Abstract
In robot-assisted catheterization, haptic feedback is important, but is currently lacking. In addition, conventional interventional surgical robotic systems typically employ a master–slave architecture with an open-loop force feedback, which results in inaccurate control. We develop herein a novel real-time master–slave (RTMS) interventional surgical robotic system with a closed-loop force feedback that allows a surgeon to sense the true force during remote operation, provide adequate haptic feedback, and improve control accuracy in robot-assisted catheterization. As part of this system, we also design a unique master control handle that measures the true force felt by a surgeon, providing the basis for the closed-loop control of the entire system. We use theoretical and empirical methods to demonstrate that the proposed RTMS system provides a surgeon (using the master control handle) with a more accurate and realistic force sensation, which subsequently improves the precision of the master–slave manipulation. The experimental results show a substantial increase in the control accuracy of the force feedback and an increase in operational efficiency during surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In conventional minimally invasive vascular interventional surgeries, a surgeon directly inserts a catheter into the blood vessels from an incision on the patient’s skin, then into a lesion using auxiliary medical images, subsequently makes a corresponding diagnosis, and determines treatment (Guo et al. 2012; Xiao et al. 2012; Saliba et al. 2008). Endovascular intervention techniques have greatly improved. However, techniques specifically related to the use of guidewires and catheters have not. In addition, physicians are exposed to radiation and its associated risks during long procedures.
Robotic catheterization technologies offer a promising solution to these issues. Various clinical trials confirmed that robotic technology enhances the use of standard endovascular intervention techniques (Antoniou et al. 2011). Compared to manual procedures, robot-assisted catheterization offers many advantages, such as highly accurate positional control, good stability of operation, and reduced radiation exposure for physicians.
During robotic surgery, doctors use master side to perform interventions remotely. Different with traditional surgery, the doctor does not need to directly grasp the catheter to insert it into the patient’s body. Master-slave robotic system structure is adopted to realize the separation of doctors and X radiation during surgery operation. In this way, radiation damage to the doctors can be reduced. However, disadvantage is the lack of haptic feedback offered to the surgeon. In conventional surgical procedures, a surgeon directly handles the surgical instruments and receives haptic information from the interaction of the instruments and the human tissue. Conversely, in robot-assisted surgical procedures, the mechanical robot, instead of the surgeon, handles the catheter. The surgeon receives no direct haptic information, which not only affects the surgeon’s hand–eye coordination, but also restricts the surgeon’s operating experience (Ahmed et al. 2010; Wang et al. 2015a; Guo et al. 2012; Carrell et al. 2012; Tsekos et al. 2007; Ma et al. 2013). The robot-assisted catheterization is also more likely to damage the patient’s organs because of improper or excessive operation and increases operation time and risk.
The differences in a surgeon’s haptic feedback during conventional and robot-assisted surgical procedures are evident. Emergent research is considering mechanisms for providing haptic feedback to a surgeon when using a surgical robotic system and improving the control accuracy based on the force feedback from the slave side during a vascular interventional surgery (Riga et al. 2011; Kesner and Howe 2011). Several vascular intervention surgical robotic systems have been used in a vascular model experiment (Ma et al. 2012; Xiao et al. 2011; Wang et al. 2013a; Singh 2011; Wang et al. 2013b). In the United States, Corindus developed the robot-assisted system CorPath 200 to support percutaneous coronary intervention (Lock and Laing 2010). The propulsion device of the guidewire/catheter does not offer a force feedback function. But the special catheter measures the stress from the proximal mechanism of the guidewire/catheter. Hansen Medical developed two vascular interventional robotic system, called Sensei and Magellan, with a powerful force feedback function measured through the end of the guidewire/catheter (Kanagaratnam et al. 2008). However, surgeons acting as the master cannot detect the true force of the guidewire/catheter, suggesting persistent accuracy issues with the system’s force feedback function. Stereotaxis developed a relatively mature system used to support magnetic navigation interventional surgery without force feedback. This system was called the Niobe system (Kiemeneij et al. 2008). Meanwhile, the Amigo system developed by Catheter Robotic included a simple force feedback. But it was capable only of force warning (Khan et al. 2013). Each of these interventional surgical robotic systems currently being used in clinical trials has issues regarding nonexistent or inaccurate force feedback.
In terms of the haptic feedback, the existing research is divided into 3 categories: no haptic feedback, only a vibration alarm, and haptic feedback. Previous studies demonstrated that the feedback force approximating the resistance of the guidewire provides doctors with an effective support. The feedback force makes the doctor’s surgery feel close to the manual operation. Under such circumstances, doctors can effectively use surgical techniques and clinical experience. Therefore, a more accurate feedback can help doctors more easily complete the operation. However, the existing haptic feedback functions are open-loop control, which will result in an inaccurate control. At the same time, At the same time, the feedback force that doctor feels are uncertain. Therefore, this study designs a new type of doctor control handle to collect the resistance the doctor encounters during an operation and a closed-loop algorithm to improve the haptic feedback accuracy.
We develop herein a real-time master–slave (RTMS) interventional surgical robotic system with a closed-loop force feedback that allows a surgeon to sense the true force during a remote operation. As part of this system, we design a unique master control handle that measures the true force felt by a surgeon and mimics conventional surgical techniques and ergonomics. Other system components include a force detection mechanism on the slave side and a real-time force feedback closed-loop control strategy on the master side and for the entire system. Section 2 describes the proposed RTMS interventional surgical robotic system. Sections 3 and 4 describe the experimental methods and results used to validate the proposed RTMS system design, respectively. Section 5 presents the concluding remarks.
2 Proposed system description
2.1 System components and functionality
This study’s laboratory team jointly developed a novel RTMS interventional surgical robotic system with a closed-loop force feedback to improve the existing vascular interventional surgical robotic systems. The system herein was designed to simulate a surgeon’s actions in a conventional vascular interventional catheterization surgery by providing the surgeon accurate force sensations and precise control. The RTMS system generally functions as follows: (Guo et al. 2012) a surgeon operates the master side from a location isolated from radiation; (Xiao et al. 2012) the position and force information is relayed from the master side to the slave side for a synchronous operation; and (Saliba et al. 2008) the position and force feedback information is relayed from the slave side to the master side, providing the basis for the closed-loop control of the entire system.
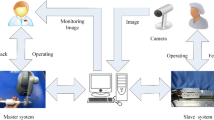
Figure 1 illustrates a diagram of the complete system structure (Wang et al. 2015a; Peng et al. 2015; Wang et al. 2015b; Guo et al. 2015; Zhang et al. 2017; Yin et al. 2016; Song et al. 2017) that includes two main platforms: (Guo et al. 2012) the slave side for guidewire insertion (used to manipulate the guidewire) and (Xiao et al. 2012) the master side for the operation (representing the surgeon console). Both platforms were designed to mimic a clinician’s experience during a hands-on operation. Surgeons tend to use their own skills when performing these types of operations; hence, the combination of a guidewire manipulator and a surgeon console represents a mode of operation familiar to clinicians. The slave side included a guidewire delivery system and a monitoring equipment. The master side included the unique master control handle, the image display developed in this study, and The Phantom. The Phantom is a haptic interface device developed by Sensable, which can be the output of the feedback force.
In the aspect of the haptic feedback, each of the interventional surgical robotic systems currently being used in clinical trials includes an open-loop control system and receives the force feedback from the slave side only. Friction in the system’s mechanical structure result in a gap between the actual force feedback information and the master side operation. Thus, the implementation of a force detection mechanism on the master side during the operation was important for improving the force feedback accuracy. The unique master control handle developed herein was designed for this purpose.
Figure 2 illustrate the master control handle structure. This device comprised a force sensor that measured the force between the surgeon’s hand and the handle, an encoder that measured the rotary velocity, two cover pieces used to grasp the handle, and a handle extension that mimicked a surgeon’s instruments. When a surgeon holds the handle extension stationary, the force acts on the force sensor along the cover pieces’ axis. When a surgeon pushes the handle extension forward, a compressional force is measured by the force senor, and the magnitude of the measured force is the force acting on the surgeon’s hand and the handle. A slider and a slide rail fix the control handle on a horizontal axis. This device can accurately measure the feedback force on the master side.
As one of two platforms in the proposed RTMS system, Fig. 3 illustrates an image of the master platform for the operation, which includes the master control handle. The master platform for the operation transmits the surgeon’s hand movements to the guidewire and receives the measured resistance force on the guidewire. A surgeon grasps the master control handle to input their operations (i.e., push, pull, or rotate the handle). During surgery, the movements of the guidewire manipulator mimics the movements of the master control handle. The guidewire manipulator in the slave side will complete the same movement of the master control handle to control the guidewire. The handle is fixed on a sliding block connected to a slide rail. Figure 3 illustrates that the Y-axis and rotation directions reflect the system’s two degrees of freedom. This novel design is consistent with human engineering during the operation process and offers a higher level of precision and accuracy attributable to the enhanced force sensor embedded in the master control handle.
The second platform in the proposed RTMS system, which is the slave platform for the guidewire insertion, acts as a manual function in the interventional surgical robotic system and directly controls the motion of the guidewire/catheter. To achieve this, we designed a unique mechanical device comprising a mobile platform, shell, body, telescopic mechanism, lock-switching mechanism, and guidewire. This device can perform axial forward, withdrawal, and rotation functions and detecting the stress force on the guidewire/catheter. Figure 4 illustrates the slave-side. Part A is a connector with the function to fix the 3-way valve. Part B is a guide wire manipulator used to grasp, relax, rotate the guide wire. Part C is a linear motion platform that controls the push and pull movements of the guide wire.
During a robot-assisted surgery using the proposed RTMS system, a surgeon primarily relies upon two types of information: (Guo et al. 2012) digital subtraction angiography images to obtain the real-time position of the catheter and (Xiao et al. 2012) haptic feedback provided by the master control handle. Thus, the slave platform for the guidewire insertion must not only be able to detect the guidewire/catheter force information, but also relay this information to the master side.
As previously noted, the slave platform for the guidewire insertion can perform axial forward, withdrawal, and rotation functions and detect the stress force on the guidewire/catheter. Figures 5 and 6 illustrate the body component and the complete slave platform for the guidewire insertion structures, respectively. We used a load mechanism comprising a force sensor, slider, slide rail, thrust bearing, linear bearing, and guidewire gripper to measure the force acting on the guidewire/catheter. The contact terminal of the force sensor was connected to the force platform mounted on the sliding bearing. The slider and the slide rail maintained the axis direction with no static friction force. The slide rail and the guidewire gripper maintained the axis direction integrity. All components connected to the guidewire can freely move in the axial direction. The force sensor remained stationary when the guidewire was under stress. The other parts connected to the guidewire maintained their integrity, and the thrust force of the guidewire was accurately measured.
2.2 Theoretical basis for closed-loop system control
Conventional interventional surgical robotic systems typically employ a master–slave architecture with an open-loop force feedback, which results in an inaccurate control. We used herein a closed-loop control to improve the accuracy. Figure 7 presents the detection force structure, where f is the force measured at the proximal end of the guidewire/catheter on the slave side; f′is the resistance force feedback relayed to the master control handle from the slave side; F is the master control handle’s push force from the surgeon; and f″ is the force measured by the force sensor embedded in the master control handle.
At time T1, when the master–slave system is is under statical state, f is primarily the static friction between the guidewire and vessel with a maximum value fmax.
The master–slave system was still in a stationary state when F < fmax. Hence, the force detected by the sensor embedded in the master side should be the same as the force detected by the sensor embedded in the slave side, f″ = f′.
The guide wire will be pushed forward in the blood vessel to enter the motion state if F > fmax.
At time T2, when the master–slave system is under motion state, the resistance of the guide wire will continuously be changed by the doctor’s operation, vascular shape, and other factors. In motion, the sensor detects the lesser of the forces on both sides. The force sensor embedded in the master side measured f″as the minimum of F or f′. F is greater than f′ because the guide wire is in the push state. Therefore, f″ = f′. The same result can be obtained when the guide wire is pulled.
In the ideal state, f″ is the haptic feedback function output, f″ = f′ = f. However, momentum, friction force and deformation of mechanical structure would lead to effects on force feed-back, which should be solved for improvement of accuracy of force feed-back.
Figure 8 illustrates the force transmission procedure. The dynamic models of the force transmission between the master side and slave sides are respectively presented as Eqs.(1) and (2):
where mm, bm, and km and ms, bs, and ks are the mass, damping, and elasticity coefficients of the master control handle and slave, respectively; xm, \( {x}_m^{\hbox{'}} \), and \( {x}_m^{\hbox{'}\hbox{'}} \) and xs, \( {x}_s^{\hbox{'}} \), and \( {x}_s^{\hbox{'}\hbox{'}} \) are the displacement, velocity, and acceleration of the master control handle and slave, respectively; fm is the force exerted on doctor from the master side; fs is the force exerted on the slave side from the guide wire; fdm is the force value from the controller sent to the master side; and fds is the force value from the slave side sent to the controller. The fm corresponds to f′, and fdm corresponds to f. An error caused by momentum, friction, and deformation was observed in the force transmission. A closed-loop control was needed to improve the accuracy of the actual force feedback function.
Figure 9 illustrates the haptic feedback on the master side. The slave detection force is the input. The force feedback device provides feedback force. The output force measured by sensor in the master control handle. A Kalman filter was used to reduce the interference from a surgeon’s shaky hands to force measurement. In addition, a simple closed-loop proportional–integral–derivative (PID) control algorithm was applied on the master side to improve the control accuracy based on the force feedback, providing a surgeon with the truest force sensations.
According to the analysis of the closed-loop dynamic model of the system. fs(t) is the input, fm(t) is the output. In order to decrease the error as soon as possible, the system choose the proper PID parameters to get the optimal control.
The PID control algorithm was again applied to improve the accuracy of the complete system’s force feedback and achieve the closed-loop force feedback control. The closed-loop force feedback system developed as part of the broader proposed RTMS interventional surgical robotic system provided good stability and increased the control accuracy in real time.
3 Experimental design
Three experiments were designed herein to verify the validity of this research. The experiment I is used to test whether the force sensor can detect the output of the haptic device. The experiment II is carried out to verify the accuracy of the force feedback in the condition of the constant input force. The experiment III is conducted to verify the performance of the RTSM in Vessel model.
First, the detected feedback force output should be validated. The PID controller should be designed based on the correct output. Second, the accuracy of the feedback force at the master control handle then needs to be verified. Finally, the feedback accuracy of the RTMS system and the operation of the volunteer efficiency improvements need to be validated. Five volunteers participated in the experiment. The volunteers performed the same operation for more than 6 times in each group of experiment. The number of tests can ensure the reliability of the result. Use the Fig. 10 to clearly describe the path of the guide wire.
The experiment I aimed to verify that the force obtained by the sensor embedded in the master control handle and the force provided by the force feedback device were equal. The first experiment is to prove that f″ is equivalent to f′. f″ is the force detected by the sensor. f′ is the feedback force output by the haptic device. The f″, which was not disturbed by the push velocity of the volunteer, must also be verified. According to our research data, the guide wire resistance during an operation is generally less than 1.2 N and mainly distributed in the region of 0–0.5 N. Therefore, these five sets of data were selected as the simulation resistance. The input value of the force feedback f′ was set as 0.1–0.5 N and maintained a constant force value throughout each experiment. This experiment was repeated 6 times by each volunteer to ensure repeatability and minimize the potential error. During the experiment, the volunteer moved the master control handle forward and backward. The force measured by the sensor embedded in the master handle was then recorded. The desired result was: when the master control handle is moved forward, f″ is the approximation of f′. When the master control handle is moved forward, f″ is the approximation of 0.
The experiment II aimed to verify the effect closed-loop force feedback on the master side. The Kalman filter was added to process f″ and reduce the interference of the volunteer’s shaky hands, which may cause impulse interference and partial fluctuation in the results. These interference and partial fluctuations came from the movement in different directions with the feedback force; hence, an appropriate threshold value was chosen to reduce the volatility of the measured force on the master side. We applied a closed-loop PID control algorithm to further reduce the potential error. This set of experiments used the expected output of the first experiment as the input. The volunteers only needed to keep pushing the master control handle. This design was made because the contrast of results in the same situation was more intuitive. The Kalman filter was then used to reduce the force detection error, while the PID control was employed to improve the accuracy of the feedback force. This experiment was again repeated for 6 times by each volunteer to ensure repeatability and minimize the potential error. The desired result was: after processing, the feedback force is equal to f′.
The experiment III aimed to verify the effect closed-loop force feedback for the complete RTMS system. An insertion experiment was performed to evaluate the effect of the haptic feedback. The experiment was completed with a model of human blood vessels (EVE, endovascular evaluator). When using the RTMS system, the volunteers directed the guidewire through the aortic arch in a relatively simple set of treatments (Fig. 10). The resistance and the completion time of each volunteer’s operations were recorded. When inserting the guidewire/catheter, the force measured at the proximal end of the guidewire/catheter on the slave side, f, and the force measured by the force sensor embedded in the master control handle, f″, were concurrently displayed on a computer monitor. These relative curves were recorded and compared with the feedback force after using the closed-loop control. The experiment was repeated twice, with and without the closed-loop PID control algorithm applied to the program.
In addition, the volunteers completed the experiment in “without feedback” and “manual” situations. In each case, each volunteer will complete more than 6 sets of operations. Three different times for each volunteer will be compared and analyzed. This experiment validated the haptic feedback to improve the effect of surgery. Table 1 illustrates the average operation completion times of five random volunteers. A manual operation was performed to use the least time. The robotic operation without a haptic feedback used the most time. The difference of these 3 operations was the different haptic. A real haptic provides the doctor’s operation with a higher efficiency. Therefore, improving the accuracy of the haptic feedback can be more effective in assisting a doctor to complete an operation.
4 Experimental results
Figure 11 presents the results of the first experiment. This experiment aimed to verify that the force provided by the force feedback device can be detected. Figure 11a, whose input was 0.5, illustrates the representative data. Figure 11b-e, whose input was 0.1–0.4, presents a random data of each situation. As can be seen in Fig. 11a, when the master control handle was pushed, the force f″ measured by the master approximated the expected value 0.5 N. Meanwhile, when the master control handle was pulled back, the force f″ measured by the master approximated the expected value 0 N. The feedback force will not be affected by the movement speed of the master control handle. A huge error between f″ and the expected value was found. However, the changing trend showed that the force provided by the force feedback device was detected. The proposed method can be used to reduce the feedback error.
Figures 12 and 13 present the results of Experiment 2 that aimed to verify the effect closed-loop force feedback on the master side (Guo et al. 2016). An experiment with the largest error was also chosen. The input of this experiment was 0.5 N. Figure 12 illustrates the feedback force after Kalman filtering. This step eliminated the effect of tremor on the force measurement. Figure 13 illustrates the comparison between the processed feedback force and the expected output. Following the application of the Kalman filter and the closed-loop PID control algorithm, the maximum force feedback error was <0.04 N.
Table 2 illustrates the maximum error under various inputs. The proposed method effectively reduced the force feedback error. This 0.04 N is the maximum error obtained in the range of 0–0.5 N. According to the experience of doctors and the bearing capacity of blood vessels, the robotic system with this maximum error reach safety requirements.
In the open-loop force feedback, the force felt by a surgeon on the master side may not be equivalent to the stress force applied to a guidewire on the slave side. Figures 14 and 15 compare a surgeon’s force sensation with the force detected on the slave side without and with the application of the closed-loop PID control algorithm to the complete RTMS system, respectively. Without the closed-loop PID control algorithm (Fig. 14), the maximum error between the master and slave sides was 0.042 N. This error was smaller than that in the last experiment because the operators were overly careful to subconsciously reduce their trembling. Therefore, the performance improvement of experiment 3 by the Kalman filter was less than that of experiment 2. With the closed-loop PID control algorithm (Fig. 15), the maximum error between the master and slave sides decreased to 0.022 N, representing an error reduction of 47.6%. The application of the closed-loop PID control algorithm to the complete RTMS system increased the control accuracy, which will provide surgeons with truer force sensations during operations.
5 Conclusion
A novel RTMS interventional surgery robot system was developed herein. The robot system had a closed loop force feedback and can make a doctor feel the real feedback force during the remote operation.
A novel master side was designed with a specific control handle. This novel master side let the operation of the surgeon with the robot system closer to the traditional clinical operation, which cannot only effectively obtain the haptic feedback information of the surgeon in the master side during the operation process, but also realize the precision force feedback control of the master–slave interventional surgery robot system. During an operation, the shaking of the doctor’s hand can cause errors in the feedback force detection. This error will be removed using Kalman filtering. The closed-loop controller herein is designed to improve the accuracy of the feedback force.
Three experiments were used for verification analysis. The rationality of the novel master side was verified. Using the novel master side with the closed-loop controller, the maximum error of the feedback force was reduced to 0.04 N when the resistance was less than 0.5 N. The closed-loop control improved the real-time precision of the haptic feedback. During an operation, surgeons can obtain a more real and precise feeling from the force feedback function to strengthen the sense of reality. Combined with this feeling and clinical experience, the operation efficiency will be improved.
References
K. Ahmed, A.N. Keeling, M. Fakhry, H. Ashrafian, R. Aggarwal, P.A. Naughton, A. Darzi, N. Cheshire, T. Athanasiou, M. Hamad, Role of virtual reality simulation in teaching and assessing technical skills in endovascular intervention. J. Vasc. Interv. Radiol. 21(1), 55–66 (2010)
G.A. Antoniou, C.V. Riga, E.K. Mayer, N.J. Cheshire, C.D. Bicknell, Clinical applications of robotic technology in vascular and endovascular surgery. J. Vasc. Surg. 53(2), 493–499 (2011)
T. Carrell, N. Dastur, R. Salter, P. Taylor, Use of a remotely steerable “robotic” catheter in a branched endovascular aortic graft. J. Vasc. Surg. 55(1), 223–225 (2012)
J. Guo, S. Guo, N. Xiao, X. Ma, S. Yoshida, T. Tamiya, M. Kawanishi, A Novel Robotic Catheter System with Force and Visual Feedback for Vascular Interventional Surgery. Int. J. Mechatron. Autom. 2(1), 15–24 (2012)
J. Guo, S. Guo, L. Shao, P. Wang, Q. Gao, Design and performance evaluation of a novel robotic catheter system for vascular interventional surgery. Int. J. Microsyst. Technol. 22(9), 2167–2176 (2015)
S Guo, M Qin, N Xiao, Y Wang, W Peng, High precise haptic device for the robotic catheter navigation system. Proceedings of 2016 I.E. international conference on mechatronics and automation. 2524–2529(2016)
P. Kanagaratnam, W.M. Koa, D.T. Wallace, et al., Experience of robotic catheter ablation in humans using a novel remotely steerable catheter sheath. J. Interv. Card. Electrophysiol. 21(1), 19–26 (2008)
S. B. Kesner and R. D. Howe, Force control of flexible catheter robots for beating heart surgery. Proceedings of 2011 I.E. international conference on robotics and automation (ICRA), 1589–1594(2011)
E.M. Khan et al., First experience with a novel robotic remote catheter system: Amigo mapping trial. J. Interv. Card. Electrophysiol. 37(2), 121–129 (2013)
F. Kiemeneij et al., Use of the Stereotaxis Niobe magnetic navigation system for percutaneous coronary intervention: Results from 350 consecutive patients. Catheter. Cardiovasc. Interv. 71(4), 510–516 (2008)
J. Lock, G. Laing. Quasistasic modeling of concentric tube robots with external loads. The Proceedings of 2010 IEEE/RSJ international conference on intelligent robots and systems. 2325–2332 (2010)
X. Ma, S. Guo, N. Xiao, J. Guo, S. Yoshida, T. Tamiya, M. Kawanishi, Development of a novel robotic catheter manipulating system with fuzzy PID control. Int. J. Intell. Mechatron. Robot. (IJIMR). 2(2), 58–77 (2012)
X. Ma, S. Guo, N. Xiao, S. Yoshida, T. Tamiya, Evaluating performance of a novel developed robotic catheter manipulating system. J. Micro-Bio Robot. 8(3–4), 133–143 (2013)
W. Peng, N. Xiao, S. Guo, Y. Wang, A Novel Force Feedback Interventional Surgery Robotic System. Proceedings of 2015 I.E. International Conference on Mechatronics and Automation. 709–714(2015)
C.V. Riga, C.D. Bicknell, M.S. Hamady, N.J. Cheshire, Evaluation of robotic endovascular catheters for arch vessel cannulation. J. Vasc. Surg. 54(3), 799–809 (2011)
W. Saliba, V.Y. Reddy, O. Wazni, et al., Atrial fibrillation ablation using a robotic catheter remote control system: Initial human experience and long-term follow-up results. J. Am. Coll. Cardiol. 51(25), 2407–2411 (2008)
I. Singh, Robotics in urological surgery: Review of current status and maneuverability, and comparison of robot-assisted and traditional laparoscopy. Comput. Aided Surg. 16(1), 38–45 (2011)
Y. Song, S. Guo, X. Yin, et al., Design and performance evaluation of a haptic interface based on MR fluids for endovascular tele-surgery. Microsyst. Technol., 1–10 (2017). https://doi.org/10.1007/s00542-017-3404-y
N.V. Tsekos, A. Khanicheh, E. Christoforou, C. Mavroidis, Magnetic resonance-compatible robotic and mechatronics systems for image-guided interventions and rehabilitation: A review study. Annu. Rev. Biomed. Eng. 9(1), 351–387 (2007)
Y. Wang, K. Hu, N. Xiao, S. Guo, A Force Acquisition Method in a catheter navigation system. Proceedings of 2013 I.E. international conference on complex medical Engineering, 633–637(2013a)
Y. Wang, N. Xiao, S. Guo, Design of a Surgeon's Controller for Catheter Navigation. Proceedings of 2013 I.E. international conference on mechatronics and automation, 974–978(2013b)
Y. Wang, S. Guo, B. Gao, Vascular Elastcity Determined Mass-spring Model for Virtual Reality Simulators. Int. J. Mechatron. Autom. 5(1), 1–10 (2015a)
Y Wang, S Guo, P Guo, et al. Study on haptic feedback functions for an interventional surgical robot system. Proceedings of 2015 I.E. International Conference on Mechatronics and Automation. 715–720(2015b)
N. Xiao, S. Guo, J. Guo, X. Xiao, and T. Tamiya, Development of a kind of robotic catheter manipulation system. Proceedings of 2011 I.E. international conference on robotics and Biomimetics (ROBIO). 32–37(2011)
N. Xiao, J. Guo, S. Guo, T. Tamiya, A. Robotic Catheter, System with real-time force feedback and monitor. Australas. Phys. Eng. Sci. Med. 35(3), 283–289 (2012)
X. Yin, S. Guo, N. Xiao, T. Tamiya, H. Hirata, H. Ishihara, Safety operation consciousness realization of MR fluids-base novel haptic Interface for teleoperated catheter minimally invasive neuro surgery. IEEE/ASME Trans. Mechatron. 21(2), 1–1 (2016)
L. Zhang, S. Guo, H. Yu, et al., Performance evaluation of a strain-gauge force sensor for a haptic robot-assisted catheter operating system. Microsyst. Technol. 23(10), 1–10 (2017)
Acknowledgements
This research is partly supported by the National Natural Science Foundation of China (61375094), National High Tech. Research and Development Program of China (No.2015AA043202).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, S., Wang, Y., Xiao, N. et al. Study on real-time force feedback for a master–slave interventional surgical robotic system. Biomed Microdevices 20, 37 (2018). https://doi.org/10.1007/s10544-018-0278-4
Published:
DOI: https://doi.org/10.1007/s10544-018-0278-4