Abstract
Microfluidic methods are frequently used to produce cell-laden microgels for various biomedical purposes. Such microfluidic methods generally employ oil-water systems. The poor distribution of crosslinking reagents in the oil phase limits the available gelation strategies. Extracting the microgel from the oil-phase also reduces its production efficiency. In this study, an aqueous two-phase system (ATPS) involving dextran (DEX) and polyethylene glycol (PEG) was used to prepare cell-laden microgel. This avoided the problems associated with an oil phase. The microgel precursor polymers and crosslinking reagents were dispersed in the DEX and PEG phases, respectively. The ultra-low interfacial tension of the ATPS hindered droplet formation. A co-flow microfluidic device was fabricated to overcome this problem. The device incorporated a square-wave-changing injection force, to improve the efficiency of droplet formation. The microgel precursor (including alginate and carboxymethyl cellulose derivatives possessing phenolic hydroxyl moieties) could be dispersed in the DEX solution at various concentrations. Uniform droplets were formed with controllable diameters, and were sequentially converted to microgel by horseradish peroxidase-catalyzed crosslinking. Cells were dispersed in the DEX phase with the microgel precursor polymer, and retained their high viability and proliferation in the resulting microgel. The solubility of gelatin derivatives in the DEX phase was low, but was sufficient to impart cell adhesion properties on the microgel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cell-laden microgels (also known as hydrogel microcapsules or hydrogel microparticles) have broad biomedical applications, including in their uses three-dimensional cell culture (Tsuda et al. 2010; Utech et al. 2015), cell delivery (Steinhilber et al. 2013; Nguyen et al. 2015), stem cell therapy (Richardson et al. 2014; Hashemi and Kalalinia 2015), cell transplants (Krishnan et al. 2014), cell cryopreservation (Huang et al. 2015a), and multicellular tissue fabrication (Sakai et al. 2012a; Alessandri et al. 2013; Liu et al. 2013). Microgels are typically produced in high throughput and low cost, using microfluidic devices that employ two-phase oil-water systems. In brief, an aqueous solution containing polymer for microgel formation and cells is injected into a continuous oil-phase that contains crosslinking reagents (Tsuda et al. 2010; Huang et al. 2015a; Sakai et al. 2012a; Liu et al. 2013). A major drawback of the oil-water system is the need to extract the microgel from the oil phase to the aqueous solution, before further application. Extraction processes are time-consuming. Numerous strategies have been reported to overcome this problem, including multiple oil depletion steps (Deng et al. 2011; Huang and He 2014; Wong et al. 2009), mechanical filtration (Hong et al. 2012), and electrophoresis (Huang et al. 2015b) by microfluidic devices. These strategies are still subject to low retrieval efficiencies. It is also difficult to disperse crosslinking reagents in the oil phase. Strategies for producing and purifying cell-laden microgels that do not involve oil are therefore required.
Many oil-free systems have been developed for this purpose, such as electrospray (Nguyen et al. 2015; Alessandri et al. 2013) and inkjet printing. However, cell damage from exposure to high voltage electric fields is a problem in the former system. Difficulties in forming stable uniform size droplets complicates the latter. Thus, an alternative oil-free system was developed in the current study, to produce cell-laden microgel and overcome the shortcomings of current oil-free systems. An aqueous two-phase system (ATPS) was employed, consisting of dextran (DEX) and polyethylene glycol (PEG) solutions at critical polymer concentrations. DEX and PEG are both approved in versatile biomedical applications (Shih 2010; Knop et al. 2010; Thomas et al. 2014; Sgouras and Duncan 1990). The solubility of many polymers and monomers in DEX and PEG-containing aqueous solutions allows for a wide variety of prospective microgel precursors and crosslinking strategies. However, it was difficult to form spherical droplets in the current DEX/PEG system. This was because stable laminar flows in the DEX and PEG-based solutions depended on Rayleigh-Taylor instability in the microfluidic device. The dependence on Rayleigh-Taylor instability resulted from the ultra-low interfacial tension of the DEX/PEG system (Atefi et al. 2014; Moon et al. 2015). To fabricate microgel particles by the ATPS, the design of the microfluidic device was modified. A periodically-changing injection force was employed, to disrupt the interface between the two aqueous phases. This is shown in Fig. 1. In this paper, we discuss the formation of polymer solution droplets in the DEX/PEG ATPS, and the horseradish peroxidase (HRP)-catalyzed crosslinking to form microgels applicable to various substrates (Sakai and Kawakami 2007; Ashida et al. 2014; Sakai et al. 2009; Kurisawa et al. 2005). We also investigate the feasibility of the ATPS for encapsulating cells in microgels, and attaching cells to the microgel surface.
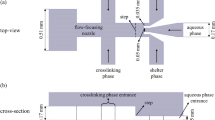
a Production of microgel using DEX/PEG-based ATPS in microfluidic device. The DEX-based solution and PEG solution with H2O2 were stained with red and blue food colorings, respectively. b Morphology of pre-gelated droplets passing through the narrowest channels in the downstream of the microfluidic device. Scale bars indicate 400 μm
2 Materials and methods
2.1 Microfluidic device fabrication
The microfluidic device was prepared by demolding cured polydimethylsiloxane (PDMS; Sylgard184, Dow Corning, Auburn, MI, USA) from master molds containing positive patterns. This was achieved using a standard soft lithography method. Photomasks were drawn by computer-aided design software (AutoCAD 2014; Autodesk, Inc., Dan Rafael, CA, USA), and printed on a transparent sheet. Photoresist SU-8 3050 (Kayaku Microchem, Tokyo, Japan) was deposited on 4-in.-diameter silicon wafers (Wanxiang Silicon-Peak Electronics Co., Ltd., Quzhou, Zhejiang, P. R. China), and spun at a given speed according to the manufacturer’s instructions to obtain the desired thickness. The wafers were then exposed to 365-nm-wavelength light through the photomasks. Solid master molds with desired patterns were obtained after a chemical development process. PDMS for generating the devices was prepared by casting PDMS polymer and curing agent at 10:1 (w/w), and then curing at 70 °C for 1 h. Demolded PDMS regions were bound on a slide glass immediately after corona plasma treatment (PIB-10 Ion Bombarder, Shinku Device Co., Ltd., Ibaraki, Japan) on both binding surfaces.
2.2 Formation of microgels using the DEX/PEG ATPS
DEX (molecular weight (MW): 70,000; Tokyo Chemical Industry, Tokyo, Japan) was dissolved in phosphate buffered saline (PBS, pH 7.4) at 10 wt.%. PEG (MW: 100,000; Sigma-Aldrich, St Louis, MO, USA) was dissolved in PBS at 7.5 wt.%. Sodium alginate (MW: 70,000; Kimica, Tokyo, Japan) was modified to give a phenolic hydroxyl (Ph) content of 1.4 × 10−4 mol-Ph/g-polymer (alginate-Ph). This modification was achieved using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS) condensation reagents, according to a previous report (Sakai and Kawakami 2007). Alginate-Ph and HRP (Wako, Osaka, Japan) were dispersed in the DEX solution at 0.5–2 wt.% and 20–200 units/mL, respectively. 1 mM hydrogen peroxide (H2O2, Wako) was added to the PEG solution, to trigger HRP-catalyzed crosslinking between alginate-Ph molecules. The DEX-based solution was injected into the H2O2-free PEG solution, to form droplets. An individual droplet then converged with another PEG droplet containing H2O2, to form the microgel. The flow rates of all solutions were controlled by a microflow controller (OB1, Elveflow, Paris, France). DEX-based solution was injected at a pressure (P DEX) of 0.6–0.9 kPa. The two PEG solutions were subjected to the same square-wave changing pressure at 0.8 s/period. Maximum pressures (P max) were 2.0–3.1 kPa, and minimum pressures (P min) were −1.1–0.4 kPa. After passing through a rolling polyvinyl chloride (PVC) tube (ID: 0.5 mm, OD: 1.5 mm; Tygon ND 100–80, Saint-Gobain, Courbevoie, France) for approximately 3 min, the resulting microgel was collected in bulk PBS, to dilute H2O2 and non-gelated polymers. A cell strainer (hole diameter: 40 μm, BD Falcon, Franklin Lakes, NJ, USA) was used to recover the microparticles from the PBS. The viscosities of the ATPS solutions were measured using a viscometer (DV-E, Brookfield, Middleboro, MA, USA). The viscosities of DEX-based solutions (μD) containing alginate-Ph at 0.5, 1, 1.5 or 2 wt.% were 59.5, 314.7, 1110 and 5600 mPa s, respectively. The viscosity of the PEG-based solution (μP) was 56.1 mPa s. The ultra-low interfacial tension (γ) was approximately 10–100 μN/m, which was estimated based on literature reports (Atefi et al. 2014; Moon et al. 2015).
A carboxymethylcellulose (CMC)-based microgel was also prepared in this study. A sodium CMC (viscosity: 400–800 mPa s, 2% in H2O (25 °C), Sigma-Aldrich, St. Louis, MO, USA) derivative possessing Ph moieties (CMC-Ph) at 2.2 × 10−4 mol-Ph/g-polymer was used. CMC-Ph was synthesized by the EDC/NHS coupling method, according to a reported procedure (Ashida et al. 2014). CMC-Ph was dispersed in DEX solution at 1 wt.% containing 100 units/mL HRP. This dispersion was sequentially injected into pure PEG solution and PEG solution containing 2 mM H2O2. Flow rates were controlled similarly to those in the preparation of alginate-Ph microparticles.
2.3 Preparation of cell-adhesive microgel
To improve the cell adhesion of the microgel, a gelatin (~300 Boom, Sigma-Aldrich) derivative possessing Ph moieties (gelatin-Ph) was dispersed in DEX solution at 0.3 or 0.6 wt.%, which contained 1 wt.% alginate-Ph and 100 units/mL of HRP. The DEX-based solution was sequentially injected into pure PEG solution and PEG solution containing 2 mM H2O2. Flow rates were controlled similarly to those in the preparation of alginate-Ph microparticles. Gelatin-Ph was synthesized by the EDC/NHS coupling method, according to a reported procedure (Sakai et al. 2009). The Ph content was 9.4 × 10−5 mol-Ph/g-polymer. Green fluorescent protein (GFP)-expressing human umbilical vein endothelial cells (GFP-HUVEC; Angio-Proteomie, Boston, MA, USA) were used to confirm cell adhesion on the microgel. Cells were cultured in MCDB107 medium (Cell Science & Technology Institute, Miyagi, Japan) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 10 ng/mL human epidermal growth factor (Sigma-Aldrich), and 10 ng/mL human recombinant fibroblast growth factor-2 (Gibco). Cells were cultured at 5 × 105 cells/mL with the microgel in a low adherent dish, and incubated under a humidified atmosphere of 5% CO2 and 95% air for 4 h. A cell strainer (pore size: 40 μm; BD Falcon, Franklin Lakes, NJ, USA) was used to remove non-adhered cells.
2.4 Cell encapsulation in the microgel
Human liver carcinoma HepG2 cells (Riken Cell Bank, Ibaraki, Japan) were used. Cells were cultured in Dulbecco’s modified Eagle’s medium (Nissui, Tokyo, Japan) containing 10% FBS under a humidified atmosphere of 5% CO2 and 95% air. Cells were mixed at 1 × 106 cells/mL with DEX solution containing 1 wt.% alginate-Ph and 100 units/mL HRP. The cell-suspended DEX-based solution was injected into H2O2-free PEG solution and PEG solution containing 2 mM H2O2. Flow rates were controlled similarly to those in the preparation of the cell-free microparticles. The resulting cell-enclosed microgel was incubated in culture medium for 6 days. The viability of cells throughout the encapsulation process was determined using trypan blue (Sigma-Aldrich), after treating with 0.2 mg/mL alginate lyase (Sigma-Aldrich) to release the cells from the microgel. Cell aggregates enclosed in the microgel were stained by Calcein-AM and propidium iodide (PI) (both from Nacalai, Osaka, Japan), to identify live and dead cells, respectively. The working concentrations of Calcein-AM and PI were both 0.5 μg/mL.
3 Results and discussion
3.1 Droplet formation
Breaking up the DEX-based solution into droplets by classical Rayleigh-Plateau instability in the PEG-based solution was difficult, because of the ultra-low interfacial tension between DEX and PEG (Atefi et al. 2014; Moon et al. 2015). A microfluidic device was therefore designed to overcome this problem. The microfluidic device contained three inlets that converged in a narrow channel, which was followed by a narrow outlet opening and a subsequent open space. One pair of inlets was located downstream in the microfluidic device (Fig. 1). The stability of the DEX/PEG ATPS showed no obvious change when injecting alginate-Ph in DEX-based solution. The narrow channel outlet after the triple inlets restricted the jetting of the DEX-based solution. The resulting jet was very narrow, and the solution readily formed droplets. The subsequent open space resulted in the jetting velocity significantly decreasing, which “pinched” off individual droplets from the jet (Ziemecka et al. 2011; Lai et al. 2011). A square-wave-changing force was used to inject the PEG-based solution, and a constant force was used to inject the DEX-based solution. The PEG-based solution periodically dispersed through the DEX-based solution, which disrupted the stable laminar flow of the DEX/PEG-based ATPS. Fig. 1a and movie S1 show droplets generated by this microfluidic device.
Droplets downstream of the microfluidic device may have become elongated, especially larger droplets (Fig. 1b). This is consistent with previous results (Moon et al. 2015). The capillary number (Ca) was defined as Ca = μP V/γ. The Ca of the current system was very high, because of the ultra-low interfacial tension. A μP = 56.1 mPa and a characteristic velocity (V) of >1 mm/s were used in this study, according to Fig. 1b and movie recordings of experiments. A γ = 10 μN/m was then used, which resulted in a value of Ca of >1. Thus, the viscous force had a larger effect than the interfacial tension on the droplets. To allow easy handling of the droplets and microgel, the pressure was controlled to yield round droplets (defined as a minimum diameter/maximum diameter of >0.8) downstream in the microfluidic device. The pressures of the DEX and PEG solutions were both controlled. First, the maximum and minimum pressures of the PEG-based solution (P max and P min, respectively) were controlled at 2.8 and 0.3 kPa, respectively. The pressure of the DEX-based solution containing 1 wt.% alginate-Ph (P DEX) was controlled from 0.75 to 0.95 kPa. The pressure of the DEX-based solution was fixed at 0.8 kPa. Either P max or P min was fixed at 2.8 or 0.3 kPa, respectively, while the injection pressure of the other PEG-based solution was varied. The increase in P DEX resulted in larger DEX-based solution droplets (Fig. 2a). Increasing P max or P min resulted in smaller droplets (Fig. 2b, c). Thus, the microfluidic device generated uniform-sized droplets of polymer solutions with diameters of 65–111 μm, simply by controlling the injection pressures.
We also varied the concentration of alginate-Ph in the DEX-based solution from 0.5–2 wt.%. The viscosity of the solution varied from 59.5 to 5600 mPa s, but the microfluidic device still generated homogenous droplets. Thus, it could be used for alginate-Ph solutions of various concentrations and viscosities. We also tried to investigate relationship between viscosity and droplet diameter. Unfortunately, the injection pressures of both DEX- and PEG- phase solutions for droplet formation trended to be stable in narrow ranges and varied in different viscosity solutions. And the variation of narrow ranges seemed no rule to follow. It was difficult to find a common pressure condition that could be applied in all the solutions with varied viscosities from 59.5 to 5600 mPa s. Thus, we aligned a pressure condition (P max = 3.5 kPa, P min = −0.2 kPa and P DEX = 0.5 kPa) and applied it in both DEX-based solutions at the viscosities of 59.5 and 314.7 mPa s (containing 0.5 wt.% alginate-Ph and wt.% alginate-Ph, respectively). Diameter of the droplet obtained from the solution with higher viscosity was smaller than the solution with lower viscosity (Fig. 2d). This result implied a tendency that the solution with lower viscosity preferred generating bigger droplets.
Polymers other than alginate were also used to prepare microgels using the APTS, including Ph-modified CMC (Ashida et al. 2014), polyvinyl alcohol (Sakai et al. 2013), amylopectin (Sakai et al. 2012b), and gelatin (Sakai et al. 2009). Similarly to alginate-Ph, CMC-Ph could be dispersed in DEX-based solution at concentrations of 0.5–2 wt.%. The other polymers did not disperse in the DEX- or PEG-based solution at concentrations high enough to form stable microgels. However, the low dispensability was still sufficient to improve the properties of the microgel, as discussed later.
3.2 Hydrogelation by HRP-catalyzed crosslinking
An HRP-catalyzed reaction was used to form the microgel. This reaction consumed H2O2 that crosslinked the Ph moieties of polymer chains. The biocompatibility, wide variety of suitable substrates, and versatile biomedical application of this reaction are well documented (Ashida et al. 2014; Sakai et al. 2009; Kurisawa et al. 2005). HRP and H2O2 were dispersed in the DEX- and PEG-based solutions, respectively. Droplets of DEX and other polymers containing Ph were solidified as a microgel when encountering H2O2 from the PEG solution. Gelation occurred after droplet formation, because H2O2 was introduced in the downstream (Fig. 1). This avoided any clotting in the microfluidic device. H2O2 can potentially have a negative effect on living cells, so the H2O2 concentration was fixed at a low level, which was based on our previous studies (Liu et al. 2013; Sakai et al. 2012a). Tygon tubing was used to transport the resulting microgel to the bulk PBS solution, which limited the exposure time to H2O2 within approximately 3 min. The HRP concentration was then increased from 20 to 200 units/mL in the DEX-based solution containing 1 wt.% alginate-Ph. Fig. 3a shows that a microgel was obtained when the HRP concentration reached 50 units/mL, but the microparticle shapes were not completely uniform. The shapes became uniform and stable when the HRP concentration was increased to 100 units/mL. Higher HRP concentrations yielded more stable microgels with higher degrees of crosslinking. These findings are similar to those from our previous studies on microgels prepared from HRP-catalyzed reactions in oil-water and air-water systems (Liu et al. 2013; Sakai et al. 2012a).
Microphotographs of alginate-based microgels prepared using different concentrations of (a) HRP ranging from 20 to 200 units/mL, with a fixed alginate-Ph concentration of 1 wt.%, and (b) alginate-Ph ranging from 0.5–2 wt.%, with a fixed HRP concentration of 100 units/mL. c Microphotographs of CMC-based microgel prepared using 100 units/mL of HRP and 1 wt.% CMC-Ph. Scale bars indicate 100 μm
The alginate-Ph concentration in the DEX-based solution was also varied in the range of 0.5–2 wt.%. Microgels formed at all alginate-Ph concentrations, when the HRP and H2O2 concentrations were 100 units/mL and 2 mM, respectively (Fig. 3b). In the case of alginate-Ph, DEX-based solution containing 1 wt.% CMC-Ph also yielded a CMC-based microgel, when the HRP and H2O2 concentrations were 100 units/mL and 2 mM, respectively (Fig. 3c). Gelatin-Ph could not be dispersed well in DEX solution at concentrations of >1 wt.%. However, it mixed with alginate-Ph at lower concentrations, which enabled mixed alginate/gelatin microgels to be obtained (not shown, will be published in future). These results suggested the potential for preparing microgels with properties tailored for individual applications. For example, microgels consisting of alginate and CMC could be degraded by the hydrolysis enzymes alginate lyase and cellulase, respectively. Cells or tissue could be released from these microgels on-demand, by using theses enzymes. Gelatin can regulate cellular behavior, so microgels containing gelatin could potentially provide cell-anchoring scaffolds.
3.3 Cell encapsulation
To prepare the cell-enclosed microgel, HepG2 cells were mixed with DEX-based solution containing alginate-Ph and HRP. The resulting mixture was injected into PEG-based solutions, in a similar process to preparing the cell-free microgel. The encapsulation process required 2 h to produce approximately 2.3 mL of cell-laden microgel containing 1 wt.% alginate-Ph (Fig. 4a). The viability of enclosed HepG2 cells was >94%, measured by trypan blue staining of the recovered cells released from the microgel after alginate lyase treatment for degradation of hydrogel membrane. The cell-enclosed microgel was then cultured for 6 days, during which cells grew into aggregates within the microgel. The viability of individual cells within aggregates was >90%, as visualized by Calcein AM/PI double staining (Fig. 4a) and measured by trypan blue staining of the recovered cells released from the microgel after alginate lyase treatment for degradation of hydrogel membrane. The cell viability and growth profile results demonstrated that the DEX/PEG APTS could yield cell-enclosed microgels under conditions sufficiently mild for living cells.
a Microphotographs of HepG2 cell-enclosed microgel immediately after production, and after culturing for 6 days. Living and dead cells were stained with Calcein-AM (green) and PI (red), respectively. b Microphotographs of HUVECs that failed to attach to the surface of the microgel containing 0.3 wt.% gelatin-Ph, and HUVECs attached to the surface of the microgel containing 0.6 wt.% gelatin-Ph (green regions indicate HUVECs expressing green fluorescent protein). Scale bars indicate 100 μm
3.4 Cell adhesion
Gelatin-Ph was introduced into the ATPS solutions, to promote cell adhesion on the microgel surface. As mentioned above, gelatin-Ph could be dispersed in both DEX- and PEG-based solutions at a very low concentration. Increasing the gelatin-Ph concentration to 1 wt.% resulted in a white precipitate (considered to be gelatin-Ph) forming at the DEX-PEG interface. We were unsure why gelatin-Ph would not disperse well in DEX solution, but viscosity was not thought to be the reason. Polymer solutions with higher viscosities (e.g. the viscosity of 1 wt.% alginate-Ph in PBS at 25 °C was 55.2 mPa s, and that of 1 wt.% CMC-Ph in PBS at 25 °C was 90.6 mPa s) than that of gelatin-Ph (viscosity of 1 wt.% gelatin-Ph in PBS at 25 °C was 26.2 mPa s) could be readily dispersed in DEX solution. Decreasing the gelatin-Ph concentration to 0.3 and 0.6 wt.% in the DEX-based solution containing 1 wt.% alginate-Ph yielded stable homogenous suspensions. The microgel was also formed from solutions under the same gelation conditions used to prepare the microgel solely from alginate-Ph. Fig. 4b shows that HUVECs did not adhere to the surface of the microgel containing 0.3 wt.% gelatin-Ph, but did adhere to the surface of the microgel containing 0.6 wt.% gelatin-Ph. Thus, a low concentration of gelatin-Ph provided the microgel with cell adhesion properties.
4 Conclusion
An aqueous two-phase system consisting of DEX and PEG was developed to prepare a cell-laden microgel. The system contained no oil phases. To overcome the ultra-low interfacial tension between the DEX and PEG solutions, a microfluidic device was developed, which involved a periodically-changing injection force. These factors promoted droplet formation, rather than simply relying on the ultra-low interfacial tension to achieve this. The microgel precursor alginate and CMC derivatives were dispersed in DEX solution at concentrations of 0.5–2 wt.%, which yielded uniform droplets using the microfluidic device. The diameter of the droplets could be controlled by changing the injection conditions. The crosslinking reagents HRP and H2O2 could also be dissolved in DEX and PEG solutions, respectively, at concentrations suitable for gelation of the resulting droplets. Cells encapsulated in the microgel retained a high viability and growth potential. Low concentrations of gelatin derivatives could be incorporated into the microgel, which promoted the cell adhesion properties of the microgel.
References
K. Alessandri, B.R. Sarangi, V.V. Gurchenkov, B. Sinha, T.R. Kiessling, L. Fetler, et al., Proc. Natl. Acad. Sci. U. S. A. 110, 14843 (2013)
T. Ashida, Y. Ojima, S. Sakai, M. Sakka, K. Sakka, K. Kawakami, et al., J. Chem. Eng. Jpn 47, 835 (2014)
E. Atefi, J.A. Mann Jr., H. Tavana, Langmuir 30, 9691 (2014)
Y. Deng, N. Zhang, L. Zhao, X. Yu, X. Ji, W. Liu, et al., Lab Chip 11, 4117 (2011)
M. Hashemi, F. Kalalinia, Life Sci. 143, 139 (2015)
S. Hong, H.J. Hsu, R. Kaunas, J. Kameoka, Lab Chip 12, 3277 (2012)
H. Huang, X. He, Appl. Phys. Lett. 105, 143704 (2014)
H. Huang, J.K. Choi, W. Rao, S. Zhao, P. Agarwal, G. Zhao, et al., Adv. Funct. Mater. 25, 6939 (2015a)
H. Huang, M. Sun, T. Heisler-Taylor, A. Kiourti, J. Volakis, G. Lafyatis, et al., Small 11, 5369 (2015b)
K. Knop, R. Hoogenboom, D. Fische, U.S. Schubert, Angew. Chem. Int. Ed. Eng. 49, 6288 (2010)
R. Krishnan, M. Alexander, L. Robles, C.E. Foster 3rd, J.R. Lakey, Rev. Diabet. Stud. 11, 84 (2014)
M. Kurisawa, J.E. Chung, Y.Y. Yang, S.J. Gao, H. Uyama, Chem. Commun. (Camb.) 14, 4312 (2005)
D. Lai, J.P. Frampton, H. Sriram, S. Takayam, Lab Chip 11, 3551 (2011)
Y. Liu, S. Sakai, M. Taya, Acta Biomater. 9, 6616 (2013)
B.U. Moon, S.G. Jones, D.K. Hwang, S.S. Tsai, Lab Chip 15, 2437 (2015)
D.K. Nguyen, Y.M. Son, N.E. Lee, Adv. Healthc. Mater. 4, 1537 (2015)
T. Richardson, P.N. Kumta, I. Banerjee, Tissue Eng. Part A 20, 3198 (2014)
S. Sakai, K. Kawakami, Acta Biomater. 3, 495 (2007)
S. Sakai, K. Hirose, K. Taguchi, Y. Ogushi, K. Kawakami, Biomaterials 30, 3371 (2009)
S. Sakai, K. Inamoto, Y. Liu, S. Tanaka, S. Arii, M. Taya, Cancer Sci. 103, 549 (2012a)
S. Sakai, Y. Liu, T. Matsuyama, K. Kawakami, M. Taya, J. Mater. Chem. 22, 1944 (2012b)
S. Sakai, M. Tsumura, M. Inoue, Y. Koga, K. Fukano, M. Taya, J. Mater. Chem. B 1, 5067 (2013)
D. Sgouras, R. Duncan, J. Mater. Sci. Mater. Med. 1, 61 (1990)
I.L. Shih, Mini-Rev. Med. Chem. 10, 1345 (2010)
D. Steinhilber, T. Rossow, S. Wedepohl, F. Paulus, S. Seiffert, R. Haag, Angew. Chem. Int. Ed. Eng. 52, 13538 (2013)
A. Thomas, S.S. Muller, H. Frey, Biomacromolecules 15, 1935 (2014)
Y. Tsuda, Y. Morimoto, S. Takeuchi, Langmuir 26, 2645 (2010)
S. Utech, R. Prodanovic, A.S. Mao, R. Ostafe, D.J. Mooney, D.A. Weitz, Adv. Healthc. Mater. 4, 1628 (2015)
E.H. Wong, E. Rondeau, P. Schuetz, J. Cooper-White, Lab Chip 9, 2582 (2009)
I. Ziemecka, V. Van Steijn, G.J.M. Koper, M.T. Kreutzer, J.H. Van Esch, Soft Matter 7, 9878 (2011)
Acknowledgements
We thank Dr. Yoshihiro Ojima, Osaka City University, Osaka, Japan, for providing an Elveflow OB1 microfluidic flow controller and for constructing the microfluids. This research was supported by the Young Researchers Career Support Program (Grant no. j881100933) of Osaka University, the Multi-disciplinary Research Laboratory System (Grant no. 1119902199) of the Graduate School of Engineering Science, Osaka University, and the Nanotechnology Platform Project (Nanotechnology Open Facilities in Osaka University) (Grant no. F-16-OS-0012) of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest directly relevant to the content of this article.
Electronic supplementary material
Movie of microgel prepared by the ATPS-based microfluidic system.
Preparation of microgel using the DEX/PEG-based aqueous system. The DEX-based solution and PEG solution with H2O2 were stained with red and blue food colorings, respectively. (MP4 3456 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Nambu, N.O. & Taya, M. Cell-laden microgel prepared using a biocompatible aqueous two-phase strategy. Biomed Microdevices 19, 55 (2017). https://doi.org/10.1007/s10544-017-0198-8
Published:
DOI: https://doi.org/10.1007/s10544-017-0198-8