to a lower number of defective sperm, including those with head vacuoles and DNA fragmentation. The findings demonstrated that in comparison to the control group, the sperm in 1.5% and 3% PVP media exhibited statistically significant increases in linear progressive motility (LPM). In particular, the LPM mean value was 38.60% and the mean velocity was 25.69 µm/s in the 1.5% PVP medium. The mean LPM and velocity in the 3% PVP medium were 41.96% and 27.24 µm/s, respectively. The control group exhibited a mean velocity of 22.93 µm/s and a mean LPM of 36.67%. In another study, a microdevice was proposed to evaluate and quantify the swimming capability and persistence of human sperm in a viscous environment (Yan et al. 2020). The device incorporated three distinct microchannels filled with various types of viscous fluids: a solution composed of 5% methylcellulose (MC) solution, another with intermediate viscosity containing an MC solution at a concentration of 3.4%, and the human tubal fluid (HTF) solution. By introducing a sperm sample into the chip, sperm swam towards these channels and were subsequently analyzed by a video recorder. The number of sperm in each area was inversely correlated to the viscosity of the fluids in the channels. By analyzing recorded videos, it transpired that the sperms found in a more viscous medium have more swimming capability. As a result, by calculating the distribution of the number of sperm in the chambers, one can evaluate the sperm sample quality. This microdevice enabled researchers for one-step high-quality sperm selection and quantitative evaluation of swimming capability, persistence, and inactivation rate (Fig. 6A). In the additional research conducted by Eamer et al. (2015), a microfluidic device was employed to examine the impact of high-viscosity media, specifically those composed of hyaluronic acid (HA) and MC, on the motility and viability of bovine and human sperm. The study revealed that bovine sperm exhibit significantly improved motility parameters when exposed to the MC medium compared with HA. Furthermore, experiments involving human sperm swimming in MC demonstrated that higher viscosities result in slower and more linear sperm movement. These findings suggest that MC is the preferred choice over HA when creating a high-viscosity environment for in vitro sperm assessment or selection based on motility.
Schematics of microfluidic devices based on the thigmotaxis behavior of sperm. (A) A circular symmetric chip with multiple viscosity solutions (5% and 4% MC and HTF) in different straight pipes, enabling evaluation and screening of sperm swimming capability, persistence, and inactivation rate. The 5% MC solution exhibited the lowest number of motile sperm, while the HTF solution showed the highest number of sperm. Reproduced from Yan et al. (2020) with permission. (B) A passive microfluidic sperm selection strategy based on the preference of highly motile sperm with high DNA integrity to turn corners and follow boundaries in a microfluidic device. The study revealed that human sperm preferring to follow boundaries on the left- or right-hand sides exhibit significantly higher DNA integrity (greater than 51%) compared to straight swimmers and a significant increase (greater than 67%) compared to sperms in raw semen. Reproduced from Eamer et al. (2016) with permission. (C) The Simple Periodic ARray for Trapping And isolatioN (SPARTAN) model-driven design uses pillar arrays to efficiently isolate highly motile and morphologically normal sperm with improved epigenetic characteristics from raw semen, offering standardized sperm selection. Reproduced from Chinnasamy et al. (2018) with permission
The subsequent environmental factor that influences the movement of sperm towards the egg is the presence of flow obstacles. Flow obstacles are formed when two laminar flows with different velocities converge. In this scenario, the stronger and more motile sperm are able to navigate towards and pass through these obstacles, while the remaining components of the seminal fluid are unable to do so. The concept of flow obstacles has been extensively studied in the literature, demonstrating its significance in separating highly motile sperms from other sperm and debris (Huang et al. 2017, 2020). Building upon this concept, Phiphattanaphiphop and Shirota have applied a methodology in their research, creating a layout that is structured along both X and Y axes (Phiphattanaphiphop et al. 2020; Shirota et al. 2016). Their innovative design has yielded several benefits, including reduced processing time for sperm samples, minimized risk of bacterial and viral contamination, and limited damage to sperm cells.
In the following, we review some studies that specifically focus on boundary-following behavior. In an article presented by Denissenko et al., the authors presented a comprehensive study on the movement patterns of migrating human sperm in microchannels (Denissenko et al. 2012). The designed microchannels consist of narrow and winding channels filled with thick fluids. They investigated both the individual and collective behaviors of the sperm in various microchannel shapes. The findings revealed that the sperm rarely swim in the central region of the channel cross-section. Instead, they tend to travel along the intersection of the channel walls, known as channel corners. When the curvature radius of the inner wall is below a threshold value of approximately 150 µ m, the sperm deviate from the inner wall. Furthermore, specific wall shapes can guide the motile sperm in a preferred direction. However, due to swimming along the corners, the area occupied by the sperm becomes essentially one-dimensional, resulting in frequent collisions.
In the research conducted by Nosrati et al., a microfluidic sperm sorter device was proposed based on sperm boundary-following behavior (Nosrati et al. 2014). The device consisted of a ring of thousands of parallel channels to create the passageways needed for sperm boundary following. The ring is sandwiched between an inner disk, where unprocessed semen is injected into the system, and an outer disk, where the sorted sperms are retrieved. The device was prefilled with a viscous medium to mimic the fluid viscosity inside the FRT. The experimental results clearly demonstrated that viable and progressive motile sperm are able to swim through the parallel channels to the outer outlet disk. The device facilitated the purification of the semen and the selection of sperm with high DNA integrity, thereby offering a clinically feasible one-step approach, enhancing sperm viability. In another study published by the same group, a similar approach to the previous design was taken, relying solely on the boundary-following behavior of sperm. Three separate microfluidic devices with almost the same design as in Nosrati et al. (2014), with minor variations, were exploited to separate right-, left-, and straight-swimming sperm (Eamer et al. 2016). Straight-swimming sperm in this experiment were those that did not approach near to boundaries in order to swim in the device. The results of the experiments showed a significant correlation between the sperm’s ability to follow boundaries and high DNA integrity. By comparing the DNA fragmentation index (DFI) results from the straight- (3.3%), right- (1.6%), and left-swimming (1.2%) populations, the authors concluded that highly motile sperm with high DNA integrity preferentially fell into both right- and left-swimming populations. On the other hand, straight-swimming sperm showed increased DFI, which foregrounds the fact that boundary-following behavior is strongly correlated with DNA integrity. The two-sided Student’s t-test further corroborated the significant difference between the straight swimmers and the boundary followers (Fig. 6B).
In another study, a relatively simple yet practical design of a simple periodic array of micropillars in a microfluidic chip was utilized for the selection of motile and morphologically normal sperm (Chinnasamy et al. 2018). By modulating the directional persistence of the sperm, the periodic array increases the spatial separation between progressive and nonprogressive motile sperm populations. In addition, the morphologically defective sperm were unable to steer through micropillars and were spatially diverged, while normal sperm were mostly packed in a rather straight trajectory. The proposed device was able to sort out highly motile and morphologically normal human sperm with lower epigenetic global methylation compared to conventional sperm-sorting methods. By analyzing the sorted samples, it was found that the percentage of morphologically normal sperm increased dramatically (52%) compared to both swim-up (24%) and raw semen (13%). The evaluation of chromatin condensation in the sorted samples also showed a three-fold increase compared to raw semen, reaching the mean of 43.5%. SPARTAN also benefited from a shortened processing time of merely 10 min, which in turn, reduced the DFI to 4–6% (Fig. 6C).
5 Non-natural mechanisms
5.1 Acoustofluidic-based methods
Acoustic microfluidic, i.e., acoustofluidic, separation techniques have gained significant relevance in applications involving biological samples due to their numerous advantages, including precise control, biocompatibility, non-invasiveness, and label-free characteristics. Acoustofluidic devices can be generally categorized into surface acoustic waves (SAW) and bulk acoustic waves (BAW). In SAW devices, interdigitated transducers (IDT) are fabricated on a piezoelectric substrate, translating electrical signals into mechanical vibrations that travel through the surface of the material. On the other hand, BAW devices are usually fabricated with a piezoelectric transducer made of lead zirconate titanate (PZT) that is physically bonded to the glass, adjacent to the microfluidic device. The generated mechanical vibrations travel instead through the bulk of the fluidic chamber or channel. Unlike SAW devices, BAW devices work in a much lower frequency range and also benefit from an easier and cost-effective fabrication (Gao et al. 2020). There have been some attempts to exploit SAW for the realization of devices aimed at sperm sorting and enhancement, which will be discussed shortly. However, BAW-based devices have been untouched, and to our knowledge, no information on their use in this field has been reported as of writing this article.
Gai et al. presented a novel and automated method for selecting high-quality sperm with desired parameters (Gai et al. 2020). By utilizing an acoustic-based continuous-flow approach, this device was able to guide larger and highly motile sperm across the channel, resulting in the selection of sperm with significantly enhanced vitality, progressive motility, and DNA integrity. In this research, a pair of IDTs were patterned on a \(128^{\circ }\) Y-cut, X-propagating lithium niobate substrate to generate standing SAW. The microfluidic chamber was cast with a higher-than-normal ratio of curing agent to monomer to minimize the deformation of the chamber in the presence of the acoustic waves. The electrodes were angled at approximately 30 degrees against the fluid flow direction, thereby exerting an oblique force that can move sperm laterally. The ensued acoustophoretic force applied on the motile sperm can overcome the experienced drag force and, thus, enable the sperm to swim along the direction of the electrodes, across the channel width, to the collection outlet (Fig. 7A). Conversely, immotile and dead sperm or other cells and debris would be unaffected. The collected sperm showed more than 50% and 60% improvement in vitality and progressive motility, respectively. Moreover, DFI experienced an approximately 20% decline in the collected sample compared with the raw semen, which was deemed statistically significant based on the t-test result. The proposed device also boasted acceptable throughput as it could sort over 60,000 sperms in less than an hour, making it suitable for carrying out both ICSI and IVF procedures. In another study, high-frequency acoustic waves were utilized to boost the motility of the sperm sample (Gai et al. 2022). In this method, a polydimethylsiloxane (PDMS)-based microfluidic chamber coupled with a pair of IDTs was fabricated to generate SAW in the device (Fig. 7B). The \(128^{\circ }\) Y-cut, X-propagating lithium niobate was patterned the same as the previous study, and the microfluidic chamber was cast using a 3D-printed master mold. The mold was silanized to prevent the attachment of PDMS during the casting process. The sperm in the chamber were exposed to acoustic waves with varying frequencies and powers. The authors concluded that the acoustic waves with a frequency of 19.28 MHz and power of 2 W had the maximum effect on enhancing sperm motility parameters. The post-treatment analysis of the sample treated with 20 s of exposure demonstrated a staggering 34% increase in curvilinear velocity (VCL). The number of motile sperms also experienced an enhancement by 32% as the treatment translated previously immotile sperms to motile. The cause of the motility enhancement after exposure to acoustic waves is not fully understood, but the major contributing factors are believed to be the alteration in metabolic activity in the sperm. This leads to a surge in ATP production within the sperm cell, resulting in the improvement of sperm motility.
A Acoustofluidic sperm selection device that displaces and separates motile and morphologically normal sperm from other cells and debris. Acoustic forces effectively overcome the viscous drag to laterally transfer sperm across the width of the microchannel. Reproduced from Gai et al. (2020) with permission. B Sperm motility boost post-acoustic actuation. IDT were utilized to generate standing SAW within the chamber housing the sperm sample, as depicted schematically. Reproduced from Gai et al. (2022) with permission
Acoustofluidics have also shown interesting potential for rapid and portable analysis of semen samples. In the study conducted by Castro et al., an acoustically driven microfluidic extensional rheometry (ADMiER) was introduced to assess the concentration of motile sperm in a bull semen sample (Castro et al. 2023). In this method, a liquid bridge filament was formed by a microliter semen sample using standing SAW pulses to measure the time it takes for the filament to thin and break under elastocapillary stresses. Comparing against the computer-assisted sperm analysis (CASA), it was demonstrated that the required time for the semen sample to break is strongly correlated with the concentration of motile sperms. Their results indicated that ADMiER is capable of resolving the motile sperm concentration with an accuracy of 93.7%, thereby rendering it an effective alternative to CASA and hemocytometer for sperm quality analysis.
5.2 Electrical-based methods
Electrophoresis and dielectrophoresis (DEP) are two well-studied methods for the manipulation and separation of particles and cells in microfluidic devices. Electrophoresis is the preferential migration of charged particles in a spatially uniform electric field in a fluid. On the contrary, DEP uses a non-uniform electric field to exert force on dielectric particles. The dielectric particle becomes polarized in the electric field, and given field inhomogeneity, it can experience unequal attractive or repulsive forces, resulting in a motion along the direction of the overwhelming force. The contributing factors in determining the direction of migration in DEP are dielectric properties, size, composition, and in the case of cells, the membrane composition (Rahman et al. 2017; Wyatt Shields Iv et al. 2015).
By employing insulator-based DEP (iDEP) with cylindrical insulating structures and direct current (DC) electric potentials, Rosales et al. were able to concentrate sperm cells and distinguish between mature and spermatogenic cells based on their shapes and electric polarizations (Rosales-Cruzaley et al. 2013). The microfluidic device used in the study consists of a microchannel with cylindrical insulating structures. The DC electric potential, ranging from 200 to 1500 V, was applied to the insulating structures (Fig. 8A). The sperm cells were introduced into the microchannel, and given the resulting non-uniform electric field, the dielectrophoretic force acted on the cells. In the case of sperm cells, positive DEP occurs when the cells are more polarizable than the medium, causing them to be attracted to the regions with higher electric field gradients. Negative DEP occurs when the sperm are less polarizable than the medium, leading to repulsion from regions with higher electric field gradients. The differences in shape between mature and spermatogenic sperm give rise to different responses in polarization, thereby separating the two groups in the device.
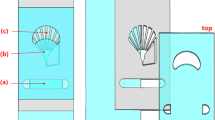
A Schematic representation of the microchannel used for trapping spermatogenic cells using insulator-based dielectrophoresis. The flow direction is left to right. Reproduced from Rosales-Cruzaley et al. (2013) with permission. B The microfluidic motile sperm counter based on the change in the measured electrical impedance, reproduced from Phiphattanaphiphop et al. (2019) with permission. C The microfluidic IVF-on-a-chip. a The PDMS microchannel and micro-structures are bonded with the ITO electrode on the actual device, which has dimensions of 5 cm × 3 cm. b The experiment involves trapping mouse oocytes and sperm on the electrodes for approximately 1 min. c The micro-structures are used for the co-incubation of oocytes and sperm for a duration of 1 h to facilitate natural insemination, reproduced from Huang et al. (2020) with permission. D The droplet-based dielectrophoretic microfluidic biochip for on-chip fertilization, reproduced from Huang et al. (2018) with permission
In another study, a microfluidic device utilizing DEP was proposed for the separation of morphologically normal sperm from the initial sample (De Wagenaar et al. 2016). As was mentioned earlier, the presence of ERC near the neck of the sperm is a common morphological anomaly that has been found to cause infertility in domestic animals. In this research, differential impedance measurement was performed on sperm passing through the examination area equipped with two electrode pairs. Using the impedance analysis, the researchers were able to identify the presence of this feature on sperm using the shape of the impedance peak. Furthermore, in order to increase the accuracy of measurement and reduce the noise associated with the sperm orientation, location, and velocity, the sperm first were focused in the middle of the channel using dielectrophoretic force. Upon the detection of sperm with the mentioned abnormality, a pair of dielectrophoretic electrodes were actuated, deflecting the abnormal sperm into the middle of the channel. The results demonstrated the feasibility of the proposed method for the separation of sperm with this type of morphological abnormality. Given the significance of motile sperm count in a sample, a microfluidic device was recently proposed for measuring this parameter (Phiphattanaphiphop et al. 2019). The proposed device was based on the previous device for the separation of motile sperm (Phiphattanaphiphop et al. 2020), where motile sperm were able to swim across the laminar flow, while immotile sperm were washed away by the fluid (Fig. 8B). The motile sperm then moved through a channel equipped with electrodes for measuring the impedance value. The microelectrodes, composed of Cr/Au, were 20 µ m wide with a 30 µ m distance, and the impedance between the electrodes was measured using an LCR meter. The passing sperm changed the value of the measured impedance, making it possible to count the number of motile sperm in real-time. The motile sperm with a velocity greater than 5 µm/s caused a noticeable change in the electrical impedance as they swam through the electrodes. The average electrical impedance of the sperms during the experiment was 110.54 k\(\Omega\). The method showed high reliability with a coefficient of determination (\(R^{2}\)) greater than 99.97%. Analyzing 10 µL of the sperm sample with the device and benchmarking the results against CASA demonstrated up to 95% accuracy in counting the concentration of motile sperm in the sample.
Huang et al. proposed a DEP-based device for carrying out the artificial insemination process on a chip (Huang et al. 2020). The electric field was generated using a set of ITO electrodes on the glass slide. In this device, the mice oocyte was fixed in place using dielectrophoretic force, and the incoming sperm were attracted to the oocyte using the same applied force. It resulted in increasing the concentration of sperm in the vicinity of the oocyte, reaching 1.5 × 106 sperm per milliliter. They concluded that the ensued increase in the concentration of nearby sperm led to an enhancement in the fertilization probability (Fig. 8C). Fertility and blastocyst rates were compared among three solutions; HTF, potassium simplex optimized medium-amino acid (KSOM-AA), and the DEP buffer solution. The fertilization rates in vitro were determined for each solution, showing no significant difference between the DEP buffer solution and the traditional culture medium. The rate of fertilization was found to be proportional to the sperm concentration in both the DEP microfluidic chip and the traditional IVF group. The development of embryos and potential harm to oocytes and sperm were assessed. The tracking of embryo development indicated no harm to oocytes and sperm after IVF in the low-conductivity medium. The blastocyst rates between the DEP microfluidic chip and traditional droplet-based IVF showed no significant difference. In another study, it was demonstrated that the sperm head and tail show different dielectrophoretic responses (Shuchat et al. 2019). Using a quadrupole electrode array, it was observed that the sperm tail experiences positive DEP, while the tail experiences negative DEP. This allows for the sperm head to distance itself from the damaging electric field. Their results proved the feasibility of utilizing DEP for safe, automated, and non-invasive sperm trapping and screening.
In a study conducted by Huang et al., researchers developed a microfluidic device for IVF-on-a-chip (Huang et al. 2018). In this study, positive DEP was used for trapping the sperm and oocytes at a specific region on the chip to promote the insemination process, as demonstrated by the previous work. Upon fertilization, the zygotes were encapsulated individually in a microdroplet emulsion and separated from unfertilized oocytes. The encapsulated zygotes were then transferred to the storage area in the device filled with the cultured medium. The results of this work demonstrated a 5% increase in the fertilization rate, and over 20% developed to the blastocyte stage, compared with the conventional IVF considering its far lower sperm–oocyte ratio (Fig. 8D).
5.3 Miscellaneous methods
In addition to the previously mentioned methods, there are also some other techniques that have proven effective in many other applications but have not been extensively studied in ART as of yet. Albeit sparse, these studies might have the potential to open up new horizons in the field of assisted reproduction. In a study conducted by Nakao et al., a commercially available microfluidic cell sorter was used to separate acrosome-reacted sperm in the sample (Nakao et al. 2020). In this device, the sperm were stained using fluorescein isothiocyanate (FITC)-labeled peanut agglutinin (PNA), which labels sperm acrosomes. The sperm sample was then sorted by the microfluidic chip based on the fluorescent signal intensity (Fig. 9A). The resulting data actuated a pulse air pressure to induce a fluid flow to deflect the passing sperm out of the main channel. The separation of the initial sample resulted in three distinct populations of sperm with varying percentages of acrosome-reacted sperms. The three populations were compared using established motility parameters, and it was found that sperm motility parameters among the three groups were not different. In the subsequent experiments, the three sorted populations were used for the fertilization of oocytes, and the results demonstrated that the population with enriched acrosome-reacted sperms had a considerably higher fertilization rate compared to others. Their results successfully indicated the potential of a microfluidic cell sorter based on sperm acrosome status for enhanced clinical outcomes.
A The microfluidic device for separation of the acrosome-reacted sperm. The acrosome-reacted sperm were sorted by a pulse of pressurized air. Reproduced from Nakao et al. (2020) with permission. B The annular laser traps sperm in a circular fashion. Reproduced from Shao et al. (2007) with permission
In another study, the authors utilized an annular laser trap to study sperm chemotactic behavior (Shao et al. 2005). The system utilized a CW Ytterbium fiber laser with a wavelength of 1070 nm, which was collimated to achieve a flat-top beam. The use of an axicon–lens pair generates an annular focus at the specimen plane, allowing for the trapping of sperm. An experiment conducted with 15 µm microbeads demonstrated the effectiveness of the annular laser ring as a trap. The subsequent experiments with dog sperm proved sperm were affected by the ring trap to some extent, but the high motility of the dog sperm, coupled with the low output power of their laser, hindered the full entrapment of sperm. In a subsequent study conducted by the same group, the proposed method was furthered for sorting and analyzing human sperm based on their motility and chemotactic behavior (Shao et al. 2007). They used the annular laser trap in conjunction with a chemo-attractant placed in the middle of the trap. They observed that sperm behave differently when exposed to the annular laser trap. Based on the observation, they concluded that sperm behavior could be categorized into two categories, one of which encompasses sperm that are barely affected by the trap and migrate towards the chemo-attractant and the other population whose motility is reduced or halted completely by the trap. By analyzing the sperm motility parameters, including VCL and average path velocity (VAP), it was deduced that the second group was comprised of significantly slower sperm compared to the first group. The results highlight the potential of optical-based methods for sperm separation and analysis (Fig. 9B). However, the effects of the laser trap on sperm vitality and the potential damage to sperm caused by irradiation-induced heating were not addressed in the article.
A summarized comparison of some of the devices presented thus far is presented in Table 1.
6 Conclusions and future prospects
Sperm morphological normality coupled with DNA integrity and motility plays an indispensable part in the fertilization process. Despite the efficacy of the current methods in assisted reproduction for enhancing the aforementioned qualities, these methods are still far from ideal, as they can induce damage to sperm cells. On account of the excellent biocompatibility, high throughput, non-invasive nature, and automation, microfluidics would be an appealing option for sperm selection and analysis in ART.
In this article, we reviewed recent technological advances in microfluidic devices for sperm sorting and screening, as well as new innovations for IVF-on-a-chip, with a focus on the sperm’s role in the fertilization process. The presented microfluidic chips can be categorized into two main groups: (1) the devices inspired by natural selection and guidance mechanisms utilized by FRT and (2) the devices based on non-natural external forces, such as acoustics. Microfluidic devices based on chemotaxis and thermotaxis can offer decent performance in terms of the motility and other important parameters of the selected sperm. However, the devices working based on chemotaxis require special reagents and careful design of the channel for creating a gradual gradient of the chemo-attractant, thereby hampering its integration into the current procedures. On the other end of the spectrum lies thermotaxis, which, in spite of its potent role as a stimulus, is only perceived by a minor fraction of the sperm. For that reason, it cannot be utilized as a stand-alone mechanism for the separation of the desired sperm. One technique to improve the prospect of thermotaxis sperm separation in clinical use can be its utilization concurrently with other stimuli and markers. For instance, given the difference in the surface electrical charge of mature and immature sperms (Simon et al. 2013), it presumably would be an option to use it alongside thermotaxis to augment the number of mature and viable sperm. Rheotaxis, on the other hand, is capable of rapid, label-free, and effective separation of motile sperm with considerable improvement in DNA integrity. It solely relies on a fluid flow to operate, basically removing any need for specific reagents and instruments. In addition to these mechanisms, there have also been some attempts to take advantage of the thigmotaxis behavior of sperm for the separation of superior populations. Some of the proposed devices mentioned earlier demonstrated impressive results in terms of DNA integrity and motility. They also benefit from easy procedures, making their incorporation into ART much more conceivable. The use of external forces has also caught on in recent years and may change the landscape of the current ART procedures. Nonetheless, their performance in terms of the quality of the sorted sperm pales in comparison with that of the naturally-inspired mechanisms.
In addition to the devices aimed at sperm sorting, some of the research demonstrated the potential of IVF-on-a-chip, integrating various stages of the ART procedures into a single chip. It can significantly save resources, offering patients lower treatment costs per cycle, while at the same time, elevating the likelihood of the fertilization rate.
Beyond the current focus on making microfluidic devices as efficient as possible in ART procedures, scaling up the proposed prototypes for real-life applications is yet another challenge that the future research has to overcome. The current universally used method for the fabrication of microfluidic devices in small-scale and academic research is soft lithography. In spite of its inexpensive nature, it relies on several manual processing steps that open the door to device-to-device variability and inconsistency, which, in turn, hinders the upscaling of its production (Lee et al. 2018). On the contrary, injection molding is at the forefront of high-throughput and large-scale device manufacturing, maintaining a minimized per-device cost. The preferred choice for casting material in this process is invariably thermoplastics, e.g., polymethyl methacrylate (PMMA), polycarbonate (PC), and polystyrene (PS), due to their low cost, durability, decent biocompatibility, and optical transparency. Moreover, the master molds in this process are typically fabricated by the high-precision milling of steel, making it precise and durable throughout hundreds of thousands of castings (Lee et al. 2018; Scott and Ali 2021). If microfluidic-enabled ART is to supersede the current paradigm, injection molding will probably be the appealing option for mass production of the devices.
The choice of the microfluidic chip materials is rather undervalued, and only a few studies examined the effects it can have on the behavior of sperm inside a microchannel. In one study, zebrafish sperm were examined in PDMS and glass microfluidic devices, and it was found that they were prone to adherence to the sidewalls (Beckham et al. 2018). The microchannels were subsequently treated with polyethylene glycol (PEG) and sperm adherence was shown to drop significantly. PEG decreases surface hydrophobicity, and the drop in the number of adhered sperm implies a probable underlying interaction between the sperm and the hydrophobic surface. In another study, the VCL of boar sperm was analyzed on glass slides coated with PS and PMMA. Sperm on the PS-coated glass had a significantly lower VCL compared to sperm on the PMMA-coated slide, which was attributed to the stronger hydrophobicity of the PS. These all highlight the need for a more thorough evaluation of the commonly used materials in microfluidic device fabrication and their potential impact on sperm behavior (Mears et al. 2014).
Apart from the well-established methods discussed hitherto, microfluidic sperm sorting and analysis can also benefit from emerging technologies such as artificial intelligence (AI). Machine learning algorithms in particular have the potential to bring about disruptive breakthroughs in the field and elevate the accuracy and robustness of sperm analysis in ART. As per recent studies, these algorithms have shown great capability to predict sperm with high DNA integrity, normal morphology, and better motility, rendering AI an invaluable tool for sperm sample analysis (You et al. 2021; McCallum et al. 2019; Hicks et al. 2019; Cherouveim et al. 2023). Unlike microfluidics, which possess remarkable dexterity in physically manipulating human gametes, AI lacks this quality. Microfluidics and AI can complement each other, providing tools of the future for sperm sorting and evaluation. Optimization and precise control over the chemo-attractant and temperature gradient or the fluid flow are only a few merits that AI-enabled devices may offer, likely shrinking the human intervention even further, thereby enhancing the performance and reliability of the devices. Pairing AI with sorting methods, such as the impedance analysis discussed earlier, may also prove effective as these algorithms can perceive even the finest details that conventional methodology cannot. These are only some ways to materialize the concept of AI-assisted microfluidics in assisted reproduction, and other possibilities might be unraveled by the future research.
Data availability
No datasets were generated or analysed during the current study.
References
M. Aboulghar, D.T. Baird, J. Collins, J.L.H. Evers, B.C.J.M. Fauser, C.B. Lambalk, E. Somigliana, A. Sunde, B. Tarlatzis, P.G. Crosignani, P. Devroey, E. Diczfalusy, K. Diedrich, L. Fraser, J.P.M. Geraedts, L. Gianaroli, A. Glasier, A. Van Steirteghem, Intrauterine insemination. Hum. Reprod. Update 15(3), 265–277 (2009). https://doi.org/10.1093/humupd/dmp003
A. Agarwal, R.A. Saleh, M.A. Bedaiwy, Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 79(4), 829–843 (2003). https://doi.org/10.1016/S0015-0282(02)04948-8
A. Agarwal, S. Baskaran, N. Parekh, C.L. Cho, R. Henkel, S. Vij, M. Arafa, M.K. Panner Selvam, R. Shah, Male infertility. The Lancet 397(10271), 319–333 (2021). https://doi.org/10.1016/S0140-6736(20)32667-2
B. Alberts, A. Johnson, J, Lewis, D. Morgan, M. Raff, K, Roberts, P. Walter, Molecular Biology of the Cell vol. 30, 4th edn., p. 100. W.W. Norton & Company, New York (2017). https://doi.org/10.1201/9781315735368
A.B. Alias, H.-Y. Huang, D.-J. Yao, A review on microfluidics: an aid to assisted reproductive technology. Molecules (Basel, Switzerland) 26(14), 4354 (2021). https://doi.org/10.3390/molecules26144354
A. Ataei, A.W.C. Lau, W. Asghar, A microfluidic sperm-sorting device based on rheotaxis effect. Microfluid. Nanofluid. 25(6), 52 (2021). https://doi.org/10.1007/s10404-021-02453-8
J. Autebert, B. Coudert, F.C. Bidard, J.Y. Pierga, S. Descroix, L. Malaquin, J.L. Viovy, Microfluidic: An innovative tool for efficient cell sorting. Methods 57(3), 297–307 (2012). https://doi.org/10.1016/j.ymeth.2012.07.002
J.M. Ayuso, M. Virumbrales-Muñoz, J.M. Lang, D.J. Beebe, A role for microfluidic systems in precision medicine. Nat. Commun. 13(1), 3086 (2022). https://doi.org/10.1038/s41467-022-30384-7
A. Bahat, M. Eisenbach, Sperm thermotaxis. Mol. Cell. Endocrinol. 252(1–2), 115–119 (2006). https://doi.org/10.1016/j.mce.2006.03.027
A. Bahat, S.R. Caplan, M. Eisenbach, Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PLoS ONE 7(7), 41915 (2012). https://doi.org/10.1371/journal.pone.0041915
J. Beckham, F. Alam, V. Omojola, T. Scherr, A. Guitreau, A. Melvin, D.S. Park, J.-W. Choi, T.R. Tiersch, W. Todd Monroe, A microfluidic device for motility and osmolality analysis of zebrafish sperm. Biomed. Microdevice 20(3), 67 (2018). https://doi.org/10.1007/s10544-018-0308-2
J.T.W. Berendsen, S.A. Kruit, N. Atak, E. Willink, L.I. Segerink, Flow-free microfluidic device for quantifying chemotaxis in spermatozoa. Anal. Chem. 92(4), 3302–3306 (2020). https://doi.org/10.1021/acs.analchem.9b05183
R.P. Bertolla, Sperm biology and male reproductive health. Sci. Rep. 10(1), 2–4 (2020). https://doi.org/10.1038/s41598-020-78861-7
E. Borges, B.F. Zanetti, A.S. Setti, D.P.D.A.F. Braga, R.R. Provenza, A. Iaconelli, Sperm DNA fragmentation is correlated with poor embryo development, lower implantation rate, and higher miscarriage rate in reproductive cycles of non-male factor infertility. Fertil. Steril. 112(3), 483–490 (2019). https://doi.org/10.1016/j.fertnstert.2019.04.029
M. Bouloorchi Tabalvandani, S. Javadizadeh, M. Badieirostami, Bio-inspired progressive motile sperm separation using joint rheotaxis and boundary-following behavior. Lab Chip 24(6), 1636–1647 (2024). https://doi.org/10.1039/D3LC00893B
S.A. Carson, A.N. Kallen, Diagnosis and management of infertility: a review. JAMA - Journal of the American Medical Association 326(1), 65–76 (2021). https://doi.org/10.1001/jama.2021.4788
J.O. Castro, M.S. Abdul Halim, L.A. Ambattu, A.R. Rezk, R. Prabhakar, R. Nosrati, L.Y. Yeo, Acoustofluidic semen analysis for veterinary male bovine infertility assessment. Flow 3, 6 (2023). https://doi.org/10.1017/flo.2022.30
H. Chang, B.J. Kim, Y.S. Kim, S.S. Suarez, M. Wu, Different migration patterns of sea urchin and mouse sperm revealed by a microfluidic chemotaxis device. PLoS ONE 8(4), 1–8 (2013). https://doi.org/10.1371/journal.pone.0060587
P. Cherouveim, C. Velmahos, C.L. Bormann, Artificial intelligence for sperm selection-a systematic review. Fertil. Steril. 120(1), 24–31 (2023). https://doi.org/10.1016/j.fertnstert.2023.05.157
T. Chinnasamy, J.L. Kingsley, F. Inci, P.J. Turek, M.P. Rosen, B. Behr, E. Tüzel, U. Demirci, Guidance and self-sorting of active swimmers: 3D periodic arrays increase persistence length of human sperm selecting for the fittest. Adv. Sci. 5(2), 1700531 (2018). https://doi.org/10.1002/advs.201700531
M. Conrad, Forbes Health, How much does IVF cost? https://www.forbes.com/health/womens-health/how-much-does-ivf-cost. Accessed 15 Nov 2023
T.G. Cooper, Cytoplasmic droplets: the good, the bad or just confusing? Hum. Reprod. 20(1), 9–11 (2005). https://doi.org/10.1093/humrep/deh555
S. Damiati, U.B. Kompella, S.A. Damiati, R. Kodzius, Microfluidic devices for drug delivery systems and drug screening. Genes 9(2), 103 (2018). https://doi.org/10.3390/genes9020103
B. De Wagenaar, S. Dekker, H.L. De Boer, J.G. Bomer, W. Olthuis, A. Van Den Berg, L.I. Segerink, Towards microfluidic sperm refinement: Impedance-based analysis and sorting of sperm cells. Lab Chip 16(8), 1514–1522 (2016). https://doi.org/10.1039/c6lc00256k
P. Denissenko, V. Kantsler, D.J. Smith, J. Kirkman-Brown, Human spermatozoa migration in microchannels reveals boundary-following navigation. Proc. Natl. Acad. Sci. U.S.A. 109(21), 8007–8010 (2012). https://doi.org/10.1073/pnas.1202934109
J. Diemer, J. Hahn, B. Goldenbogen, K. Müller, E. Klipp, Sperm migration in the genital tract-In silico experiments identify key factors for reproductive success. PLoS Comput. Biol. 17(7), 1–17 (2021). https://doi.org/10.1371/journal.pcbi.1009109
M.R. Doostabadi, E. Mangoli, L.D. Marvast, F. Dehghanpour, B. Maleki, H. Torkashvand, A.R. Talebi, Microfluidic devices employing chemo- and thermotaxis for sperm selection can improve sperm parameters and function in patients with high DNA fragmentation. Andrologia 54(11), 1–15 (2022). https://doi.org/10.1111/and.14623
L. Eamer, R. Nosrati, M. Vollmer, A. Zini, D. Sinton, Microfluidic assessment of swimming media for motility-based sperm selection. Biomicrofluidics 9(4) (2015). https://doi.org/10.1063/1.4928129
L. Eamer, M. Vollmer, R. Nosrati, M.C. San Gabriel, K. Zeidan, A. Zini, D. Sinton, Turning the corner in fertility: High DNA integrity of boundary-following sperm. Lab Chip 16(13), 2418–2422 (2016). https://doi.org/10.1039/c6lc00490c
M. Eisenbach, L.C. Giojalas, Sperm guidance in mammals - An unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 7(4), 276–285 (2006). https://doi.org/10.1038/nrm1893
M.A. Fischer, J. Willis, A. Zini, Human sperm DNA integrity: correlation with sperm cytoplasmic droplets. Urology 61(1), 207–211 (2003). https://doi.org/10.1016/S0090-4295(02)02098-8
B.M. Friedrich, F. Jülicher, Chemotaxis of sperm cells. Proc. Natl. Acad. Sci. U.S.A. 104(33), 13256–13261 (2007). https://doi.org/10.1073/pnas.0703530104
M.G. Funaro, H.H. Kim, S. Mazel, A. Bolyakov, M. Goldstein, P.N. Schlegel, D.A. Paduch, A novel sorting technology allows for highly efficient selection of sperm without chromatin damage. Syst. Biol. Reprod. Med. 59(3), 172–177 (2013). https://doi.org/10.3109/19396368.2013.777135
J. Gai, R. Nosrati, A. Neild, High DNA integrity sperm selection using surface acoustic waves. Lab Chip 20(22), 4262–4272 (2020). https://doi.org/10.1039/d0lc00457j
J. Gai, E. Dervisevic, C. Devendran, V.J. Cadarso, M.K. O’Bryan, R. Nosrati, A. Neild, High-frequency ultrasound boosts bull and human sperm motility. Adv. Sci. 9(11), 2104362 (2022). https://doi.org/10.1002/advs.202104362
Y. Gao, M. Wu, Y. Lin, J. Xu, Acoustic microfluidic separation techniques and bioapplications: A review. Micromachines 11(10), 1–21 (2020). https://doi.org/10.3390/mi11100921
A. Gergely, E. Kovanci, L. Senturk, E. Cosmi, L. Vigue, G. Huszar, Morphometric assessment of mature and diminished-maturity human spermatozoa: sperm regions that reflect differences in maturity*. Hum. Reprod. 14(8), 2007–2014 (1999). https://doi.org/10.1093/humrep/14.8.2007
C. Gnoth, B. Maxrath, T. Skonieczny, K. Friol, E. Godehardt, J. Tigges, Final ART success rates: a 10 years survey. Hum. Reprod. (Oxford, England) 26(8), 2239–46 (2011). https://doi.org/10.1093/humrep/der178
F. Gode, T. Bodur, F. Gunturkun, A.S. Gurbuz, B. Tamer, I. Pala, A.Z. Isik, Comparison of microfluid sperm sorting chip and density gradient methods for use in intrauterine insemination cycles. Fertil. Steril. 112(5), 842–8481 (2019). https://doi.org/10.1016/j.fertnstert.2019.06.037
E. Gomez, D.W. Buckingham, J. Brindle, F. Lanzafame, D.S. Irvine, R.J. Aitken, Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J. Androl. 17(3), 276–87 (1996)
M.T. Guo, A. Rotem, J.A. Heyman, D.A. Weitz, Droplet microfluidics for high-throughput biological assays. Lab Chip 12(12), 2146–2155 (2012). https://doi.org/10.1039/c2lc21147e
R.R. Henkel, W.B. Schill, Sperm preparation for ART. Reprod. Biol. Endocrinol. 1, 108 (2003). https://doi.org/10.1186/1477-7827-1-108
A. Heydari, M. Zabetian Targhi, I. Halvaei, R. Nosrati, A novel microfluidic device with parallel channels for sperm separation using spermatozoa intrinsic behaviors. Sci. Rep. 13(1), 1185 (2023). https://doi.org/10.1038/s41598-023-28315-7
S.A. Hicks, J.M. Andersen, O. Witczak, V. Thambawita, P. Halvorsen, H.L. Hammer, T.B. Haugen, M.A. Riegler, Machine learning-based analysis of sperm videos and participant data for male fertility prediction. Sci. Rep. 9(1), 16770 (2019). https://doi.org/10.1038/s41598-019-53217-y
W.V. Holt, A. Fazeli, Sperm transport and selection in mammals vol. 2016, pp. 1–7. Elsevier Inc., (2018). https://doi.org/10.1016/b978-0-12-801238-3.64463-x
H.Y. Huang, Y.L. Lai, D.J. Yao, Dielectrophoretic microfluidic device for in vitro fertilization. Micromachines 9(3) (2018). https://doi.org/10.3390/mi9030135
H.Y. Huang, P.W. Huang, D.J. Yao, Enhanced efficiency of sorting sperm motility utilizing a microfluidic chip. Microsyst. Technol. 23(2), 305–312 (2017). https://doi.org/10.1007/s00542-015-2495-6
H.Y. Huang, C.Y. Lu, I.W. Wang, D.J. Yao, Motility-driven sperm-sorting microfluidic chip with little cell damage for oligozoospermia patients. Sens. Mater. 32(8), 2585–2596 (2020). https://doi.org/10.18494/SAM.2020.2643
H.Y. Huang, W.L. Kao, Y.W. Wang, D.J. Yao, Using a dielectrophoretic microfluidic biochip enhanced fertilization of mouse embryo in vitro. Micromachines 11(8), 1–11 (2020). https://doi.org/10.3390/MI11080714
C.H. Huang, C.H. Chen, T.K. Huang, F. Lu, J.Y. Jen Huang, B.R. Li, Design of a gradient-rheotaxis microfluidic chip for sorting of high-quality Sperm with progressive motility. iScience 26(8), 107356 (2023). https://doi.org/10.1016/j.isci.2023.107356
G. Huszar, L. Vigue, Incomplete development of human spermatozoa is associated with increased creatine phosphokinase concentration and abnormal head morphology. Mol. Reprod. Dev. 34(3), 292–298 (1993). https://doi.org/10.1002/mrd.1080340309
K. Ishimoto, E.A. Gaffney, Fluid flow and sperm guidance: A simulation study of hydrodynamic sperm rheotaxis. J. R. Soc. Interface 12(106), 20150172 (2015). https://doi.org/10.1098/rsif.2015.0172
H. Kang, T. An, D. Lee, B. Kim, Gravity and rheotaxis based sperm sorting device employing a cam-actuated pipette mechanism. Rev. Sci. Instrum. 90(8), 084101 (2019). https://doi.org/10.1063/1.5096793
N. Kashaninejad, M.J.A. Shiddiky, N. Nguyen, Advances in microfluidics-based assisted reproductive technology: from sperm sorter to reproductive system-on-a-chip. Adv. Biosyst. 2(3), 1700197 (2018). https://doi.org/10.1002/adbi.201700197
J. Keating, C.E. Grundy, P.S. Fivey, M. Elliott, J. Robinson, Investigation of the association between the presence of cytoplasmic residues on the human sperm midpiece and defective sperm function. Reproduction 110(1), 71–77 (1997). https://doi.org/10.1530/jrf.0.1100071
Y.J. Ko, J.H. Maeng, B.C. Lee, S. Lee, S.Y. Hwang, Y. Ahn, Separation of progressive motile sperm from mouse semen using on-chip chemotaxis. Anal. Sci. 28(1), 27–32 (2012). https://doi.org/10.2116/analsci.28.27
Y.J. Ko, J.H. Maeng, S.Y. Hwang, Y. Ahn, Design, fabrication, and testing of a microfluidic device for thermotaxis and chemotaxis assays of sperm. SLAS Technol. 23(6), 507–515 (2018). https://doi.org/10.1177/2472630318783948
O.M. Kocur, P. Xie, S. Cheung, S. Souness, M. McKnight, Z. Rosenwaks, G.D. Palermo, Can a sperm selection technique improve embryo ploidy? Andrology 11(8), 1605–1612 (2023). https://doi.org/10.1111/andr.13362
N. Kumar, A.K. Singh, The anatomy, movement, and functions of human sperm tail: An evolving mystery. Biol. Reprod. 104(3), 508–520 (2021). https://doi.org/10.1093/biolre/ioaa213
U.N. Lee, X. Su, D.J. Guckenberger, A.M. Dostie, T. Zhang, E. Berthier, A.B. Theberge, Fundamentals of rapid injection molding for microfluidic cell-based assays. Lab Chip 18(3), 496–504 (2018). https://doi.org/10.1039/C7LC01052D
M. Lee, J.W. Park, D. Kim, H. Kwon, M.J. Cho, E.J. Lee, T.E. Shin, D.K. Kim, S. Lee, D.G. Byeun, J.J. Ko, J.H. Lee, J.K. Choi, Viscous cervical environment-on-a-chip for selecting high-quality sperm from human semen. Biomedicines 9(10), 1439 (2021). https://doi.org/10.3390/biomedicines9101439
C.A. Leisinger, G. Adaniya, M.R. Freeman, E.J. Behnke, M. Aguirre, M.D. VerMilyea, M.C. Schiewe, Effect of microfluidic sperm separation vs. standard sperm washing processes on laboratory outcomes and clinical pregnancy rates in an unselected patient population. Reprod. Med. 2(3), 125–130 (2021). https://doi.org/10.3390/reprodmed2030013
S. Lepine, S. McDowell, L.M. Searle, B. Kroon, D. Glujovsky, A. Yazdani, Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst. Rev. 7(7), 010461 (2019). https://doi.org/10.1002/14651858.CD010461.pub3
E.T.Y. Leung, C.L. Lee, X. Tian, K.K.W. Lam, R.H.W. Li, E.H.Y. Ng, W.S.B. Yeung, P.C.N. Chiu, Simulating nature in sperm selection for assisted reproduction. Nat. Rev. Urol. 19(1), 16–36 (2022). https://doi.org/10.1038/s41585-021-00530-9
Z. Li, W. Liu, T. Qiu, L. Xie, W. Chen, R. Liu, Y. Lu, K. Mitchelson, J. Wang, J. Qiao, J. Cheng, The construction of an interfacial valve-based microfluidic chip for thermotaxis evaluation of human sperm. Biomicrofluidics 8(2), 024102 (2014). https://doi.org/10.1063/1.4866851
K. Li, R. Li, Y. Ni, P. Sun, Y. Liu, D. Zhang, H. Huang, Novel distance-progesterone-combined selection approach improves human sperm quality. J. Transl. Med. 16(1), 1–10 (2018). https://doi.org/10.1186/s12967-018-1575-7
R. Lottero-Leconte, C.A. Isidro Alonso, L. Castellano, S.P. Martinez, Mechanisms of the sperm guidance, an essential aid for meeting the oocyte. Transl. Cancer Res. 6(S2), 427–430 (2017). https://doi.org/10.21037/tcr.2017.03.68
Z. Lu, S. Wang, Z. Sun, R. Niu, J. Wang, In vivo influence of sodium fluoride on sperm chemotaxis in male mice. Arch. Toxicol. 88(2), 533–539 (2014). https://doi.org/10.1007/s00204-013-1099-0
N.S. Mane, D.B. Puri, S. Mane, V. Hemadri, A. Banerjee, S. Tripathi, Separation of motile human sperms in a T-shaped sealed microchannel (2022). https://doi.org/10.1007/s13534-022-00229-9
C. McCallum, J. Riordon, Y. Wang, T. Kong, J.B. You, S. Sanner, A. Lagunov, T.G. Hannam, K. Jarvi, D. Sinton, Deep learning-based selection of human sperm with high DNA integrity. Commun. Biol. 2(1), 250 (2019). https://doi.org/10.1038/s42003-019-0491-6
M. Mears, T.M. Kennelly, J.R. Howse, D.S. Tarmey, M. Geoghegan, A.A. Pacey, Reduced curvilinear velocity of boar sperm on substrates with increased hydrophobicity. Theriogenology 81(5), 764–769 (2014). https://doi.org/10.1016/j.theriogenology.2013.12.014
D.J. Miller, Review: The epic journey of sperm through the female reproductive tract. Animal 12(s1), 110–120 (2018). https://doi.org/10.1017/S1751731118000526
N. Morishita, M. Miura, Y. Kobayashi, R. Matsunaga, T. Maeda, M. Ochi, T. Horiuchi, P-039 Male age is associated with sperm DNA integrity: Selection of high DNA integrity sperm by microfluidics sorting is critical to clinical outcomes in older patients. Hum. Reprod. 37(Supplement 1), 107–036 (2022). https://doi.org/10.1093/humrep/deac107.036
D. Mortimer, The functional anatomy of the human spermatozoon: relating ultrastructure and function. Mol. Hum. Reprod. 24(12), 567–592 (2018). https://doi.org/10.1093/molehr/gay040
S. Nakao, T. Takeo, H. Watanabe, G. Kondoh, N. Nakagata, Successful selection of mouse sperm with high viability and fertility using microfluidics chip cell sorter. Sci. Rep. 10(1), 8862 (2020). https://doi.org/10.1038/s41598-020-65931-z
R. Ní Dhuifin, D.K. Griffin, T. Moodley, The efficacy of hyaluronic acid binding (HAB) in the treatment of male infertility: a systematic review of the literature. DNA 2(3), 149–171 (2022). https://doi.org/10.3390/dna2030011
R. Nosrati, M. Vollmer, L. Eamer, M.C. San Gabriel, K. Zeidan, A. Zini, D. Sinton, Rapid selection of sperm with high DNA integrity. Lab Chip 14(6), 1142–1150 (2014). https://doi.org/10.1039/c3lc51254a
R. Nosrati, P.J. Graham, B. Zhang, J. Riordon, A. Lagunov, T.G. Hannam, C. Escobedo, K. Jarvi, D. Sinton, Microfluidics for sperm analysis and selection. Nat. Rev. Urol. 14(12), 707–730 (2017). https://doi.org/10.1038/nrurol.2017.175
S. Oehninger, T.F. Kruger, Sperm morphology and its disorders in the context of infertility vol. 2, pp. 75–92. American Society for Reproductive Medicine (2021). https://doi.org/10.1016/j.xfnr.2020.09.002
W. Ombelet, Global access to infertility care in developing countries: A case of human rights, equity and social justice. Hum. Reprod. 3(4), 257–266 (1988)
W. Ombelet, J. Onofre, IVF in Africa: what is it all about? Facts, Views & Vision in ObGyn 11(1), 65–76 (2019)
M.F. Orsolini, S.A. Meyers, P. Dini, An update on semen physiology, technologies, and selection techniques for the advancement of in vitro equine embryo production: Section i. Animals 11(11) (2021). https://doi.org/10.3390/ani11113248
I. Oseguera-López, S. Ruiz-Díaz, P. Ramos-Ibeas, S. Pérez-Cerezales, Novel techniques of sperm selection for improving IVF and ICSI outcomes. Front. Cell Dev. Biol. 7(November), 298 (2019). https://doi.org/10.3389/fcell.2019.00298
A. Pacheco, A. Blanco, F. Bronet, M. Cruz, J. García-Fernández, J.A. García-Velasco, Magnetic-activated cell sorting (Macs): A useful sperm-selection technique in cases of high levels of sperm dna fragmentation. J. Clin. Med. 9(12), 3976 (2020). https://doi.org/10.3390/jcm9123976
S. Pérez-Cerezales, S. Boryshpolets, M. Eisenbach, Behavioral mechanisms of mammalian sperm guidance. Asian J. Androl. 17(4), 628–632 (2015). https://doi.org/10.4103/1008-682X.154308
S. Pérez-Cerezales, R. Laguna-Barraza, A.C. De Castro, M.J. Sánchez-Calabuig, E. Cano-Oliva, F.J. De Castro-Pita, L. Montoro-Buils, E. Pericuesta, R. Fernández-González, A. Gutiérrez-Adán, Sperm selection by thermotaxis improves ICSI outcome in mice. Sci. Rep. 8(1), 2902 (2018). https://doi.org/10.1038/s41598-018-21335-8
C. Phiphattanaphiphop, K. Leksakul, R. Phatthanakun, A. Suthummapiwat, Real-time single cell monitoring: Measurement and counting of motile sperm using LCR impedance- integrated microfluidic device. Micromachines 10(10), 647 (2019). https://doi.org/10.3390/mi10100647
C. Phiphattanaphiphop, K. Leksakul, R. Phatthanakun, T. Khamlor, A novel microfluidic chip-based sperm-sorting device constructed using design of experiment method. Sci. Rep. 10(1), 17143 (2020). https://doi.org/10.1038/s41598-020-73841-3
J. Pihl, J. Sinclair, M. Karlsson, O. Orwar, Microfluidics for cell-based assays. Mater. Today 8(12), 46–51 (2005). https://doi.org/10.1016/S1369-7021(05)71224-4
N.A. Rahman, F. Ibrahim, B. Yafouz, Dielectrophoresis for biomedical sciences applications: A review. Sensors (Switzerland) 17(3), 449 (2017). https://doi.org/10.3390/s17030449
K. Rappa, J. Samargia, M. Sher, J.S. Pino, H.F. Rodriguez, W. Asghar, Quantitative analysis of sperm rheotaxis using a microfluidic device. Microfluid. Nanofluid. 22, 100 (2018). https://doi.org/10.1007/s10404-018-2117-6
A.K. Rengan, A. Agarwal, M. Linde, S.S. Plessis, An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod. Biol. Endocrinol. 10(1), 92 (2012). https://doi.org/10.1186/1477-7827-10-92
J. Riordon, F. Tarlan, J.B. You, B. Zhang, P.J. Graham, T. Kong, Y. Wang, A. Lagunov, T. Hannam, K. Jarvi, D. Sinton, Two-dimensional planar swimming selects for high DNA integrity sperm. Lab Chip 19(13), 2161–2167 (2019). https://doi.org/10.1039/c9lc00209j
J.E. Rodríguez-Gil, Photostimulation and thermotaxis of sperm: Overview and practical implications in porcine reproduction. Theriogenology 137, 8–14 (2019). https://doi.org/10.1016/j.theriogenology.2019.05.031
J. Romero-Aguirregomezcorta, E. Sugrue, L. Martínez-Fresneda, D. Newport, S. Fair, Hyperactivated stallion spermatozoa fail to exhibit a rheotaxis-like behaviour, unlike other species. Sci. Rep. 8(1), 16897 (2018). https://doi.org/10.1038/s41598-018-34973-9
E. Rosales-Cruzaley, P.A. Cota-Elizondo, D. Sánchez, B.H. Lapizco-Encinas, Sperm cells manipulation employing dielectrophoresis. Bioprocess Biosyst. Eng. 36(10), 1353–1362 (2013). https://doi.org/10.1007/s00449-012-0838-6
R. Samuel, H. Feng, A. Jafek, D. Despain, T. Jenkins, B. Gale, Microfluidic-based sperm sorting & analysis for treatment of male infertility. Translational Andrology and Urology 7(Suppl 3), 336–347 (2018). https://doi.org/10.21037/tau.2018.05.08
I.R. Sarbandi, A. Lesani, M. Moghimi Zand, R. Nosrati, Rheotaxis-based sperm separation using a biomimicry microfluidic device. Sci. Rep. 11(1), 18327 (2021). https://doi.org/10.1038/s41598-021-97602-y
S. Scott, Z. Ali, Fabrication methods for microfluidic devices: an overview. Micromachines 12(3), 319 (2021). https://doi.org/10.3390/mi12030319
B. Shao, J.M. Vinson, E.L. Botvinick, S.C. Esener, M.W. Berns, Axicon-based annular laser trap for studies on sperm activity. Optical Trapping and Optical Micromanipulation II 5930, 59300 (2005). https://doi.org/10.1117/12.613116
B. Shao, L.Z. Shi, J.M. Nascimento, E.L. Botvinick, M. Ozkan, M.W. Berns, S.C. Esener, High-throughput sorting and analysis of human sperm with a ring-shaped laser trap. Biomed. Microdevice 9(3), 361–369 (2007). https://doi.org/10.1007/s10544-006-9041-3
K. Shirota, F. Yotsumoto, H. Itoh, H. Obama, N. Hidaka, K. Nakajima, S. Miyamoto, Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil. Steril. 105(2), 315–3211 (2016). https://doi.org/10.1016/j.fertnstert.2015.10.023
S. Shuchat, S. Park, S. Kol, G. Yossifon, Distinct and independent dielectrophoretic behavior of the head and tail of sperm and its potential for the safe sorting and isolation of rare spermatozoa. Electrophoresis 40(11), 1606–1614 (2019). https://doi.org/10.1002/elps.201800437
L. Simon, S.Q. Ge, D.T. Carrell, Sperm selection based on electrostatic charge. Methods Mol. Biol. 927, 269–278 (2013). https://doi.org/10.1007/978-1-62703-38-0_25
M. Stimpfel, I. Verdenik, B. Zorn, I. Virant-Klun, Magnetic-activated cell sorting of non-apoptotic spermatozoa improves the quality of embryos according to female age: a prospective sibling oocyte study. J. Assist. Reprod. Genet. 35(9), 1665–1674 (2018). https://doi.org/10.1007/s10815-018-1242-1
S.S. Suarez, A.A. Pacey, Sperm transport in the female reproductive tract. Hum. Reprod. Update 12(1), 23–37 (2006). https://doi.org/10.1093/humupd/dmi047
Y. Sun, P. Sethu, Microfluidic adaptation of density-gradient centrifugation for isolation of particles and cells. Bioengineering 4(3), 67 (2017). https://doi.org/10.3390/bioengineering4030067
G.A. Thouas, D.K. Gardner, Laboratory techniques in IVF. In: Kovacs, G. (ed.) The Subfertility Handbook: A Clinician’s Guide, Second Edition, 2nd edn., pp. 193–210. Cambridge University Press, Melbourne. Chap. 18 (2010). https://doi.org/10.1017/CBO9780511919244.019
F.B. Tian, L. Wang, Numerical modeling of sperm swimming. Fluids 6(2) (2021). https://doi.org/10.3390/FLUIDS6020073
G. Transport, In: Reference Module in Biomedical Sciences. Elsevier (2014). https://doi.org/10.1016/B978-0-12-801238-3.05427-1
C.K. Tung, F. Ardon, A.G. Fiore, S.S. Suarez, M. Wu, Cooperative roles of biological flow and surface topography in guiding sperm migration revealed by a microfluidic model. Lab Chip 14(7), 1348–1356 (2014). https://doi.org/10.1039/c3lc51297e
C.-K. Tung, L. Hu, A.G. Fiore, F. Ardon, D.G. Hickman, R.O. Gilbert, S.S. Suarez, M. Wu, Microgrooves and fluid flows provide preferential passageways for sperm over pathogen Tritrichomonas foetus. Proc. Natl. Acad. Sci. 112(17), 5431–5436 (2015). https://doi.org/10.1073/pnas.1500541112
S.A. Vasilescu, L. Ding, F.Y. Parast, R. Nosrati, M.E. Warkiani, Sperm quality metrics were improved by a biomimetic microfluidic selection platform compared to swim-up methods. Microsyst. Nanoeng. 9(1), 37 (2023). https://doi.org/10.1038/s41378-023-00501-7
J. Wang, M. Sauer, In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Ther. Clin. Risk Manag. 2(4), 355–364 (2006)
L. Weng, IVF-on-a-chip: recent advances in microfluidics technology for in vitro fertilization. SLAS Technology 24(4), 373–385 (2019). https://doi.org/10.1177/2472630319851765
WHO, Infertility Prevalence Estimates 1990-2021, pp. 1–98 (2023). https://www.who.int/publications/i/item/978920068315
T. Wu, Y. Wu, J. Yan, J. Zhang, S. Wang, Microfluidic chip as a promising evaluation method in assisted reproduction: A systematic review. Bioeng. Trans. Med. 9(2), 10625 (2024). https://doi.org/10.1002/btm2.10625
C. Wyatt Shields Iv, C.D. Reyes, G.P. López, Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 15(5), 1230–1249 (2015). https://doi.org/10.1039/c4lc01246a
W. Xiao, M. Yu, Y. Yuan, X. Liu, Y. Chen, Thermotaxis of mammalian sperm. Mol. Hum. Reprod. 28(8), 027 (2022). https://doi.org/10.1093/molehr/gaac027
M. Yaghoobi, M. Azizi, A. Mokhtare, A. Abbaspourrad, Progressive bovine sperm separation using parallelized microchamber-based microfluidics. Lab Chip 21(14), 2791–2804 (2021). https://doi.org/10.1039/d1lc00091h
Y. Yan, H. Liu, B. Zhang, R. Liu, A PMMA-based microfluidic device for human sperm evaluation and screening on swimming capability and swimming persistence. Micromachines 11(9), 793 (2020). https://doi.org/10.3390/MI11090793
Y. Yan, B. Zhang, Q. Fu, J. Wu, R. Liu, A fully integrated biomimetic microfluidic device for evaluation of sperm response to thermotaxis and chemotaxis. Lab Chip 21(2), 310–318 (2021). https://doi.org/10.1039/d0lc00845a
H. Yin, D. Marshall, Microfluidics for single cell analysis. Curr. Opin. Biotechnol. 23(1), 110–119 (2012). https://doi.org/10.1016/j.copbio.2011.11.002
J.B. You, Y. Wang, C. McCallum, F. Tarlan, T. Hannam, A. Lagunov, K. Jarvi, D. Sinton, Live sperm trap microarray for high throughput imaging and analysis. Lab Chip 19(5), 815–824 (2019). https://doi.org/10.1039/c8lc01204k
J.B. You, C. McCallum, Y. Wang, J. Riordon, R. Nosrati, D. Sinton, Machine learning for sperm selection. Nat. Rev. Urol. 18(7), 387–403 (2021). https://doi.org/10.1038/s41585-021-00465-1
M. Zaferani, S.H. Cheong, A. Abbaspourrad, Rheotaxis-based separation of sperm with progressive motility using a microfluidic corral system. Proc. Natl. Acad. Sci. U.S.A. 115(33), 8272–8277 (2018). https://doi.org/10.1073/pnas.1800819115
M. Zaferani, G.D. Palermo, A. Abbaspourrad, Strictures of a microchannel impose fierce competition to select for highly motile sperm. Sci. Adv. 5(2), 2111 (2019). https://doi.org/10.1126/sciadv.aav2111
S. Zeaei, M. Zabetian Targhi, I. Halvaei, R. Nosrati, High-DNA integrity sperm selection using rheotaxis and boundary following behavior in a microfluidic chip. Lab Chip 23(9), 2241–2248 (2023). https://doi.org/10.1039/d2lc01190e
Y. Zhang, R.R. Xiao, T. Yin, W. Zou, Y. Tang, J. Ding, J. Yang, Generation of gradients on a microfluidic device: Toward a high-Throughput investigation of spermatozoa chemotaxis. PLoS ONE 10(11), 1–14 (2015). https://doi.org/10.1371/journal.pone.0142555
Z. Zhang, J. Liu, J. Meriano, C. Ru, S. Xie, J. Luo, Y. Sun, Human sperm rheotaxis: A passive physical process. Sci. Rep. 6, 23553 (2016). https://doi.org/10.1038/srep23553
A. Zini, M.K. O’Bryan, L. Israel, P.N. Schlegel, Human sperm nadh and nadph diaphorase cytochemistry: correlation with sperm motility. Urology 51(3), 464–468 (1998). https://doi.org/10.1016/S0090-4295(97)00631-6
J. Žvirblytė, L. Mažutis, In: Caballero, D., Kundu, S.C., Reis, R.L. (eds.) Microfluidics for cancer biomarker discovery, research, and clinical application, pp. 499–524. Springer, Cham (2022). https://doi.org/10.1007/978-3-031-04039-9_20
ZyMōt fertility Inc. https://www.zymotfertility.com. Accessed 15 Nov 2023
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.B.T. and M.B.; methodology, M.B.T. and S.J.; investigation, M.B.T., Z.S., Z.H., S.J. and S.A.F.; data curation, Z.S. and Z.H.; supervision, M.B.T. and M.B.; writing-original draft preparation, M.B.T., Z.S., Z.H. and S.A.F.; writing-review and editing, M.B.T., S.A.F. and M.B.; project administration, M.B.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouloorchi Tabalvandani, M., Saeidpour, Z., Habibi, Z. et al. Microfluidics as an emerging paradigm for assisted reproductive technology: A sperm separation perspective. Biomed Microdevices 26, 23 (2024). https://doi.org/10.1007/s10544-024-00705-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10544-024-00705-2