Abstract
This study aimed to investigate the drug delivery efficacy and bio-effectiveness of a novel photodynamic therapy (PDT)-matrix drug delivery system for cholangiocarcinoma (CCA). Metallic stents were coated with polyurethane (PU) as the first layer. A 2-hydroxyethyl methacrylate (2-HEMA)/ethylene glycol dimethacrylate (EGDMA)/benzoyl peroxide (BPO) layer and a poly(ethylene-co-vinyl acetate) (PEVA)/poly(n-butyl methacrylate) (PBMA)/polyvinylpyrrolidone K30 (K30) layer containing various concentrations of Photofrin were then incorporated onto the stent as the second and third layers. After incubating the layered membranes with cultured CCA cell line, the release of Photofrin, cell viability, the intracellular uptake of Photofrin, reactive oxygen species (ROS) generation, and apoptosis were determined. Using a single-layer diffusion model, the maximum release of Photofrin from the 5 to 10% K30 formulas was 80 and 100%, respectively, after 24 h. When using the multiple-layer diffusion model, the released Photofrin showed an initial burst of the loading dose from the PEVA/PBMA/K30 layer. In the immobilized model, less than 5% of the Photofrin from the 2-HEMA/EGDMA/BPO layer was released over the 24-h period. Cell viability decreased linearly with increasing Photofrin concentrations, and ROS generation and apoptosis were shown to increase significantly with increasing Photofrin concentrations, until the concentration of Photofrin reached a saturation point of 1.5 μg/ml. This new, multiple-layered, PDT-based stent with dual-release mechanisms is a promising treatment for CCA and cancer-related ductal stenosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cholangiocarcinoma (CCA), most commonly arising at the bifurcation of the right and left bile ducts, is the second most common primary hepatic malignancy worldwide (Anderson et al. 2004). CCA is generally an unresectable and locally invasive tumor that occludes the biliary tree and leads to the development of cholangitis and liver failure once diagnosed. Surgery with complete resection is the only potential cure treatment for CCA. Unfortunately, curative resection is only achievable in less than 30% of patients (Jarnagin et al. 2001; Witzigmann et al. 2006). For inoperable patients with advanced CCA, who usually have biliary obstruction, the main purpose of therapy is to relieve jaundice and cholangitis, and to improve their quality of life by biliary duct stenting. However, this procedure only slightly improves the survival time of the patients. Chemotherapy using a combination of gemcitabine and cisplatin may be considered; however, in the ABC-02 trial, the benefit of this combination was not satisfactory(Valle et al. 2010). Photodynamic therapy (PDT) is a promising modality for treating CCA(Moole et al. 2017}. The principle of PDT is to administer a nontoxic photosensitizer that preferentially accumulates within the tumor and becomes cytotoxic when the tumor is directly exposed to a specific wavelength light. The advantages of PDT include its safety, its minimal side effects compared with radiotherapy or chemotherapy, and the feasibility of local treatment for diseases such as gastrointestinal cancer (Chahal and Baron 2006; Gao et al. 2010). PDT alone or in combination with biliary duct stenting or chemotherapy has been reported to be effective in the palliative treatment of biliary obstruction, with prolongation of the survival time of patients with CCA(Harewood et al. 2005; Witzigmann et al. 2006; Fuks et al. 2009). In a retrospective analysis of patients with advanced hilar CCA, PDT extended not only the median survival but also the stent patency period (Cheon et al. 2012). Skin photosensitivity is the main side effect of PDT (Harrod-Kim 2006), and a drawback of PDT is that patients have to spend several days in subdued lighting following treatment to prevent complications from skin necrosis. It is essential to develop a new drug-eluting stent (DES) with PDT that exerts anti-tumor effects with fewer side effects.
The use of metallic stents has significantly reduced the need for restenting and has proven to be cost effective compared with surgical bypass in treating CCA patients(Martin et al. 2002). Conventional stents work as simple endoluminal scaffolds, without anti-tumor effects. However, ingrowing tumors can eventually infiltrate the lumen and again occlude the bile duct. Treatments for unresectable CCA using PDT rely on systemic injection of a photosensitizer that circulates throughout the entire body, with little control over the concentration in the tumor. Local, rather than systemic, cancer treatment could be a more reasonable therapy and could decrease systemic side effects during treatment for unresectable CCA. To decrease the side effects during PDT and to provide additional anti-cancer therapy via a metallic stent, we created a novel PDT-matrix drug delivery system (PDT-matrix DDS) by coating a photosensitizer on the surface of a newly designed metallic stent to combine PDT and palliative stenting (Fig. 1). This PDT-matrix DDS stent could be deployed in an obstructed bile duct via the endoscopic or percutaneous transhepatic route to relieve bile duct obstruction. In addition, the photosensitizer coated on the stent could be released and subsequently activated by a light source at a specific wavelength using endoscopy to treat the CCA. The PDT-matrix DDS was prepared by mixing Photofrin with poly(ethylene-co-vinyl acetate) (PEVA), poly(n-butyl methacrylate) (PBMA), and 2-hydroxyethyl methacrylate (2-HEMA) to create a multiple-layered PDT-based stent with dual-release mechanisms. This PDT-matrix DDS-based stent could release the photosensitizer in a controlled and local manner into CCA, without systemic exposure, providing both local cancer therapy without the systemic side effects of PDT and prolonged stent patency. In this study, the Photofrin release profiles, the cytotoxicity of the release of Photofrin during PDT, and the anti-cancer efficacy against the TFK-1 human CCA cell line were evaluated.
Photofrin-eluting stent. a Schematic illustration of a Photofrin-loaded cover stent. b Photograph of a cover stent. The bare stent was 10 mm wide and up to 95 mm long (upper). The bare stent was coated with PU as the primary membrane (middle). The second layer consisted of 2-HEMA/EGDMA/BPO (1%:0.5%), with 5 μg/ml Photofrin coated on the PU layer. The third layer consisted of PEVA/PBMA (67%:33%), with 10% K30 and 5 mg/ml Photofrin coated on the 2-HEMA layer (lower). Abbreviations: PU, polyurethane; PEVA, poly(ethylene-co-vinyl acetate); PBMA, poly(n-butyl methacrylate); K30, polyvinylpyrrolidone K30
2 Materials and methods
2.1 Materials
Polyurethane (PU), 2-HEMA, ethylene glycol dimethacrylate (EGDMA), benzoyl peroxide (BPO), PEVA, PBMA, polyvinylpyrrolidone K30 (K30), and tetrahydrofuran (THF) were purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI 1640 was purchased from Invitrogen (Carlsbad, CA, USA). Photofrin was purchased from Axcan Pharma (Quebec, Canada).
2.2 Preparation of the diffusion layer
The PDT-matrix DDS was prepared by mixing the photosensitizer with biodegradable polymer matrixes. A Photofrin-eluting membrane was then prepared using the dip-coating method. PEVA and PBMA were dissolved in THF at a ratio of 67%:33% to make a 0.5% matrix solution, which served as the dipping solution. Additional entities, such as different ratios of Photofrin and K30, were added to the dipping solution, which was mixed thoroughly to obtain a homogeneous solution. The concentration of Photofrin in the polymer solution was 50, 75, or 100 μg/ml, and the concentration of K30 in the polymer solution was 5% or 10%.
2.3 Preparation of the immobilized layer
In the immobilized model, Photofrin was immobilized in a hydrogel matrix through copolymerization with an acrylic monomer (2-HEMA). A hydrogel matrix through free radical copolymerization with an acrylic monomer (2-HEMA). Photofrin immobilized model was polymerized from 2-HEMA and Photofrin via Van Der Waals’ force. EGDMA and BPO were then added to the 2-HEMA solution at a ratio of 1%:0.5%, 0.7%:0.8%, or 0.5%:1%. After thoroughly mixing the materials, the coated membranes were heated at 70°C for 2 h to polymerize the 2-HEMA monomers with the Photofrin. Preparation of the 2.4 Photofrin-eluting stent.
Photofrin-eluting membranes were fabricated using the previously described dip-coating method (Lee et al. 2012). PU, 2-HEMA/EGDMA/BPO/Photofrin and PEVA/PBMA/K30/Photofrin were separately dissolved in THF and then stirred to obtain homogenous dipping solutions. A bare Ni-Ti stent (a generous gift from Prof. Fuh-Yu Chang) was fixed on a Teflon bar, and then dipped into the PU solution, withdrawn, and air dried. The PU membrane was used as the primary layer to be coated on the stent. The PU-coated stent was then submerged in the immobilized-layer solution, withdrawn, and heated at 70°C for 2 h. The outer layer was formed by coating the stent with the PEVA/PBMA/K30/Photofrin dipping solution, and all final products were air dried for over 3 h. The air-dried membranes were then carefully peeled from the Teflon bar and further evaluated.
2.4 In vitro release of Photofrin
To investigate the release rate of Photofrin, the membranes coated with 2-HEMA, PEVA, PBMA and K30 were placed in phosphate-buffered saline (PBS) solution at pH 7.2 for 1 h to 4 weeks. The Photofrin stents were placed individually in the capped polypropylene tubes with 10 ml PBS at pH 7.2 and incubated at 37°C with constant shaking at 75 Hz in a water bath. At every time point post-drug releasing, 500 μl supernatant was extracted carefully from the tube after the stent suspension was centrifuged at 6500 rpm for 15 min. The Photofrin concentration in each tube was measured with the same UV/VIS spectrometer as used in the standard preparation. After each analysis, the supernatant solution was put back to the releasing tube in order to maintain the releasing medium at the same volume. The amount of Photofrin released from the membranes was then measured using a UV-Vis spectrophotometer at 395 nm (Quant, BioTek, Winooski, VT, USA)(Leunig et al. 1993; Moon et al. 2011; Yoo et al. 2012).
2.5 PDT
The human extrahepatic bile duct cancer cell line TFK-1 (RCB2537, a human cell line derived from an extrahepatic bile duct carcinoma from Tohoku University; RIKEN Cell Bank, Japan) was maintained in RPMI 1640 medium (GIBCO-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 mg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. Cells growing in 6-well plates were co-incubated with Photofrin-containing matrix membranes in serum-free medium in the dark, and after 24 h of incubation, the cells were rinsed with PBS. PDT was then performed with a light-emitting diode (LED) light source at 630 nm. For the subsequent experiments, the cells were irradiated at a fluence rate of 13.6 mW/cm2. Cells in the control group were incubated in the same medium but without Photofrin.
2.6 Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Kumamoto, Japan) was used to measure the cytotoxicity of TFK-1 cells. The CCK-8 assay was used to measure cytotoxicity under starved conditions, which are based on the conversion of a water-soluble tetrazolium salt, 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H–tetrazolium, monosodium salt, to a water-soluble formazan dye upon reduction by dehydrogenases in the presence of an electron carrier. TFK-1 cells were seeded into 96-well microplates (1 × 104 cells per well), incubated at 37°C, and then treated with serum-free medium containing various concentrations of Photofrin-coated membranes for 24 h. Afterward, the cells were irradiated at 0–8 J/cm2 at 630 nm. After PDT administration, the medium was refreshed with complete medium, and cell viability was assessed using a Cell Counting Kit-8. CCK-8 solution (10 μl) was added to each well, followed by incubation for 2 h at 37°C. The absorbance at 450 nm was determined by a multiplate reader (Lambda Bio-20; Beckman). Cell viability was expressed as a percentage of that of the control (untreated) cells.
2.7 Measurement of intracellular reactive oxygen species (ROS)
TFK-1 cells were cultured in a 6-well microplate at a density of 1 × 104 cells per well for 24 h at 37°C. Cellular ROS generation was then determined using a Cellular Reactive Oxygen Species Detection Assay Kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions.
2.8 Cellular photosensitizer uptake assay
Cells were cultured in a 12-well microplate at a density of 1 × 105 cells per well for 24 h and then co-incubated with various concentrations of Photofrin, which were coated on matrix membranes, for 24 h at 37°C in the dark. The cells were harvested and then washed with and resuspended in PBS. Aliquots were retained from each sample to perform protein assays using the Bio-Rad method. The photosensitizer was extracted from the cell pellets using methanol and vigorous vortex mixing. The total levels of photosensitizer in the TFK-1 cells were measured using fluorescence spectroscopy (PerkinElmer, USA), with an excitation wavelength of 395 nm, an emission wavelength of 633 nm, and extracted cells without added photosensitizer as a blank.
2.9 Apoptosis assay
Cells were cultured in a 6-well microplate at a density of 1 × 104 cells per well for 24 h at 37°C and then co-incubated with various concentrations of Photofrin, which were coated on matrix membranes, for 24 h at 37°C in the dark. After exposing the TFK-1 cells to PDT at a rate of 5 J/cm2 for 24 h, they were treated with trypsin-EDTA, and the cell lysates were collected by centrifugation at 1000 rpm for 5 min at 4°C, followed by incubation with 10 μl of annexin V and 5 μl of propidium iodine (PI) in binding buffer at room temperature for 15 min. Apoptosis assays were then performed using a MEBCYTO Apoptosis Kit (MBL, Nagoya, Japan) according to the manufacturer’s instructions. Apoptotic cells were assayed based on annexin V and PI staining and detected using an LSR II flow cytometer (BD Biosciences, San Diego, CA, USA). The data were analyzed using FACSDiva software (BD Biosciences, San Diego, CA, USA).
2.10 Statistical analysis
The results are expressed as the means ± standard deviations. Comparative studies of means were performed using t-test analysis. Significance was accepted at p < 0.05.
3 Results
3.1 Photofrin release profile of the PDT-matrix DDS
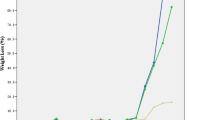
The release profiles of Photofrin from the PEVA/PBMA (67%:33%) matrix as well as from matrixes with different concentrations of Photofrin at different percentages of K30 were determined. After 24 h of in vitro incubation, the maximum release of Photofrin from a matrix containing 5% K30 or 10% K30 was 80% and 100%, respectively (Fig. 2). The addition of K30 accelerated the release of Photofrin from the polymeric matrix. In addition, the Photofrin release profile showed an initial burst of approximately 60% of the Photofrin within 1 h and a continual release to a maximum of 80% of the Photofrin after 24 h of incubation (Fig. 2).
Establishment of the in vitro Photofrin release profile of a polymeric matrix modified with various concentrations of Photofrin. a Release profile of PEVA/PBMA with 5% K30 or 10% K30 and 50 μg/ml Photofrin. b Release profile of PEVA/PBMA with 5% K30 or 10% K30 and 100 μg/ml Photofrin. c Release profile of PEVA/PBMA with or without K30 and with 10 μg/ml Photofrin over a 4-weeks period. The data represent the means ± SEMs (n = 3–4). Abbreviations: PEVA, poly(ethylene-co-vinyl acetate); PBMA, poly(n-butyl methacrylate); K30, polyvinylpyrrolidone K30
3.2 Slow Photofrin release profile of the polymeric matrix
The release profiles of Photofrin from 2-HEMA were then investigated. By controlling the ratio of the EGDMA/BPO mixture, the release profiles of Photofrin in this model (with different EGDMA/BPO ratios in the 2-HEMA solution) were established (Fig. 3). After 4 weeks of in vitro incubation, the observed maximum release rate was 13% in 1%:0.5% EGDMA/BPO, which demonstrated that Photofrin could be slowly released by the p-HEMA matrix. This model provides a drug-loading pattern with a slow-release rate that is suitable for long-term treatment.
Establishment of the in vitro Photofrin release profile of the model consisting of a polymeric hydrogel modified with immobilized Photofrin. The stability of Photofrin immobilized by 2-HEMA, with different EGDMA/BPO ratios, including 1%:0.5%, 0.7%:0.8%, and 0.5%:1%, is shown. The total amounts released during in vitro incubation were less than 5% after 24 h and less than 14% after 4 weeks. The data represent the means ± SEMs (n = 3). Abbreviations: 2-HEMA, 2-hydroxyethyl methacrylate; EGDMA, ethylene glycol dimethacrylate; BPO, benzoyl peroxide
3.3 Phototoxicity of PDT and intracellular Photofrin levels in TFK-1 cells
To investigate the phototoxicity of Photofrin-PDT, various doses of PDT and various concentrations of Photofrin were used to treat TFK-1 cells for 24 h. The release profiles for the various concentrations of Photofrin in the PEVA/PBMA/10% K30 membranes were then measured in the culture medium. We observed that 80% of Photofrin was released after 24 h (Fig. 4a), and this result was similar to those from our previous kinetics studies. The increased cytotoxicity in TFK-1 cells corresponded with increasing doses of PDT and increasing concentrations of Photofrin (Fig. 4b). The observed cell survival rates were 3–79%, and the LD50 values were approximately 6 μg/ml, 3 μg/ml, and 1.2 μg/ml Photofrin at 2 J/cm2, 5 J/cm2 and 8 J/cm2, respectively. The intracellular Photofrin levels were then assessed by determining the fluorescence intensities, which were expressed as the percentage of the Photofrin levels measured in cells incubated with or without Photofrin-containing matrix membranes (Fig. 4c). These results demonstrated that higher doses of PDT and higher concentrations of Photofrin are related to increases in cell damage and intracellular Photofrin levels. In addition, the Photofrin levels in TFK-1 cells can be increased by increasing the amount of Photofrin that is coated on the membranes.
Relationships between various concentrations of Photofrin and phototoxicity and intracellular Photofrin levels in TFK-1 cells. a In vitro release profiles of Photofrin in culture medium, showing the percentage of the initial coated concentration of Photofrin. b Cell viability was detected by CCK-8 assays after various doses of PDT. c Quantitative analysis of the relative fluorescence intensity of cell extracts, expressed as the percentage of levels measured in cells with or without the Photofrin-containing matrix. All of the values indicate the mean fluorescence intensity. Untreated cells were used as a control. The data represent the means ± SEMs (n = 4–5, *p < 0.05). Abbreviations: PDT, photodynamic therapy; CCK-8, Cell Counting Kit-8
3.4 PDT-induced endogenous ROS in TFK-1 cells
To investigate the relationship between cell viability and intracellular ROS levels, TFK-1 cells were pre-incubated with Photofrin-coated membranes for 24 h, and the ROS generated in the cells after 5 J/cm2 PDT were measured using dichloro-dihydro-fluorescein diacetate (DCFH-DA), a fluorogenic probe designed to reliably measure ROS in live cells, and a microplate reader. The amounts of ROS generated in TFK-1 cells treated with Photofrin-coated membranes are summarized in Fig. 5a. The total amount of intracellular ROS in cells treated with higher concentrations of Photofrin (such as 12 μg/ml) increased in proportion to the Photofrin concentration (Fig. 5a).
Effect of Photofrin on ROS production and apoptosis in TFK-1 cells during Photofrin-PDT. a Intracellular ROS levels assessed with DCFH-DA; untreated cells were used as a control. The mean fluorescence intensity is expressed as the percentage of levels measured in untreated cells. b Percentage of apoptotic cells induced by Photofrin-PDT (5 J/cm2). The data represent the means ± SEMs (n = 3–5, **p < 0.01, ***p < 0.001). Abbreviations: ROS, reactive oxygen species; PDT, photodynamic therapy; DCFH-DA: 2,7-dichloro-dihydro-fluorescein diacetate
3.5 PDT-induced TFK-1 cell apoptosis
To analyze TFK-1 cell death after treatment with Photofrin-coated membranes and PDT, the TFK-1 cells were first pre-incubated with Photofrin-coated membranes for 24 h. After PDT (5 J/cm2), the number of apoptotic cells was determined by annexin V staining and flow cytometry. The number of apoptotic cells gradually increased with increasing Photofrin concentrations (maximum of 1.5 μg/ml), and over 20% cell death was observed at 12 μg/ml Photofrin (Fig. 5b). These results indicated that the percentage of apoptotic cells increased in a Photofrin concentration-dependent manner after PDT via co-incubation with Photofrin-coated membranes.
3.6 Establishment of a prototype of a multi-layered, drug-eluting cover stent
A schematic illustration of the Photofrin-loaded DDS is shown in Fig. 5a. A prototype cover stent was prepared by dipping the metallic stent in PU solution, in a 2-HEMA/EGDMA/BPO/Photofrin matrix solution, and finally, in a PEVA/PBMA/K30/Photofrin matrix solution (Fig. 1b). The results demonstrated that PU, the 2-HEMA/EGDMA/BPO/Photofrin matrix and the PEVA/PBMA/K30/Photofrin matrix could be coated on the stent. Therefore, the preparation of a prototypical multi-layered cover stent coated with PU-PEVA/PBMA was established in this study.
4 Discussion
Most patients with extrahepatic CCA are diagnosed in the advanced stages, when surgical resection is not feasible. In view of the poor prognosis of these patients, palliative treatment by endoscopic or percutaneous biliary stenting is the traditional management. However, the stent can become blocked over time due to tumor ingrowth, and even with a stent, the prognosis is still dismal. PDT and metallic stent placement are the promising approaches for prolonging the life of CCA patients (Singh and Patel 2006). An uncoated metallic stent offers simple mechanical support without an anti-tumor effect. Chahal P. et al. suggested that PDT with stenting could hold promise for managing locally unresectable CCA (Chahal and Baron 2006). DESs for gastrointestinal cancer have recently been investigated (Kim et al. 2009; Lee et al. 2012), and it has been found that the physicochemical and compositional properties of the drug loading and of the releasing film critically affect the release of the drug(Kim et al. 2009). Yoo J.J. et al. have developed a 5-aminolevulinic acid-poly(vinyl alcohol) nanofiber-coated metallic stent for potential therapeutic use in CCA(Yoo et al. 2012). In the present study, our Photofrin-releasing membrane had a triple-layered structure composed of PU as the backing layer, a Photofrin-releasing 2-HEMA/EGDMA/BPO layer for sustained release of Photofrin, and a Photofrin-releasing PEVA/PBMA/K30 layer (Fig. 1). The PU layer provides a physical foundation and acts as a barrier to minimize bile duct re-obstruction. The second layer, composed of 2-HEMA/EGDMA/BPO, provides a homogeneous and sustained release pattern for multiple rounds of further PDT. Finally, the third layer, composed of PEVA/PBMA/K30, is the major drug-loading layer for initial PDT. Previous studies demonstrated that PDT is an important palliative option for patients with unresectable extrahepatic CCA(Ortner 2009) and that long-term therapy via multiple PDT procedures is feasible and safe for patients with unresectable extrahepatic CCA(Hoblinger et al. 2011). Local administration of a photosensitizer combined with a metallic stent could be a more feasible treatment option compared with systemic administration for the treatment of unresectable extrahepatic CCA with bile duct obstruction; in particular, local treatment could provide better accumulation and a more favorable biodistribution of the photosensitizer with fewer systemic side effects (Tomizawa and Tian 2012; Yoo et al. 2012). In the present study, we evaluated the feasibility of using an eluting matrix membrane with PDT using TFK-1 cells (a human CCA cell line). The release profiles of our designs showed that at higher concentrations of Photofrin and higher percentages of K30, Photofrin release increased proportionally. The release rate reached approximately 80% after 24 h of incubation (Figs. 2a–c), which was similar to that of other DESs(Moon et al. 2011; Lee et al. 2012). In addition, we found that increasing the percentage of K30 could increase Photofrin release. A previous study indicated that long-term PDT is beneficial and safe for treating CCA(Hoblinger et al. 2011); therefore, we designed the HEMA/EGDMA/BPO/Photofrin matrix to provide long-term Photofrin release (Fig. 3). We also demonstrated that Photofrin-PDT had a strong impact on cell viability. The cytotoxicity caused by our PDT-matrix DDS correlated positively with the concentration of Photofrin and the dose of PDT (Fig. 4b), and in the absence of light irradiation, increased intracellular Photofrin levels were observed for the Photofrin-containing matrix membranes (Fig. 4c). A previous study has shown that PDT could induce cell apoptosis(Castano et al. 2006); therefore, we used annexin V-FITC and PI staining to determine the number of apoptotic TFK-1 cells, including those in the early and late apoptosis stages. We demonstrated that Photofrin from Photofrin-eluting membranes combined with PDT could effectively induce an apoptotic response in TFK-1 cells and that the percentages of apoptotic cells increased in a Photofrin concentration-dependent manner after PDT via co-incubation with Photofrin-coated membranes (Fig. 5b).
Currently, PDT is a palliative strategy for unresectable CCA(Gerhards et al. 2001; Cheng et al. 2002; Yoo et al. 2012). PDT has been shown to improve the quality of life, biliary drainage, and survival of patients with CCA(Gerhards et al. 2001; Zoepf et al. 2001; Baron 2008; Cheon et al. 2012; Tomizawa and Tian 2012), and early PDT after diagnosis and multiple rounds of PDT have been shown to improve survival benefits (Cheon et al. 2012). Our Photofrin-releasing membrane has a triple-layered structure composed of PU as the backing layer, a Photofrin-releasing 2-HEMA/EGDMA/BPO layer for sustained release of Photofrin, and a Photofrin-releasing PEVA/PBMA/K30 layer. Our newly designed metallic stent, which includes a novel PDT-matrix DDS, provides an opportunity of continuous relief from malignant obstruction and constant local tumor control via multiple sessions of PDT.
5 Conclusion
The combination of a matrix for PDT that has a dual-release profile and that covers a metallic stent could be a good option for treating unresectable CCA and cancer-related biliary tract obstruction. Further studies determining the effect of the combination of the PDT-matrix DDS and chemotherapeutic drugs on patient survival are worthwhile. This new hybrid treatment method might become a new standard of care for CCA.
Abbreviations
- 2-HEMA:
-
2-hydroxyethyl methacrylate
- BPO:
-
Benzoyl peroxide
- CCA:
-
Cholangiocarcinoma
- DDS:
-
Drug delivery system
- DES:
-
Drug-eluting stent
- EGDMA:
-
Ethylene glycol dimethacrylate
- K30:
-
Polyvinylpyrrolidone K30
- PBMA:
-
Poly(n-butyl methacrylate)
- PDT:
-
Photodynamic therapy
- PEVA:
-
Poly(ethylene-co-vinyl acetate)
- ROS:
-
Reactive oxygen species
References
C.D. Anderson, C.W. Pinson, J. Berlin, R.S. Chari, Oncologist 9, 43–57 (2004)
T.H. Baron, Clin. Gastroenterol. Hepatol. 6, 266–267 (2008)
A.P. Castano, P. Mroz, M.R. Hamblin, Nat. Rev. Cancer 6, 535–545 (2006)
P. Chahal, T.H. Baron, Curr. Opin. Gastroenterol. 22, 551–560 (2006)
J.L. Cheng, M.J. Bruno, J.J. Bergman, E.A. Rauws, G.N. Tytgat, K. Huibregtse, Gastrointest. Endosc. 56, 33–39 (2002)
Y.K. Cheon, T.Y. Lee, S.M. Lee, J.Y. Yoon, C.S. Shim, HPB (Oxford) 14, 185–193 (2012)
D. Fuks, E. Bartoli, R. Delcenserie, T. Yzet, P. Celice, C. Sabbagh, D. Chatelain, J.P. Joly, N. Cheron, J.L. Dupas, J.M. Regimbeau, J. Gastroenterol. Hepatol. 24, 1745–1752 (2009)
F. Gao, Y. Bai, S.R. Ma, F. Liu, Z.S. Li, J. Hepatobiliary Pancreat. Sci. 17, 125–131 (2010)
M.F. Gerhards, D. den Hartog, E.A. Rauws, T.M. van Gulik, D. Gonzalez Gonzalez, J.S. Lameris, L.T. de Wit, D.J. Gouma, Eur. J. Surg. 167, 274–280 (2001)
G.C. Harewood, T.H. Baron, A. Rumalla, K.K. Wang, G.J. Gores, L.M. Stadheim, P.C. de Groen, J. Gastroenterol. Hepatol. 20, 415–420 (2005)
P. Harrod-Kim, J. Vasc. Interv. Radiol. 17, 1441–1448 (2006)
A. Hoblinger, T. Gerhardt, M.A. Gonzalez-Carmona, R. Huneburg, T. Sauerbruch, V. Schmitz, Eur. J. Med. Res. 16, 391–395 (2011)
W.R. Jarnagin, Y. Fong, R.P. DeMatteo, M. Gonen, E.C. Burke, B.J. Bodniewicz, B.M. Youssef, D. Klimstra, L.H. Blumgart, Ann. Surg. 234, 507–517; discussion 517–509 (2001)
T.G. Kim, H. Lee, Y. Jang, T.G. Park, Biomacromolecules 10, 1532–1539 (2009)
J.W. Lee, S.G. Yang, K. Na, Int. J. Pharm. 427, 276–283 (2012)
A. Leunig, F. Staub, J. Peters, A. Heimann, O. Kempski, A.E. Goetz, Res. Exp. Med. (Berl) 193, 361–370 (1993)
R.C. Martin 2nd, G.C. Vitale, D.N. Reed, G.M. Larson, M.J. Edwards, K.M. McMasters, Surg. Endosc. 16, 667–670 (2002)
H. Moole, H. Tathireddy, S. Dharmapuri, V. Moole, R. Boddireddy, Y. Yedama, S. Dharmapuri, A. Uppu, N. Bondalapati, A. Duvvuri, World J. Gastroenterol. 23(7), 1278–1288 (2017)
S. Moon, S.G. Yang, K. Na, Biomaterials 32, 3603–3610 (2011)
M.A. Ortner, Curr. Opin. Gastroenterol. 25, 472–476 (2009)
P. Singh, T. Patel, Curr. Opin. Gastroenterol. 22, 294–299 (2006)
Y. Tomizawa, J. Tian, Dig. Dis. Sci. 57, 274–283 (2012)
J. Valle, H. Wasan, D.H. Palmer, D. Cunningham, A. Anthoney, A. Maraveyas, S. Madhusudan, T. Iveson, S. Hughes, S.P. Pereira, M. Roughton, J. Bridgewater, N. Engl. J. Med. 362, 1273–1281 (2010)
H. Witzigmann, F. Berr, U. Ringel, K. Caca, D. Uhlmann, K. Schoppmeyer, A. Tannapfel, C. Wittekind, J. Mossner, J. Hauss, M. Wiedmann, Ann. Surg. 244, 230–239 (2006)
J.J. Yoo, C. Kim, C.W. Chung, Y.I. Jeong, D.H. Kang, Int. J. Nanomedicine 7, 1997–2005 (2012)
T. Zoepf, R. Jakobs, J.C. Arnold, D. Apel, A. Rosenbaum, J.F. Riemann, Am. J. Gastroenterol. 96, 2093–2097 (2001)
Acknowledgements
This study was supported by the National Research Program for Biopharmaceuticals (NRPB), Republic of China (R.O.C.), Taiwan (Project No. 100INP015-1 and 100INP015-2). The stent used in this study was provided by Professor Fuh-Yu Chang, Department of Mechanical Engineering, National Taiwan University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Rights and permissions
About this article
Cite this article
Liang, PC., Huang, KW., Tung, CC. et al. A novel photodynamic therapy-based drug delivery system layered on a stent for treating cholangiocarcinoma. Biomed Microdevices 20, 3 (2018). https://doi.org/10.1007/s10544-017-0249-1
Published:
DOI: https://doi.org/10.1007/s10544-017-0249-1