Abstract
Zebrafish is a model organism for various sensory-motor biological studies. Rheotaxis, or the ability of zebrafish to orient and swim against the water stream, is a common behavior that involves multiple sensory-motor processes such as their lateral line and visual systems. Due to the lack of a controllable and easy-to-use assay, zebrafish rheotaxis at larval stages is not well-understood. In this paper, we report a microfluidic device that can be used to apply the flow stimulus precisely and repeatedly along the longitudinal axis of individual zebrafish larvae to study their coaxial rheotaxis. We quantified rheotaxis in terms of the response rate and location along the channel at various flow velocities (9.5–38 mm.sec−1). The larvae effectively exhibited a similarly high rheotactic response at low and medium velocities (9.5 and 19 mm.sec−1); however, at high velocity of 38 mm.sec−1, despite sensing the flow, their rheotactic response decreased significantly. The flow velocity also affected the response location along the channel. At 9.5 mm.sec−1, responses were distributed evenly along the channel length while, at 19 and 38 mm.sec−1, the larvae demonstrated higher rheotaxis responses at the anterior and posterior ends of the channel, respectively. This result shows that although the response is similarly high at low and medium flow velocities, zebrafish larvae become more sensitive to the flow at medium velocity, demonstrating a modulated rheotactic behavior. Employing our device, further investigations can be conducted to study the sensory-motor systems involved in rheotaxis of zebrafish larvae and other fish species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zebrafish (Danio rerio) is an emerging model organism in biological studies for various applications such as drug discovery (Calum and MacRae 2015), neural circuit investigations (Issa et al. 2011), and behavioral assays (Kalueff and Cachat 2010). Zebrafish larva’s high genetic homology to humans, small size, transparency, and rapid embryonic development are among the most important advantages of zebrafish as a model organism (Basu and Sachidanandan 2013). Moreover, at larval stage, their behavioral functionalities such as escape (Olszewski et al. 2012), avoidance (Pelkowski et al. 2011), turning (Huang et al. 2013; Olive et al. 2016; Suli et al. 2012), and overall locomotor behaviors (Budick and O’Malley 2000; Colwill and Creton 2011) are fully evolved (Higashijima 2008; McLean and Fetcho 2004), providing a platform in which the biological pathways involved in sensing environmental cues and responding to them can be investigated.
One of the most common and important behaviors among many of the aquatic species is rheotaxis. It is defined as animals’ ability to sense the fluid flow surrounding their bodies and to respond to it spontaneously by turning and swimming against the stream (Olive et al. 2016). Zebrafish larvae have been shown to demonstrate rheotaxis for avoiding the predators attack, holding their position during a flow strike, and migrating upstream in the rivers and oceans (Arnold 1974; Mchenry et al. 2009; Olive et al. 2016; Olszewski et al. 2012; Suli et al. 2012). Using relatively complicated experimental setups to control the stimulating flow, it has been shown that zebrafish larvae utilize several sensory systems such as the lateral line, visual and vestibular systems to respond to the mechanical stimulation exerted by the water current (Olive et al. 2016; Suli et al. 2012).
Existing rheotaxis screening setups can be divided into two major configurations based on their flow stimulation modalities, i.e. (i) using a fluid suction source-point to generate a radially inward flow in a tank (Olive et al. 2016; Olszewski et al. 2012) and (ii) exposing the larvae to streamlined flow along the axis of a chamber (Mchenry et al. 2009; Suli et al. 2012). Some challenges associated with these setups are the large fluid velocity variations between the tested larvae depending on their radial location on the platform, lack of control over flow direction with respect to larvae’s initial orientation, and involvement of multiple stimuli such as flow and visual cues (e.g. flow generation source). Moreover, current studies have mostly focused on group response investigations while the behavior of individual zebrafish larva differs from their group-based responses (De Paiva et al. 2012). Technologies to address the above-mentioned challenges are needed in order to enable systematic investigation of individual larva’s rheotaxis and its biological basis.
Microfluidic platforms have enhanced our ability to perform controlled, quantitative and high throughput biological assays on model organisms including the zebrafish larvae (Gupta and Rezai 2016; Yang et al. 2016). These technologies have been employed to investigate the effects of many stimuli such as chemical (Candelier et al. 2015; Nady et al. 2017), mechanical (Ahmad et al. 2012), electrical (Peimani et al. 2017) and optical (Monesson-Olson et al. 2014) stimulations on the zebrafish larvae. In this paper, we have employed microfluidics to develop a simple, effective, and efficient device to study individual zebrafish larvae’s rheotaxis. Our device provides several advantages over the conventional methods by allowing placement of a single semi-confined larva along the axis of a channel, application of a streamlined and repeatable flow with a constant average velocity and direction axially towards the larva in the channel, and quantification of their rheotactic response in a simple manner. Altogether, this technology can be used in the future to interrogate the biological pathways involved in rheotaxis of zebrafish larvae and other aquatic species with improved control over stimulus and accuracy in behavioral quantification.
2 Methods
2.1 Zebrafish preparation
Zebrafish (Danio rerio) of the Tupfel long fin (TL) strain were kept at 28°C on a 12 h light-dark cycle at their adult stage in a recirculation system (Aquaneering, CA, USA). They were fed ad libitum with brine shrimps (Brine Shrimp Direct, Odgen, Utah, USA) twice a day. Animal husbandry and breeding was carried out according to the guidelines of the Canadian Council for Animal Care (CCAC) after approval of the ACC protocol (GZ 2014–19 (R3)). After collection of the eggs, the embryos were raised under 28° condition in egg water, i.e. 60 mg.l−1 of instant ocean sea salt (Instant Ocean, Blacksburg, VA, USA), containing 0.1% methylene blue (M291–100 Fisher Scientific, CA). All experiments including zebrafish larvae were kept to a minimum following guidelines approved by York University’s Biosafety Committee (PR Biosafety Permit 02–19).
2.2 Microfluidic device
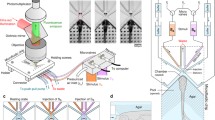
Our experimental setup consisted of a microfluidic device (Fig. 1), two syringe pumps (LEGATO 111 and 110, KD Scientific Inc., MA, USA), and an inverted microscope (BIM-500FLD, Bioimager Inc., Canada) with a camera (GS3-U3-23S6M-C, Point Grey Research Inc., Canada) that was connected to a computer. The microfluidic device was used to study the rheotaxis of zebrafish larvae. The syringe pumps were used to apply the desired water flow rates for loading and flow stimulation of the larvae. The microscope and camera were used for recording the larvae’s rheotactic behavior in the device.
The microfluidic device used to study the rheotaxis of 5–7 days post fertilization (dpf) zebrafish larvae. It consisted of a tilted inlet tube for loading the larva, a U-shaped expanding channel for retaining the larva in the device, and a main channel for rheotaxis studies. Water flow in the side channel helped conveniently loading the zebrafish larva into the main channel with length, width, and height of 63.3 mm, 1.6 mm, and 0.55 mm, respectively. The main channel was divided into three sections 1, 2, and 3, representing the spatial locations at which the larvae responded to the flow at different flow velocities. The setup included two syringe pumps, a microscope connected to a camera, and the microfluidic device
To fabricate the device in Fig. 1, de-bubbled 10 to 1 ratio base to reagent polydimethylsiloxane or PDMS pre-polymer (Sylgard 184 kit from Dow Corning, MI, USA) was casted on a 3D–printed master mold with a negative replica design of the device. Subsequently, PDMS was cured at room temperature for 24 h, peeled off the master and oxygen-plasma bonded to a flat glass slide. The device consisted of a 45° angle inlet tube for smooth and convenient loading of larvae; a side channel (0.1 mm wide, 0.2 mm deep, and 26 mm long) to assist the loading process by precluding the larvae from colliding with the base and walls of the channel; a main channel (1.6 mm wide, 0.55 mm deep, and 63.3 mm long) for zebrafish rheotaxis assays; and a U-shaped channel narrowing from 1.6 to 0.9 mm that acted as a fluidic valve to keep the larva inside the device. To assess larvae’s sensitivity to flow, we tracked the location of the rheotactic response by dividing the main channel into three equal-length sections 1 (initial position), 2 (mid-channel), and 3 (posterior) as shown in Fig. 1.
2.3 Experimental procedure and rheotaxis quantification
Zebrafish larvae at 5–7 dpf were transferred individually into the main channel of the device using the two syringe pumps set to flow rates of 10 and 3 ml.min−1 at the angled and side channels, respectively. The syringe pumps were turned off once the larva reached section 1 of the main channel. Larvae were allowed to recover from the loading process for 60 s. After the exploration phase and when larvae turned towards the outlet tube, rheotaxis was evoked by flow velocity in the range of 9.5–38 mm.sec−1 injected from the angled inlet set to flow rates of 0.5–2 ml.min−1. These velocities encompassed conditions evoking rheotaxis before the larvae were hydrodynamically carried out of the device. The response of each larva was monitored and video recorded for 30 s. A response was defined as a full 180° rotation and reorientation in the channel against the flow. Videos were analyzed for determination of the rheotaxis rate and location in the main channel (i.e. within sections 1, 2 and 3 in Fig. 1). Finally, the tested larva was ejected through the outlet and the device was reused for experimental repeats.
2.4 Statistics
To satisfy statistical requirements, the control (no flow) and rheotaxis experiments at three velocity settings were repeated three times with a total of N = 34 zebrafish larvae per condition. The rheotaxis response results were presented in the form of the average response ± SD (Standard Deviation). For statistical analysis between each two data sets, two-tailed Student t-test was conducted with the assumption of unequal variances using the Microsoft Excel software (Microsoft Corp., WA, USA).
3 Results and discussions
The conventional techniques used to study a group of larvae’s rheotaxis inside open platforms are not able to elucidate the effect of the flow velocity and direction accurately, due to the complexity of controlling the fluid flow and larvae’s intricate responses to various flow conditions. For instance, it has been shown that rheotaxis occurs with a peak rate when the water stream is directed coaxially towards the tail of the zebrafish larvae parallel to their body axis (Olszewski et al. 2012). However, a number of questions remain unaddressed such as whether and how individual zebrafish larva respond to flow in enclosed and semi-confined environments with no flow source visual cues. Thus, in this paper we intended to answer these questions with a simple microfluidic device that was used to quantitatively study the rheotaxis response frequency and place preference, a parameter used in zebrafish chemical screening assays (Ek et al. 2016; Swain et al. 2004).
3.1 Rheotaxis of zebrafish larva in a channel
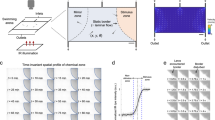
To provide a simple assay to study the coaxial rheotactic behavior of 5–7 dpf zebrafish larvae in an enclosed semi-confined environment, we developed the microfluidic device shown in Fig. 1. A semi-confined larva could be positioned along the main channel in this device and stimulated with a streamlined, controllable and constant-velocity water flow. The larvae were loaded individually into the main channel and positioned at section 1 of the channel (Fig. 1) with their heads facing the outlet. A 60 s recovery time with no flow in the device was provided so that the larvae became habituated to the device environment. During the recovery phase, the larvae were able to explore the device environment by conveniently turning in the main channel. Afterwards, they were stimulated by different tail-to-head flows and their positive rheotactic response (i.e. complete 180° orientation against the flow direction) was investigated at different locations in the channel. Control groups of larvae that were not exposed to any flow after loading into the device were also tested. For instance, once a larva was exposed to a 19 mm.sec−1 flow, it exhibited positive rheotaxis within 1.3 s of stimulation as shown in Fig. 2.
Bright field images of a 7 dpf zebrafish larva (a) before, (b–c) during, and (d) after rheotactic orientation upon stimulation by a water flow velocity of 19 mm.sec−1 in section 1 of the device. The flow direction is from right to the left of the pictures (tail-to-head). It is observed that the larva tends to display rheotaxis by swimming against the flow within 1.3 s
The U-bend design and the large length of the main channel in comparison with its small cross-section in our device enabled us to exclude any visual cues in the assay, stimulate the larvae with unidirectional flows, and apply the flow at a constant rate along the axis of the zebrafish in order to evoke and study a complete positive rheotaxis. Furthermore, the larvae were assayed individually, avoiding any potential group-based influence on their rheotaxis behavior. These experimental modalities are not achievable with conventional rheotaxis screening setups.
3.2 Effect of flow velocity on rheotaxis of zebrafish larva in a channel
It has been shown that the zebrafish larvae can sense the flow immediately within 15 ms of stimulation (Mchenry et al. 2009); however, we were interested in investigating the coaxial rheotactic response thoroughly from the sensing moment to full reorientation against the flow direction in a semi-confined channel. Moreover, we asked if the magnitude of the flow velocity affects zebrafish coaxial rheotaxis in a channel. Accordingly, we tested the effect of coaxial flow velocity in the range of 9.5–38 mm.sec−1 on the rheotactic response of 5–7 dpf zebrafish larvae as shown in Fig. 3. Any flow velocity lower than 9.5 mm.sec−1 appeared not to evoke a robust positive rheotactic response. Suli et al. (2012) hypothesized that this decrease in rheotaxis might be due to the undeveloped hair cells in zebrafish larvae’s superficial neuromasts. Velocities higher than 38 mm.sec−1 also failed to induce a robust rheotaxis in the channel, following the same behavioral observations in the literature that rheotaxis only occurs within a range of flow velocities (Olszewski et al. 2012). One possible explanation can be the dominant role of fluid shear that leads to ejection of the larva from the main channel before it demonstrates a positive rheotaxis response.
Positive rheotaxis of zebrafish larvae (N = 34 larvae per condition) inside an enclosed channel in response to average flow velocities of 9.5, 19, and 38 mm.sec−1. The control group was not exposed to any flow in the device and the reported response is for any arbitrary re-orientation. The results show no desire in the larvae for rotation and their preference to remain in the initially loaded orientation without any flow in the channel; however, once the flow velocities of 9.5 and 19 mm.sec−1 were applied, the rheotactic behavior increased significantly (***: two-tailed t-test, p-value < 0.001). At 38 mm.sec−1, the zebrafish larvae demonstrated a low rheotactic response which was not different from the random movement of control larvae (two-tailed t-test, p-value > 0.05)
As shown in Fig. 3, out of the 34 larvae tested at each flow velocity, 69 ± 10.3%, 68.4 ± 9.4% and 32.5 ± 8.0% were able to show a positive rheotactic response at 9.5, 19, and 38 mm.sec−1 velocities, respectively. The control animals that were not exposed to any flow preferred to stay in their initial orientation along the channel while exploring the surrounding environment and showing only a random turning of 19.40 ± 10.1%. This low rate of movement indicated that the responses to other stimuli (e.g. visual cues) were kept at a minimum and relatively constant range in our device. Flow velocities of 9.5 and 19 mm.sec−1 were effective and sufficiently strong to induce similar rheotactic responses that were statistically different from the random turning of the control larvae (two-tailed t-test, p-value < 0.001). However, at the higher flow velocity of 38 mm.sec−1, there was a significant drop in rheotactic response while no statistical difference was measured comparing to the control group (two-tailed t-test, p-value > 0.05). The reason for the low rheotactic response at 38 mm.sec−1 is that the flow velocity was sufficiently strong to eject the larva from the channel before it could reveal a positive rheotaxis.
Although the literature has shown that increasing the flow velocity results in linear enhancement of rheotaxis in a non-confined platform (Olive et al. 2016; Olszewski et al. 2012; Suli et al. 2012), our findings demonstrate that the rheotaxis response of zebrafish larvae first plateaus and then diminishes at high flow velocities in an enclosed channel. These differences may stem from variations in the type of assays (internal versus external flows), ranges of flow velocities tested, channel confinement to ensure coaxial exposure to flow, and the fact that we investigated a thorough rheotactic reorientation in the channel as opposed to the instantaneous turning against and adjustment along the flow direction. For instance, Olive et al. (2016) examined the rheotaxis of zebrafish larvae at a velocity range of ~230–685 mm.sec−1, while we studied the coaxial rheotaxis at much lower flow rates and demonstrated larvae’s sensitivity to approximately 25 times slower velocities.
3.3 Effect of flow velocity on rheotaxis location in the channel
Based on our observations, we became interested in investigating if different flow velocities in a coaxial rheotaxis assay would influence the larvae’s response location in the channel. Moreover, since the rheotaxis response was similarly high at 9.5 and 19 mm.sec−1, we hypothesized that the flow sensitivity of the larva could also be delineated in more detail by tracking the response location. For this, we determined the number of responses occurred in sections 1, 2, and 3 of the channel (Fig. 1) for the responding zebrafish larvae at flow velocities reported in the previous section. The results are shown in Fig. 4.
Spatial distribution of rheotactic response of zebrafish larvae (N = 14 larvae per condition) along the three sections of the main channel (1: anterior, 2: mid-channel, and 3: posterior location). The control larvae tended to randomly reorient mostly within section 1 of the device where they were initially loaded. At the lowest flow velocity of 9.5 mm.sec−1, the spatial distribution of response was uniform across the three sections, whereas at 19 mm.sec−1, a large portion of responses took place at section 1 immediately upon exposure to flow. This contends that the larvae become more sensitive to the flow once exposed to a medium flow velocity of 19 mm.sec−1 compared to 9.5 mm.sec−1. The response to 38 mm.sec−1 mostly occurred in section 3, which implies that the larvae failed to overcome the flow strength and were carried out of the device before they could appropriately respond
It is shown in Fig. 4 that for the control group of larvae, an arbitrary orientation at no flow was observed mostly in section 1 (initial loading position) of the channel with a response distribution of 83, 17, and 0% in sections 1, 2, and 3, respectively. When the larvae were exposed to the lowest flow velocity of 9.5 mm.sec−1, their rheotactic response was evenly distributed among the three sections (response in 1: 37%, 2: 30% and 3: 33%). However, application of a medium-level flow velocity of 19 mm.sec−1 led to responses mainly in sections 1 and 2 of the channel with a considerable drop at section 3 (response in 1: 53%, 2: 40% and 3: 7%). This demonstrated that the zebrafish larvae became more sensitive to the medium flow velocity and showed an early response in the channel despite having a similar rheotaxis rate to low-level flow (Fig. 3). At 38 mm.sec−1, most of the rheotaxis took place in section 3 showing that the larvae were predominately carried away by the flow without being able to successfully display rheotaxis in a timely manner (response in 1: 25%, 2: 25% and 3: 50%). This outcome also suggests that high flow velocity can postpone the rheotactic reaction by taking the larva away from its initial position alongside the channel, although the flow sensation was most often immediate. To expand our study in the future, we will extend the length of the channel and investigate the effect of the channel width on zebrafish larvae rheotaxis.
3.4 Viability of Zebrafish larvae after Rheotaxis assay in the channel
To assess if the zebrafish larvae could survive after microfluidic-based rheotaxis and if the device had any negative effect on the larvae, we assessed larvae’s health status after exposure to the device and flow stimulation. The assay included a set of flow-exposed larvae at the highest velocity of 38 mm.sec−1 compared to the zebrafish that were kept intact in water throughout the experiment (N = 15 per condition). After 4 days of post-experimental observation, more than 70% of the larvae in both groups survived the assay with no statistical significant discerning between them (two tailed t-test, p-value > 0.05). This implied that neither the device nor the utilized flow velocities cause any detrimental effect on the larvae.
4 Conclusions
We have developed a microfluidic device in which the coaxial rheotactic behavior of individual zebrafish larvae could be quantitatively examined in a simple, controllable, precise, and repeatable manner. The design of the channel enabled us to expose the larvae to constant-velocity and directionally consistent flows while eliminating the possibility of visual responses as opposed to the conventional rheotaxis screening setups. The results suggested that the larva fails to effectively display rheotaxis within the channel at high velocity of 38 mm.sec−1. Whereas, the larvae showed higher rate of coaxial rheotaxis at lower flow velocities of 9.5 and 19 mm.sec−1. The locations of rheotaxis occurrence along the channel was uniformly distributed between the three sections at the low velocity of 9.5 mm.sec−1. However, at the higher velocities of 19 and 38 mm.sec−1, the larvae tended to display rheotaxis at the initial loading position and further downstream of the channel, respectively. This finding states that despite the similar high rheotaxis rates, the larva reveals more sensitivity by responding to the velocity of 19 mm.sec−1 right away, unlike 9.5 mm.sec−1.
Here, the larvae were experimented individually, ruling out any possibility of behavioral alterations in a group. In the future, researchers can benefit from our device to explore the regulation, time dependency and effectiveness of flow velocity on the zebrafish rheotactic behavior. Although our device is designed for single larva studies, it is also amenable to high throughput investigations simply by parallelizing the screening microchannel into tens of side-by-side units. Thin walls between channels can provide flow isolation while allowing the larvae to see each other to resemble group-based assays. Furthermore, investigating this behavior can also open a window for examination of potential inductive neurons of lateral line system or other sensory systems of the zebrafish larvae involved in coaxial rheotaxis.
References
F. Ahmad, L.P.J.J. Noldus, R.A.J. Tegelenbosch, M.K. Richardson, Behaviour 149, 1241 (2012)
G.P. Arnold, Biol. Rev. Camb. Philos. Soc. 49, 515 (1974)
S. Basu, C. Sachidanandan, Chem. Rev. 113, 7952–7980 (2013)
S.A. Budick, D.M. O’Malley, J. Exp. Biol. 203, 2565 (2000)
R.T.P. Calum, A. MacRae, Nat. Rev. Drug Discov. 14, 721 (2015)
R. Candelier, M. Sriti Murmu, S. Alejo Romano, A. Jouary, G. Debrégeas, G. Sumbre, Sci Rep 5, 12196 (2015)
R.M. Colwill, R. Creton, Rev. Neurosci. 22, 63 (2011)
D. De Paiva, D. Forsin, R. Armando, D. Cunha, A. Rosa, D.F. Baptista, Bull Env. Contam. Toxicology 88, 1009 (2012)
F. Ek, M. Malo, M.Å. Andersson, C. Wedding, J. Kronborg, P. Svensson, S. Waters, P. Petersson, R. Olsson, ACS Chem. Neurosci. 7, 633 (2016)
B. Gupta, P. Rezai, Micromachines 7, 123 (2016)
S.I. Higashijima, Develop. Growth Differ. 50, 407 (2008)
K.H. Huang, M.B. Ahrens, T.W. Dunn, F. Engert, Curr. Biol. 23, 1566 (2013)
F.A. Issa, G. O’Brien, P. Kettunen, A. Sagasti, D.L. Glanzman, D.M. Papazian, J. Exp. Biol. 214, 1028 (2011)
A. V. Kalueff, J. M. Cachat, Eds, Zebrafish Models in Neurobehavioral Research, Illustrate (Humana Press, 2010). http://www.springer.com/gp/book/9781607619215
M.J. Mchenry, K.E. Feitl, J.A. Strother, W.J. Van Trump, W.J. Van Trump, Biol. Lett. 5, 477 (2009)
D.L. McLean, J.R. Fetcho, J. Comp. Neurol. 480, 38 (2004)
B.D. Monesson-Olson, J. Browning-Kamins, R. Aziz-Bose, F. Kreines, J.G. Trapani, PLoS One 9, 1 (2014)
A. Nady, A. R. Peimani, G. Zoidl, P. Rezai, A microfluidic device for partial immobilization, chemical exposure and behavioural screening of zebrafish larvae. Lab Chip. (2017). https://doi.org/10.1039/C7LC00786H
R. Olive, S. Wolf, A. Dubreuil, V. Bormuth, G. Debrégeas, R. Candelier, Front. Syst. Neurosci. 10, 14 (2016)
J. Olszewski, M. Haehnel, M. Taguchi, J.C. Liao, PLoS One 7, e36661 (2012)
A. R. Peimani, G. Zoidl, P. Rezai, Zebrafish Larva’s Cyclic Electrotaxis Behavior and Its Dependency on Dopamine Level Enabled by a Novel Microfluidic Assay, International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2017), October 22–26 2017 (Savannah, Georgia, 2017), pp. 1094–1095
S.D. Pelkowski, M. Kapoor, H.A. Richendrfer, X. Wang, R.M. Colwill, R. Creton, Behav. Brain Res. 223, 135 (2011)
A. Suli, G.M. Watson, E.W. Rubel, D.W. Raible, PLoS One 7, 1 (2012)
H.A. Swain, C. Sigstad, F.M. Scalzo, Neurotoxicol. Teratol. 26, 725 (2004)
F. Yang, C. Gao, P. Wang, G.-J. Zhang, Z. Chen, Lab Chip 7, 1106 (2016)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peimani, A.R., Zoidl, G. & Rezai, P. A microfluidic device for quantitative investigation of zebrafish larvae’s rheotaxis. Biomed Microdevices 19, 99 (2017). https://doi.org/10.1007/s10544-017-0240-x
Published:
DOI: https://doi.org/10.1007/s10544-017-0240-x