Abstract
A major goal in the development of point-of-care (POC) devices is to build them as portable to provide a rapid and effective determination for disease pathogens. In nucleic acid testing, an optical detection system used to monitor the product of nucleic acid amplification has always been a bulky accessory. In this work, we developed a handheld, automatic and detection system-free thermal digital microfluidic (DMF) device for DNA detection by loop-mediated isothermal amplification (LAMP). Droplet manipulation and real-time temperature control systems were integrated into a handheld device. The control software could be installed into any tablet and communicate with the device via Bluetooth. In the experimentation, we loaded 2-μl samples with an electrowetting force into sandwich-structured DMF chips, thereby considerably reducing reagent consumptions. After an on-chip LAMP reaction, we added a highly concentrated SYBR Green I droplet and mixed it with a reaction droplet to enable product detection with the naked eye. This step prevented aerosol contamination by avoiding the exposure of the reaction droplet to the air. Using a blood parasite Trypanosoma brucei as a model system, this system showed similar results as a commercial thermal cycler and could detect 40 copies per reaction of the DNA target. This low-cost, compact device removed the bulky optical system for DNA detection, thus enabling it to be well suited for POC testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Point-of-care (POC) testing, or near-patient testing, is required for improving disease management, especially in limited-resource areas where a centralised laboratory is difficult to access (Drain et al. 2014). Portability, low cost, robustness and the capacity to rapidly generate accurate and reliable results are the requirements for POC devices. Through microfluidic techniques, sophisticated diagnostic methods can be miniaturised into POC testing. Microfluidics allows a precise control and manipulation of sub-microliter sizes of fluids and integration of complex diagnostic methodologies, such as blood chemistries, immunoassays, nucleic acid amplification tests and flow cytometry (Pandey et al. 2018; Sharma et al. 2015). Unloading the burden of external valves, pumps and pressure supplies for droplet manipulation in the traditional channel microfluidic techniques, digital microfluidics (DMF) utilises electronics to manipulate an individual microdroplet on an array of electrodes on the basis of an electrowetting force (Gao et al. 2013). The salient features of DMF include small reagent consumption, rapid heat transfer, highly automatic and small footprint, thereby making DMF an ideal platform for developing POC devices.
Nucleic acid amplification test can detect a trace amount of genetic materials (DNA or RNA) of various pathogens from an early stage of disease (Niemz et al. 2011). Polymerase chain reaction is the most extensively explored nucleic acid amplification methodology on DMF (Coelho et al. 2017a). However, the thermal cycling condition adds a complexity to the device setting. Recently, several isothermal amplification methods, such as rolling circle amplification (RCA), recombinase polymerase amplification (RPA) and loop-mediated isothermal amplification (LAMP), have been integrated into DMF (Coelho et al. 2017b; Kalsi et al. 2015; Kuhnemund et al. 2014; Wan et al. 2017). Among these methods, LAMP has the easiest assay setting and lowest cost which makes it the most popular isothermal nucleic acid test for detecting viruses, bacteria, fungi and parasites (Iwamoto et al. 2003; Njiru et al. 2008b; Song et al. 2016; Sun et al. 2010).
LAMP is conducted by a set of four or six primers and a DNA polymerase with strand displacement activity (Bst) for DNA amplification under an isothermal condition (Nagamine et al. 2002; Notomi et al. 2000). LAMP produces mass of DNA products with high sensitivity and specificity under a short period, allowing an easy product detection with the naked eye by observing turbidity, or with the aid of a metal indicator or a high concentration of DNA-intercalated dye (Goto et al. 2009; Mori et al. 2001; Parida et al. 2006; Tomita et al. 2008). The determination of turbidity with the naked eye may be subjective, and the colour change in a metal indicator would be difficult to distinguish on-chip given the small volume and thickness of a reaction droplet. Therefore, the high concentration of a DNA-intercalated dye would be an optimal selection for product detection on-chip. A previous study has found that a high concentration of SYBR Green I inhibited a LAMP reaction (Oscorbin et al. 2016). Therefore, a DNA dye must be added after the reaction, thus posing another problem of aerosol contamination given the opening of the tube. Several studies have pre-loaded wax or agar in the reaction tube to isolate the high concentration of SYBR Green I from the reaction solution (Karthik et al. 2014; Liang et al. 2013). After the LAMP reaction, the wax or agar was melted under a high temperature to release SYBR Green I into the products and emitted fluorescence for naked-eye visualisation. Nevertheless, running through the entire protocol is complex.
Several studies have focused on developing a simple and compact LAMP device using channel microfluidic techniques or low-cost electronic set up (Craw et al. 2015; Mauk et al. 2018; Myers et al. 2013; Velders et al. 2018). Channel microfluidics has the benefits of brief test time (<60 min), reduced reagent consumption and ease of use. This technique uses either an embedded fluorescence detection system or naked-eye colorimetry for result read-outs. The disadvantages of channel microfluidics lie in the controllability of the individual reaction droplets when compared to digital microfluidics. When the reaction droplets are inputted, no subsequent manipulation can be performed. Other naked-eye fluorescence optics require a post-reaction product exposure in the air to add in a high concentration of fluorescence dye (Velders et al. 2018).
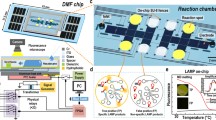
In this work, we addressed this issue by simply using the characteristics of DMF for precisely controlling individual droplets. Here, we present a prototype of a handheld DMF device called LampPort for a POC LAMP reaction. Figure 1a illustrated the composition of the handheld device. A control software could be installed on an external control system with the hardware control via Bluetooth. For LAMP product detection, as depicted in Figs. 1b, e, a highly concentrated SYBR Green I dye was added and mixed with the droplet after the LAMP reaction to enable the product observation with the naked eye. This process preventd carry-over contamination and unloads the fluorescence microscope for product observation. Moreover, given the absence of air inside the reaction chamber in the DMF chip, the aerosol could not form, thus completely preventing aerosol contamination. The system has been tested for detecting a unicellular protozoa, Trypanosoma brucei (T. brucei), which causes African trypanosomiasis in humans and animals.
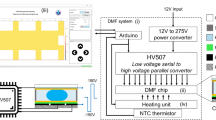
Overview of a handheld digital microfluidic (DMF) device for loop-mediated isothermal amplification (LAMP). a 3D schematic of the components of the device. DC: direct current. AC: alternating current. HV: high-voltage output. Fq: frequency range. Av: voltage amplification. Op-AMP: operational amplifier. Sig-Gen: signal generating. IC: integrated circuit. Mgmt: management. b Schematic of the LAMP reaction and endpoint SYBR Green I denotation of positive LAMP products. Bst: Bst DNA polymerase. dNTP: deoxynucleotides. c Side view of the DMF chip. Cr: chromium electrode. ITO: indium tin oxide. d Droplet actuation and heating control electronics. PTC: positive temperature coefficient. e Schematic of the naked-eyed visualisation of the LAMP results. NTC: no-template control. POS: positive sample
2 Materials and methods
2.1 DMF chip fabrication
The DMF chip was a sealed disposable chip with the LAMP reaction spots. It had a sandwiched structure of a bottom plate patterned with an array of square chromium (Cr) electrodes and a top plate made of ITO glass with the droplet immerged in hexadecane oil, as displayed in Fig. 1c. Furthermore, 10 μm-thick SU-8 was coated on the bottom plate as the dielectric layer. Then, 100 μm-thick patterned SU-8 fences were coated on top of the dielectric layer to restrict the droplets at the desired locations during experiments (Dong et al. 2017). Finally, 100 nm Teflon was coated on both bottom and top plates to create a hydrophobic surface for smooth droplet transportation. The bottom and top plates were separated by a 300 μm laser-cut frame glass spacer and sealed with UV glue. Thus an enclosed reaction chamber was formed to prevent potential contamination during and after the reaction. Hexadecane oil and LAMP reaction mixture were loaded through the inlet holes (1 mm diameter) drilled using a laser cutter on the top plate and sealed by a transparent tape to prevent evaporation during isothermal LAMP reaction. The detailed fabrication and assembly process are demonstrated in Fig. S1 (supplementary material).
2.2 LampPort device settings
As schematically displayed in Fig. 1a, the main body of the device consisted of: (i) an electronic control circuits on a printed circuit board (PCB) with power management circuits, physical relays and mounted with a preconfigured field-programmable gate array (FPGA), (ii) a signal generating module and (iii) a chip holder with a low-cost ceramic positive-temperature-coefficient (PTC) heater. In the signal generating module, the AC actuation signal was provided by a signal generating circuit and amplified to a certain voltage for droplet transportation by a transformer. The actuation voltage needed to be higher than the threshold voltage and lower than the breakdown voltage. Higher actuation voltage ensured higher velocity of droplet moving and less droplet partitioning into oil. Here, 140 V was applied through empirical determination. The PTC heater was powered by a 12-V DC voltage. A film thermocouple temperature sensor was inserted between the heater and the aluminium heat sink for real-time temperature sensing to control the heater power supply and stabilise on-chip temperature at 65 °C. The other temperature sensor was inserted on top of the DMF chip to ensure that the DMF chip reached the set temperature. The control electronics of the entire system was illustrated in Fig. 1d. We developed a computer program written in C# and compiled in Microsoft Visual Studio© to automate the manipulation by controlling the power valves on the PCB through the FPGA.
2.3 Primers and LAMP assay
To test the POC LampPort system, T. brucei was used as a model system. T. brucei is a species of parasitic protozoan that belongs to the genus Trypanosoma. It causes African trypanosomiasis, also known as sleeping sickness in humans or Nagana’s disease in other wild and domestic animals. This disease is spread by the bite of tsetse flies and infects the central nervous system (Njiru et al. 2008a). A rapid, convenient and portable diagnostic of T. brucei is in high demand in sub-Saharan Africa, where medical facilities are limited.
In this work, we used a LAMP assay system modified from the assay described by Njiru (Njiru et al. 2008b). All the primers were purchased from Invitrogen (Table 1).
Three sets of primers that recognise eight independent sequences of the target gene were used in the assay for a specific DNA amplification. This process can enhance the specificity of the LAMP assay when comparing it with a two-pair set of primers (Nagamine et al. 2002). Our previous work has shown that two pairs of primers could easily cause non-specific amplification (Wan et al. 2017).
The LAMP reaction buffer (1×) (NEB) used in this work consisted of 20 mM Tris-HCl (pH 8.8), 10 mM (NH4)2SO4, 2 mM MgSO4 and 0.1% Tween X-100. Off-chip LAMP was performed in 10 μl reaction mixture containing 1× reaction buffer (NEB), 8 mM MgSO4 (in total), 1.6 μM each FIP and BIP, 0.4 μM each F3 and B3, 0.8 μM each LF and LB, 1.4 mM each dNTP (TaKaRa), 16 Unit Bst (NEB), 0.4× SYBR Green I (Invitrogen, diluted to 1000×, 10× stock solution with TE buffer), ddH2O and various concentrations of T. brucei genomic DNA. In addition, a no-template control (NTC) sample with a TE buffer, rather than DNA, was included in each run as a negative control for detecting contamination amplification in the reaction. The off-chip real-time LAMP was run in 10 μl per reaction in a conventional PCR machine (Bio-Rad CFX96™). The on-chip LAMP was conducted with the same mixture as the off-chip counterpart but with a total volume of 2 μl each reaction loading on the DMF chip and run in the LampPort system. The off- and on-chip LAMP reactions were run at 65 °C for 1 h.
2.4 Serial dilutions of the LAMP
T. brucei was enriched and eluted from the blood of infected mice through DEAE-cellulose columns (Lanham and Godfrey 1970). The T. brucei genomic DNA was extracted and purified by a traditional DNA isolation method. Concentration of the purified DNA was measured using Quant-iT™ PicoGreen dsDNA Assay Kit (Invitrogen, USA) and SpectraMax M Series Multi-Mode microplate reader (Molecular Devices, USA) according to the manufacturer’s protocol. DNA serial dilutions were prepared using a Tris-EDTA (TE) buffer (Fluka Analytical, Switzerland). The DNA copy number was calculated as follows:
where
- X:
-
amount of amplicon (ng);
- N:
-
length of dsDNA amplicon (approximately 35 Mb/haploid genome for T. brucei (Berriman et al. 2005; Jackson et al. 2010)); and
- 650 g/mol:
-
average mass of 1 bp dsDNA.
The products of the serial dilutions of the on-chip LAMP were detected with the naked eye under UV light. Images were captured using an iPhone 6 under the UV light. Image J was used to process and analyse fluorescence intensity.
2.5 Visualisation of the LAMP products with the naked eye
After the LAMP on-chip reaction for the NTC sample (containing TE buffer) and POS sample (containing 4000 copies per drop), two 1 μl 1000× SYBR Green I droplets were added to the DMF chip through the inlet. The before-reaction and end-point images of the on-chip LAMP products were captured using an iPhone 6 under ambient light.
3 Results and discussion
3.1 System setup and thermal control
Figure 2a depicts the composition of the LampPort system. The overall size of the main body was 270 mm × 68 mm × 52 mm. The LampPort system consisted of four major parts, namely, (i) electronic control board mounted by the chip holder and the FPGA, (ii) a signal-generating module to provide droplet actuation signals, (iii) a highly reconfigurable DMF chip with patterned multiple LAMP reaction spots in an enclosed reaction chamber and and (iv) software for communication between an operator and a device through the FPGA which enabled the droplet manipulation and temperature controls on the DMF chip. The whole device could be powered by a 12 V DC through a boost cable from a 5 V power bank. The control software could be installed in any computer, tablet or smartphone if needed. The assembled LampPort system was presented in Fig. S2 (supplementary material).
The demonstrated DMF chip for the handheld LampPort had six reaction spots located around the inlet holes through which the LAMP master mix and 1000× SYBR Green I were loaded into the reaction chamber (Fig. 1e). When the droplets were transported to the designated location, the actuation signal was replaced with a low-voltage DC (12 V) as the electric heating signal. The temperature was monitored by a temperature sensor inserted between the DMF chip and the electric heater for real-time feedback to adjust the heating power supply to maintain a stable reaction temperature at 65 °C. The infrared image captured using the thermal imager confirmed that the temperature on the DMF chip reached 65 °C (Fig. 2b).
In our DMF system, the reaction droplets were surrounded by hexadecane for isolating each reaction. Sample inlet holes were far from the reaction spots to avoid any aerosol leakage (Fig. 1e). With an electrowetting force, aqueous droplets could be freely transported from one electrode to another in oil to mix them without unsealing the DMF chip. That made DMF an ideal platform for adding SYBR Green I after reaction without contaminating the environment. The samples were loaded through the inlet holes and transported to the reaction spots for the LAMP reaction. After the reaction, 1000× SYBR Green I could be loaded via the inlet hole and transported to each reaction spot for product identification with the naked eye. The droplet actuation on LampPort is demonstrated in Video S1.
3.2 On-chip LAMP reaction and detection limit evaluation
False positive results in LAMP reactions, either by aerosol contamination or by non-specific DNA amplification, have been a critical problem in molecular diagnostics laboratories. In our previous work, we reported a DMF system for LAMP reaction with a molecular beacon as the product detection probe (Wan et al. 2017). In such a work, carry-over contamination was eliminated by running the reaction isolated from the environment with other reaction droplets using hexadecane oil on the DMF chip, and the molecular beacon probe guaranteed a specific product detection. However, a fluorescence microscope was still required for product detection, thereby hindering the system for the POC test. Another recent work for the LAMP on the DMF integrated sample preparation on-chip (Coelho et al. 2017b). However, their product detection method which required a withdrawal of the post-amplification droplet still posed a risk of contamination. In the present work, hexadecane oil was utilised on the DMF chip to prevent aerosol contamination. Three sets of primers, rather than two sets, were used in the LAMP assay to increase the DNA amplification specificity.
The DNA detection limits of the LAMP assay off- and on-chip were determined with serial dilutions of T. brucei DNA, as exhibited in Fig. 3. The off-chip LAMP was tested in real-time fluorescence detection, as presented in Fig. 3a. The lowest detection limit was 20 copies of DNA molecules in a 10 μL reaction. In the LAMP reaction, the amplified DNA segment was extended with time. Simultaneously, a short segment was produced. Therefore, the fluorescence signal in the real-time LAMP was not strictly an exponential curve as observed in the real-time PCR. For the determination of on-chip LAMP detection sensitivity, the product was detected at the end point under UV light from the LampPort system. Figure 3b illustrates that the samples with an initial DNA concentration of 40 copies exhibit a clear fluorescence signal over the samples with low DNA concentrations or NTC. The fluorescence intensity of the original image captured under the UV light was analysed using Image J. With the RGB image, the relative fluorescence intensity was calculated using grey values. The RGB pixels were converted to grey values using the following formula: V = (R + G + B)/3. Figure 3b depicts the significant difference in the mean grey values between the negative products (NTC, DNA copy number 0.4 and 4) and the positive products (DNA copy number 40, 400 and 4000). The detection limit was comparable with the off-chip counterparts. That indicated that the LampPort system did not inhibit the reaction when considerably reducing the reaction volume by fivefold. It also confirmed the LampPort system was an accurate and specific DNA detection system using LAMP reaction. We did not observe any non-specific amplification in the off- and on-chip tests with low copy numbers of DNA. This phenomenon confirmed that the three sets of primers used in this work significantly increased the amplification specificity.
Serial dilutions of LAMP reactions. a Off-chip serial dilutions in real time. Reactions were run in duplicate. b On-chip serial dilutions at the end point. The mean grey value of the post-reaction droplet was analysed from an image captured under UV light (365 nm). Inset shows the endpoint image of on-chip serial dilutions captured under UV light using iPhone 6. NTC: no-template control
3.3 Naked-eye visualisation of the LAMP product
To demonstrate the naked-eye observation in the LampPort system, the LAMP product was detected by loading SYBR Green I at the end point of the reaction and comparing the fluorescence of the positive sample with the NTC sample on the DMF chip. The addition of droplets to existing droplets on-chip without unsealing the DMF chip is demonstrated in Video S2. Figure 4 presents the chip images captured using a cell phone (iPhone 6) under the ambient light. In Fig. 4a, before adding SYBR Green I, the NTC and positive (POS) samples had no green fluorescence, whereas the POS sample displayed certain turbidity. Given that the binding of SYBR Green I with the double-stranded DNA could initiate a colour change from orange to green, the positive reaction turned green, whereas the negative remained orange after loading 1 μL of 1000× SYBR Green I to the samples, as exhibited in Fig. 4b. A strong differentiation in the fluorescence for the NTC and POS samples was observed under sunlight (Fig. 4c). The colour change after mixing the post-reaction droplet and the 1000× SYBR Green I is presented in Video S3. The naked-eye visualisation was tested several times in different DMF chips and under various light conditions (e.g. ambient light, sunlight, and through UV light) with high reproducibility. The reproducibility is displayed in Fig. S3 (supplementary information).
End-point evaluation of on-chip LAMP reactions (a) before and (b) after adding SYBR Green I. These images were captured using iPhone 6 (a, b) under the ambient light and (c) under the sunlight. The SYBR Green I concentration in (a) was 1000×. A black adhesive tape was attached to the bottom of the DMF chip to enhance the naked-eye visualisation. NTC: no-template control. POS: positive sample
The elimination of the bulky microscopic optical module in our DMF device remarkably simplified the requirement of running the LAMP, thus demonstrating its potential for being utilised as a POC device.
4 Conclusions
We built a handheld DMF device for LAMP reaction with a fully automated electronic control being highly efficient, low cost and contamination-free. By exploiting the DMF technology, we added a highly concentrated SYBR Green I after completing the LAMP reaction to observe the positive or negative DNA amplification with the naked eye. Using T. brucei genomic DNA as the pathogen target, we successfully demonstrated that the on-chip detection sensitivity can be as low as 40 copies of DNA molecules in a 2 μL droplet, comparable with that running off-chip (20 copies per reaction). By further integrating the sample preparation on-chip in the future, the POC device can serve areas with limited resources through rapid disease diagnostics and promote extensive medical care for preventing infectious diseases.
References
M. Berriman et al., The genome of the African trypanosome Trypanosoma brucei. Science 309, 416–422 (2005). https://doi.org/10.1126/science.1112642
B. Coelho, B. Veigas, E. Fortunato, R. Martins, H. Aguas, R. Igreja, P.V. Baptista, Digital microfluidics for nucleic acid amplification. Sensors (Basel) 17 (2017a). https://doi.org/10.3390/s17071495
B.J. Coelho, B. Veigas, H. Aguas, E. Fortunato, R. Martins, P.V. Baptista, R. Igreja, A digital microfluidics platform for loop-mediated isothermal amplification detection. Sensors (Basel) 17 (2017b). https://doi.org/10.3390/s17112616
P. Craw, R.E. Mackay, A. Naveenathayalan, C. Hudson, M. Branavan, S.T. Sadiq, W. Balachandran, A simple, low-cost platform for real-time isothermal nucleic acid amplification. Sensors (Basel) 15, 23418–23430 (2015). https://doi.org/10.3390/s150923418
C. Dong, Y. Jia, J. Gao, T. Chen, P.I. Mak, M.I. Vai, R.P. Martins, A 3D microblade structure for precise and parallel droplet splitting on digital microfluidic chips. Lab Chip 17, 896–904 (2017). https://doi.org/10.1039/c6lc01539e
P.K. Drain, E.P. Hyle, F. Noubary, K.A. Freedberg, D. Wilson, W.R. Bishai, W. Rodriguez, I.V. Bassett, Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 14, 239–249 (2014). https://doi.org/10.1016/S1473-3099(13)70250-0
J. Gao, X. Liu, T. Chen, P.I. Mak, Y. Du, M.I. Vai, B. Lin, R.P. Martins, An intelligent digital microfluidic system with fuzzy-enhanced feedback for multi-droplet manipulation. Lab Chip 13, 443–451 (2013). https://doi.org/10.1039/c2lc41156c
M. Goto, E. Honda, A. Ogura, A. Nomoto, K. Hanaki, Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 46, 167–172 (2009). https://doi.org/10.2144/000113072
T. Iwamoto, T. Sonobe, K. Hayashi, Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J. Clin. Microbiol. 41, 2616–2622 (2003)
A.P. Jackson, M. Sanders, A. Berry, J. McQuillan, M.A. Aslett, M.A. Quail, B. Chukualim, P. Capewell, A. MacLeod, S.E. Melville, W. Gibson, J.D. Barry, M. Berriman, C. Hertz-Fowler, The genome sequence of Trypanosoma brucei gambiense, causative agent of chronic human african trypanosomiasis. PLoS Negl. Trop. Dis. 4, e658 (2010). https://doi.org/10.1371/journal.pntd.0000658
S. Kalsi, M. Valiadi, M.N. Tsaloglou, L. Parry-Jones, A. Jacobs, R. Watson, C. Turner, R. Amos, B. Hadwen, J. Buse, C. Brown, M. Sutton, H. Morgan, Rapid and sensitive detection of antibiotic resistance on a programmable digital microfluidic platform. Lab Chip 15, 3065–3075 (2015). https://doi.org/10.1039/c5lc00462d
K. Karthik, R. Rathore, P. Thomas, T.R. Arun, K.N. Viswas, K. Dhama, R.K. Agarwal, New closed tube loop mediated isothermal amplification assay for prevention of product cross-contamination. MethodsX 1, 137–143 (2014). https://doi.org/10.1016/j.mex.2014.08.009
M. Kuhnemund, D. Witters, M. Nilsson, J. Lammertyn, Circle-to-circle amplification on a digital microfluidic chip for amplified single molecule detection. Lab Chip 14, 2983–2992 (2014). https://doi.org/10.1039/c4lc00348a
S.M. Lanham, D.G. Godfrey, Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp. Parasitol. 28, 521–534 (1970)
C. Liang, S. Cheng, Y. Chu, H. Wu, B. Zou, H. Huang, T. Xi, G. Zhou, A closed-tube detection of loop-mediated isothermal amplification (LAMP) products using a wax-sealed fluorescent intercalator. J. Nanosci. Nanotechnol. 13, 3999–4005 (2013)
M.G. Mauk, J. Song, C. Liu, H.H. Bau, Simple approaches to minimally-instrumented, microfluidic-based point-of-care nucleic acid amplification tests. Biosensors (Basel) 8 (2018). https://doi.org/10.3390/bios8010017
Y. Mori, K. Nagamine, N. Tomita, T. Notomi, Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 289, 150–154 (2001). https://doi.org/10.1006/bbrc.2001.5921
F.B. Myers, R.H. Henrikson, J.M. Bone, L.P. Lee, A handheld point-of-care genomic diagnostic system. PLoS One 8, e70266 (2013). https://doi.org/10.1371/journal.pone.0070266
K. Nagamine, T. Hase, T. Notomi, Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 16, 223–229 (2002)
A. Niemz, T.M. Ferguson, D.S. Boyle, Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 29, 240–250 (2011). https://doi.org/10.1016/j.tibtech.2011.01.007
Z.K. Njiru, A.S. Mikosza, T. Armstrong, J.C. Enyaru, J.M. Ndung'u, A.R. Thompson, Loop-mediated isothermal amplification (LAMP) method for rapid detection of Trypanosoma brucei rhodesiense. PLoS Negl. Trop. Dis. 2, e147 (2008a). https://doi.org/10.1371/journal.pntd.0000147
Z.K. Njiru, A.S. Mikosza, E. Matovu, J.C. Enyaru, J.O. Ouma, S.N. Kibona, R.C. Thompson, J.M. Ndung'u, African trypanosomiasis: Sensitive and rapid detection of the sub-genus Trypanozoon by loop-mediated isothermal amplification (LAMP) of parasite DNA. Int. J. Parasitol. 38, 589–599 (2008b). https://doi.org/10.1016/j.ijpara.2007.09.006
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:E63, 63e
I.P. Oscorbin, E.A. Belousova, A.I. Zakabunin, U.A. Boyarskikh, M.L. Filipenko, Comparison of fluorescent intercalating dyes for quantitative loop-mediated isothermal amplification (qLAMP). Biotechniques 61, 20–25 (2016). https://doi.org/10.2144/000114432
C.M. Pandey, S. Augustine, S. Kumar, S. Kumar, S. Nara, S. Srivastava, B.D. Malhotra, Microfluidics based point-of-care diagnostics. Biotechnol. J. 13 (2018). https://doi.org/10.1002/biot.201700047
M.M. Parida, S.R. Santhosh, P.K. Dash, N.K. Tripathi, P. Saxena, S. Ambuj, A.K. Sahni, P.V. Lakshmana Rao, K. Morita, Development and evaluation of reverse transcription-loop-mediated isothermal amplification assay for rapid and real-time detection of Japanese encephalitis virus. J. Clin. Microbiol. 44, 4172–4178 (2006). https://doi.org/10.1128/JCM.01487-06
S. Sharma, J. Zapatero-Rodriguez, P. Estrela, R. O'Kennedy, Point-of-care diagnostics in low resource settings: Present status and future role of microfluidics. Biosensors (Basel) 5, 577–601 (2015). https://doi.org/10.3390/bios5030577
J. Song, M.G. Mauk, B.A. Hackett, S. Cherry, H.H. Bau, C. Liu, Instrument-free point-of-care molecular detection of Zika virus. Anal. Chem. 88, 7289–7294 (2016). https://doi.org/10.1021/acs.analchem.6b01632
J. Sun, M.J. Najafzadeh, V. Vicente, L. Xi, G.S. de Hoog, Rapid detection of pathogenic fungi using loop-mediated isothermal amplification, exemplified by Fonsecaea agents of chromoblastomycosis. J. Microbiol. Methods 80, 19–24 (2010). https://doi.org/10.1016/j.mimet.2009.10.002
N. Tomita, Y. Mori, H. Kanda, T. Notomi, Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 3, 877–882 (2008). https://doi.org/10.1038/nprot.2008.57
A.H. Velders, C. Schoen, V. Saggiomo, Loop-mediated isothermal amplification (LAMP) shield for Arduino DNA detection. BMC Res Notes 11, 93 (2018). https://doi.org/10.1186/s13104-018-3197-9
L. Wan, T. Chen, J. Gao, C. Dong, A.H. Wong, Y. Jia, P.I. Mak, C.X. Deng, R.P. Martins, A digital microfluidic system for loop-mediated isothermal amplification and sequence specific pathogen detection. Sci. Rep. 7, 14586 (2017). https://doi.org/10.1038/s41598-017-14698-x
Acknowledgements
This work was supported by FDCT110/2016/A3 and AMSV SKL Fund from the Macao Science and Technology Development Fund (FDCT), MYRG2017-00022-AMSV and SRG2016-00072-AMSV from the University of Macau, and #31720103918 from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wan, L., Gao, J., Chen, T. et al. LampPort: a handheld digital microfluidic device for loop-mediated isothermal amplification (LAMP). Biomed Microdevices 21, 9 (2019). https://doi.org/10.1007/s10544-018-0354-9
Published:
DOI: https://doi.org/10.1007/s10544-018-0354-9