Abstract
The circulating micronutrient pattern in type 2 diabetes mellitus (T2DM) may impact glycemic control and insulin resistance; however, there is a scarcity of studies that have evaluated the circulating micronutrient pattern in the T2DM population. Therefore, our objective was to identify circulating micronutrient pattern and their association with markers of glycemic control and insulin resistance in individuals with T2DM. We developed a cross-sectional observational study involving adults with T2DM in Sergipe, Brazil. We assessed plasma levels of magnesium, zinc, calcium, potassium, and serum 25-hydroxyvitamin D. Additionally, also measured fasting glucose levels, the percentage of glycated hemoglobin (%HbA1c), and calculated the homeostatic model assessment for insulin resistance (HOMA-IR). Patterns of body reserve were established using principal component analysis and categorized into quartiles. Binary logistic regression models were employed. We evaluated 114 individuals (63.7% women), with a median age and body mass index of 49 years and 29.6 kg/m², respectively. Two circulating micronutrient patterns were identified, explaining 62.5% of the variance: Pattern 1 (positive contributions from magnesium, zinc, calcium, and potassium) and Pattern 2 (positive contributions from 25-hydroxyvitamin D and zinc, with a negative contribution from potassium). Lowest quartile for Pattern 1 and Pattern 2 exhibiting a 4.32-fold (p = 0.019) and 3.97-fold (p = 0.038) higher likelihood of increasing HOMA-IR and %HbA1c values, respectively, compared to the larger quartiles. However, no associations were found between these patterns and fasting glucose values. Lowest quartile for both patterns of micronutrients was associated with inadequate metabolic control in individuals with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus is characterized by chronic hyperglycemia, a condition resulting from the malfunction of insulin secretion and/or action (American Diabetes Association 2019). The progression of this disease is often linked to deficiencies in micronutrients that play a crucial role in carbohydrate metabolism and insulin resistance (Bilous and Donnelly 2010). Vitamin D, magnesium (Mg), zinc (Zn), calcium (Ca), and potassium (K) are key players in insulin synthesis and secretion, each operating through distinct mechanisms (Chausmer 1998; Pittas et al. 2007; Ozcaliskan Ilkay et al. 2019; Coregliano-Ring et al. 2022). Additionally, these micronutrients contribute to antioxidant and anti-inflammatory protection, potentially reducing insulin resistance (Zelko et al. 2002; Kim et al. 2015; Pfeffer et al. 2018; Grudet et al. 2020; Liu and Dudley 2020).
Vitamin D interacts with vitamin D receptors located in pancreatic beta cells, playing a direct role in insulin synthesis (Zeitz et al. 2003; Neelankal John and Jiang 2018) and aiding in the homeostasis of Ca, Mg, and K (Pittas et al. 2007; Christakos et al. 2017). Both Ca and K participate in the exocytosis of insulin granules, leading to the subsequent secretion of the hormone (Saad 2002; Berridge 2017; Szymczak-Pajor et al. 2020). Meanwhile, Mg facilitates the translocation of glucose transporters 4 (GLUT4) and the phosphorylation of insulin receptors (Huang 2014). Furthermore, Zn aids in the storage of insulin molecules as Zn crystals, which exhibit greater structural stability (Chausmer 1998).
Consequently, a combined deficiency of vitamin D, Mg, Zn, Ca, and K may lead to poor glycemic control and insulin resistance in individuals with type 2 diabetes mellitus (Medalle et al. 1976; Ahmed and Mohammed 2019). No studies have been identified in the literature that assess the circulating concentration of these micronutrients collectively, thereby establishing patterns of micronutrient reserves in individuals with type 2 diabetes mellitus.
The hypothesis of this study posits that adequate circulating micronutrient pattern of vitamin D, Mg, Zn, Ca, and K are associated with better glycemic control in individuals with type 2 diabetes mellitus. Thus, our objective was to establish circulating micronutrient patterns and examine their relationship with glycemic control and insulin resistance in individuals with type 2 diabetes mellitus.
Materials and methods
Participants and study design
An observational, cross-sectional study was conducted involving 114 individuals with type 2 diabetes mellitus. Eligibility criteria for inclusion in the study comprised individuals aged between 19 and 59 years, of both sexes, residing in Sergipe, Brazil. Participants were recruited from Primary Health Care Units and through voluntary participation, following promotion of the research via digital media and within the community. The sample size was considered to provide satisfactory statistical power (0.94), with alpha and beta errors of 0.05, based on an expected outcome of an odds ratio (OR) of 4.09 for percentage of glycated hemoglobin (%HbA1c) (Li et al. 2018). The G-power 3.1.9.7 software was utilized to estimate the statistical power of the sample.
Individuals who consumed tobacco and alcohol daily were excluded, as were those who had ingested vitamin and mineral supplements or medications, including multivitamins and corticosteroids within the 3 months leading up to the data collection date. The exclusion criteria also encompassed individuals with self-reported chronic non-communicable diseases such as cancer, rheumatoid arthritis, chronic kidney disease, and thyroid disorders. Those with self-reported acute inflammatory conditions or infections were similarly excluded. Women who were undergoing hormone replacement therapy were also not included in the study.
Information regarding socioeconomic status, lifestyle, and medical history was collected using a structured questionnaire. Anthropometric measurements, including body weight and height, were recorded to compute the body mass index (BMI) and waist circumference (WC). Additionally, bioelectrical impedance was employed to evaluate body composition, specifically the percentage of body fat.
The assessment of individuals included the measurement of serum concentrations of 25-hydroxyvitamin D (25(OH)D) and plasma levels of Zn, Mg, Ca, and K. Glycemic control markers, such as fasting glucose and %HbA1c, insulin, C-peptide, and parathyroid hormone (PTH) were also evaluated. To assess insulin resistance, the Homeostasis Model Assessment for insulin resistance (HOMA-IR) was calculated.
Individuals diagnosed with type 2 diabetes mellitus, who met the inclusion criteria, were pre-contacted via telephone or through community health agents. They were invited to a designated location to complete a socioeconomic questionnaire, undergo an anthropometric assessment and body composition evaluation, and provide biological samples.
Ethical aspects
The participants reviewed and signed the informed consent form. The Ethics and Research Committee of Federal University of Sergipe granted approval for the study, under opinion number 3.012.056, in accordance with the principles outlined in the Declaration of Helsinki.
Anthropometric and body composition measurements
BMI was calculated using measured weight and height and classified in accordance with the World Health Organization (WHO) (Organization 2000). WC was determined using a non-stretchable measuring tape (Cescorf®, Porto Alegre, Brasil), positioned midway between the last rib and the iliac crest, and classified following WHO standards (World Health Organization 2011).
The percentage of body fat was determined using a tetrapolar bioelectrical impedance (Bioimpedance Analyzer BIA 310, Biodynamics Corporation, Shoreline, WA, USA). This involved the placement of four electrodes: two on the right hand (one on the wrist and one on the hand) and two on the right foot (one on the ankle and one on the foot) (Ward 2019). Participants were advised to abstain from strenuous physical activities, as well as the consumption of coffee, chocolate, and tea, on the day preceding the examination. The examination was rescheduled for women who were in their menstrual period. Individuals with a body fat percentage exceeding 30% were classified as having excess fat (Lohman 1992).
Collection of biological samples
Participants were directed to undergo a 10-h overnight fast. Subsequently, 15 mL of blood was drawn and distributed into various tubes for analysis. Tubes containing ethylenediaminetetraacetic acid (Vacuette, Greiner Bio-One, Brazil) were used to determine %HbA1c. Tubes devoid of anticoagulants (Vacuette, Greiner Bio-One, Brazil) were employed for assessing serum concentrations of 25(OH)D, PTH, fasting glucose, insulin, and C-peptide. Additionally, demineralized polypropylene tube with a 30% sodium citrate anticoagulant were utilized for the analysis of Zn, Mg, Ca, and K in plasma.
To control contamination, all materials (including glassware, plastics, tips, tubes) utilized for mineral analyses were demineralized in a 20% nitric acid bath for a minimum of 12 h and subsequently rinsed 10 times with deionized water (Morte et al. 2012). Sterile materials were employed for other analyses.
Assessment of plasma micronutrient concentrations
The Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) with axial configuration (model ES-720, Agilent, Mulgrave, Australia) was employed to determine the concentration of minerals such as Zn (202.548 nm), Mg (279.553 nm), Ca (211.276 nm), and K (766.491 nm) in plasma.
Sample digestion was carried out in a temperature-controlled block digester equipped with polytetrafluoroethylene (PTFE) flasks and lids (model TE007-A, TECNAL, São Paulo, SP, Brazil). The samples underwent a 2-h digestion process at 150 °C in a digestion block utilizing 0.5 mL of the sample, 2 mL of pure nitric acid, and 3 mL of ultrapure water (resistivity of 18.2 MΩ.cm) (Gehaka, Master System All, SP, Brazil). Following digestion, the samples were diluted to a final volume of 15 mL using ultrapure water. Blank samples were also prepared for each digestion, adhering to the same analysis conditions (Castro et al. 2009). Each set of triplicates was analyzed three times, with three readings obtained from each analysis.
To assess the precision and accuracy of the method, we conducted addition and recovery tests at four levels (5 mg/L, 50 mg/L, 500 mg/L, and 1000 mg/L) for the elements Zn, Mg, Ca, and K, aiming to represent the concentration ranges of these minerals in plasma samples. Precision, expressed by the relative standard deviation, was < 10%, and accuracy ranged from 87% (K) to 119% (Ca), demonstrating that the method is suitable for determining these elements in human plasma (Santos et al. 2021). Additionally, a certified reference material [Seronorm Trace Elements Serum (Sero, Billingstad, Norway)] was reanalysed together with a blank and an intermediate calibration standard every 10 samples.
The limits of detection (LOD) and limits of quantification (LOQ) for the micronutrients were computed to verify the method’s reliability (Silva et al. 2002; Skoog et al. 2002). The LOD and LOQ values for Zn analysis were 0.004 and 0.013 µg/dL; for Mg, 0.001 and 0.005 mg/dL; for Ca, 0.774 and 2.555 mg/L; and for K, 0.033 and 0.111 mmol/L, respectively.
All mineral data were collected in milligrams per liter and subsequently converted to the corresponding units used in the reference cutoff points: Mg > 2.07 mg/dL (Rosanoff et al. 2022); Zn ideally falls within 70–110 µg/dL (Gibson 2005); K is ideal between 4 and 5 mmol/L (Kratz et al. 2004); and Ca > 41.9 mg/L (Deng et al. 2008).
Assessment of serum concentration of 25(OH)D and PTH
The chemiluminescence method was utilized to determine serum concentrations of 25(OH)D and PTH, using the DXI800 – Beckman Coulter® equipment and corresponding kits. Individuals were classified as having sufficient concentrations if 25(OH)D values were ≥ 30 ng/mL and as having insufficient/deficient concentrations if values were < 30 ng/mL (Holick et al. 2011). Participants with PTH values exceeding 65 pg/mL were characterized as having hyperparathyroidism (Khundmiri et al. 2016).
Assessment of biochemical markers of glycemic control
The %HbA1c was measured using immunoturbidimetric inhibition, and fasting glycemia was assessed using an enzymatic colorimetric method using commercial kits (Labtest®, Lagoa Santa, Minas Gerais, Brazil). Indicators of poor glycemic control include %HbA1c values ≥ 7% and fasting glycemia values ≥ 130 mg/dL (Elsayed et al. 2023).
Insulin and C-peptide serum concentrations were assessed using the Architect i1000SR immunoassay analyzer (Abbott®, Abbott Park, IL, USA) with chemiluminescence. Commercial kits from Abbott® were used for this purpose.
The HOMA-IR was determined using serum concentrations of C-peptide and fasting glucose, utilizing the HOMA calculator from the University of Oxford, United Kingdom. This calculation was based on the updated homeostasis model (Levy et al. 1998). Insulin resistance was identified when HOMA-IR values exceeded 2.71 (Geloneze et al. 2009).
Statistical analysis
A descriptive analysis was performed on the data. Categorical variables are presented in absolute frequencies (n) and relative percentages (%). Continuous variables are expressed as either mean and standard deviation or as median (25th–75th percentile), depending on the data distribution determined by the Kolmogorov–Smirnov test (Kolmogorov and Smirnov 1933).
In order to discern circulating micronutrient pattern, the data was initially standardized to account for the variability in the units of measurement for the evaluated micronutrients. The standardization process was conducted on a scale from − 1 to + 1, utilizing the z-score standardization formula (z = (x - µ) / σ). In this formula, z signifies the standardized value, x denotes the original value, µ is the mean of the original values, and σ represents the standard deviation of the original values (Kreyszig et al. 2011).
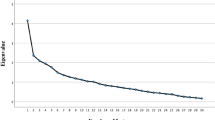
The distribution of circulating nutrient reserves was determined using principal component analysis, which was based on z-score data. The selection of factors was guided by the Kaiser criterion (eigen value > 1) and scree plot analysis, which was visualized through an eigen value plot that accounted for the total variance associated with each value (Ledesma et al. 2015). To establish the reliability of the factor analysis, Bartlett’s test of sphericity and the Kaiser–Meyer–Olkin (KMO) test were employed. A p-value of < 0.05 in Bartlett’s test and a p-value of > 0.50 in the KMO test signified a valid factor analysis (Kaiser 1974).
The varimax method of orthogonal rotation was employed to analyze factor loadings. A direct correlation with the pattern was indicated by factor loadings ≥ 0.25, while an inverse correlation with the pattern was suggested by loadings ≤ − 0.25 (Cunha et al. 2010).
Additionally, the discerned patterns of body micronutrient reserves were categorized into quartiles and incorporated into binary logistic regression models. These models considered the following risk values for dependent variables: fasting glucose > 130 mg/dL, %HbA1c ≥ 7%, and HOMA-IR > 2.71. The models were calibrated for age, duration since diagnosis, and BMI. The Hosmer and Lemeshow test were employed to evaluate the model fit (Hosmer and Lemesbow 1980).
Statistical analyses were conducted using SPSS Statistics version 28 software, with a p-value of < 0.05 deemed significant.
Results
A total of 114 individuals diagnosed with type 2 diabetes mellitus were evaluated. The median age was 49 years (ranging from 44 to 54 years), and the median duration of diagnosis was 6 years (ranging from 2 to 11 years). Most of the participants were women (63.7%). According to BMI, 44.6% of the sample was classified with obesity, while 62.4% were classified with obesity based on body fat percentage. Poor glycemic control was observed in 59.6% and 59.3% of individuals when assessed using fasting glucose and %HbA1c, respectively. Insulin resistance was observed in 41.2% of the evaluated individuals. All individuals (100%) exhibited Mg deficiency, while deficiencies in vitamin D, Zn, and Ca were observed in 42.1%, 49.1%, and 28.1% of the sample, respectively. The majority of individuals (98.2%) had excessive K levels (> 5 mmol/L), while 1.8% had a deficiency (< 4 mmol/L). Regarding PTH concentration, 97.3% of individuals had normal secretion levels. Table 1 presents the main clinical, anthropometric, and biochemical characteristics related to micronutrient concentrations and glycemic control markers.
Principal component analysis identified two circulating micronutrient patterns, accounting for a total of 62.52% of the variance (Fig. 1).
Pattern 1 accounts for 40.7% of the variance, demonstrating a positive correlation between factor loading and plasma levels of Zn, Mg, Ca, and K. Conversely, Pattern 2 accounts for 21.8% of the variance, showing a positive correlation with 25(OH)D and Zn but a negative correlation with K. Table 2 presents the factor loading attributed to the components of these identified patterns.
Assessment of circulating micronutrient patterns by quartiles revealed that the lowest quartile of Pattern 1 had 4.32 times higher odds (p = 0.019) of insulin resistance, as evaluated by HOMA-IR, compared to the highest quartile. Similarly, the lowest quartile of Pattern 2 had 3.97 times higher odds (p = 0.038) of an increase in %HbA1c compared to the highest quartile. No associations were found between the quartiles of Patterns 1 and 2 and the outcome variables in the other tested models (Table 3).
Discussion
Individuals with type 2 diabetes mellitus who have lower quartiles of circulating micronutrient patterns, as indicated by 25(OH)D, Zn, Mg, Ca, and K, often exhibit inadequate metabolic control. This is evidenced by elevated %HbA1c and HOMA-IR values.
No studies were found in the literature that identified associations between circulating micronutrient patterns and markers of glycemic control and insulin resistance in individuals with type 2 diabetes mellitus. However, isolated deficiency of the micronutrients included in the identified patterns is associated with inadequate metabolic control in individuals with type 2 diabetes, which can worsen in the joint presence of nutrient deficiencies.
Separately, the observed deficiency or insufficiency of 25(OH)D, along with Zn, Mg, and Ca deficiencies in this study, aligns with findings from other studies involving individuals with type 2 diabetes mellitus (Chutia and Lynrah 2015; Hassan et al. 2016; Farooq et al. 2020; Hasanato 2020). An increase in %HbA1c was associated with Zn deficiency (Farooq et al. 2020), and lower concentrations of 25(OH)D (Santos et al. 2018) and Ca serum levels (Hassan et al. 2016) were found to be inversely correlated with %HbA1c in adults with type 2 diabetes mellitus. The homeostasis of Ca metabolism, as assessed by plasma Ca concentrations in this study, was upheld by the high prevalence of individuals with adequate PTH secretion. Moreover, a deficiency of Mg in individuals with type 2 diabetes mellitus was linked to higher %HbA1c values (Zahra et al. 2021).
Furthermore, similar to our findings, individuals with type 2 diabetes and insulin resistance have been shown to have higher K concentrations (Kim et al. 2015). However, these body reserves of K can be influenced by inadequate intake of food sources, the presence of conditions that decrease absorption and alter K metabolism, as well as the use of thiazide diuretic medications. Nonetheless, this relationship seems to be influenced by the duration of medication use, diabetes diagnosis time, and the individual’s age (Coregliano-Ring et al. 2022).
Vitamin D, Zn, Mg, Ca, and K play integral roles in promoting glycemic control by acting together in different mechanisms of insulin secretion and activity. Both Vitamin D and Zn influence insulin gene activity, thereby aiding in hormone synthesis (Chausmer 1998; Vardatsikos et al. 2013; Huang 2014; Bertoccini et al. 2018). Furthermore, Vitamin D is involved in the conversion of the proinsulin molecule into insulin within the pancreas (Morró et al. 2020). Concurrently, Zn enters the insulin vesicles via the Zn transporter Znt8, resulting in the storage of insulin molecules as zinc crystals, which enhances their structural stability (Chausmer 1998). Consequently, these micronutrients facilitate insulin synthesis and secretion, the uptake of circulating glucose, and the subsequent reduction in %HbA1c values and insulin resistance (Chausmer 1998; Pittas et al. 2007; Ozcaliskan Ilkay et al. 2019; Palacios et al. 2020).
The 1,25(OH)D, active form of vitamin D, it binds to its receptor and regulates the influx of Ca into the beta cell, exocytosis of insulin granules and the subsequent secretion of the hormone (Pittas et al. 2007; Mauricio et al. 2008; Vondra and Hampl 2021). This process also depends on sufficient concentrations of K (Saad 2002). The closure of ATP-sensitive K channels results in membrane depolarization, which activates voltage-dependent Ca channels. This, in turn, triggers insulin exocytosis due to increased intracellular Ca levels (Zeitz et al. 2003; Berridge 2015, 2017; Szymczak-Pajor et al. 2020).
Conversely, elevated K levels can induce membrane hyperpolarization, which diminishes the calcium influx into the cell, thereby inhibiting insulin secretion (Ekmekcioglu et al. 2016). A study involving individuals with type 2 diabetes mellitus found a correlation between low K intake and increased micronutrient excretion and a higher mortality rate (Yeung et al. 2022).
Insulin’s role in glucose uptake begins with its binding to its receptor, which subsequently promotes the translocation of GLUT4 to the cell membrane. Mg aids this process by facilitating the phosphorylation of insulin receptors, triggering the translocation of GLUT4. This action enhances the uptake of circulating glucose by peripheral tissues, thereby aiding in maintaining appropriate glycemic control (Morakinyo et al. 2018). Furthermore, the bioavailability of vitamin D is also associated with the body’s Mg reserve (Medalle et al. 1976; Reddy and Edwards 2017). Therefore, Mg deficiency observed in 100% of individuals evaluated in our study, may contribute to lower vitamin D activity in glycemic control.
Moreover, the correlation between the lower quartile of circulating micronutrient patterns, as explained by Zn, Mg, Ca, and K, and an increase in HOMA-IR, may be attributed to their antioxidant and anti-inflammatory activity. The management of oxidative stress and low-grade inflammation, often observed in this population, contributes to the binding of insulin to the receptor and the phosphorylation of its substrates (Chausmer 1998; Prasad 2009; Jomova et al. 2022).
Our research revealed that individuals with type 2 diabetes mellitus who fall into the lower quartiles of body micronutrient reserves, as determined by concentrations of 25(OH)D, Zn, Mg, Ca, and K, are more likely to experience inadequate glycemic control and insulin resistance. Furthermore, a prior study involving the same population indicated that insufficient dietary intake of Mg, Zn, Ca, and K was linked to an increased probability of elevated %HbA1c (Brandão-Lima et al. 2018).
The present study did not evaluate outcomes in relation to the use of medications or the class of oral antidiabetic agents used by the study population, which is a limitation. Despite this, the study is pioneering in its identification of circulating micronutrient patterns and their associations with glycemic control markers in a sample of individuals with type 2 diabetes mellitus, demonstrating satisfactory statistical power. This research provides a foundation for the development of further studies of a similar nature in diverse populations. It also supports the initiation of randomized clinical trials that consider circulating micronutrient patterns when determining nutritional strategies for glycemic control in individuals with type 2 diabetes mellitus.
Conclusions
In summary, two distinct patterns of circulating micronutrient pattern were identified in individuals with type 2 diabetes mellitus. A higher likelihood of increased HOMA-IR was associated with lower combined concentrations of Mg, Zn, Ca, and K. Conversely, lower concentrations of 25(OH)D and Zn were linked to a higher likelihood of elevated %HbA1c. These associations were observed regardless of age, BMI, and time of diagnosis.
The results obtained highlight the necessity for additional similar studies in diverse samples of individuals with type 2 diabetes mellitus. Moreover, these findings support the development of randomized clinical trials that consider body micronutrient reserves patterns when determining nutritional strategies for glycemic control in individuals with type 2 diabetes mellitus.
References
Ahmed F, Mohammed A (2019) Magnesium: the Forgotten Electrolyte-A Review on Hypomagnesemia. Med Sci 7:56
American Diabetes Association (2019) Introduction: standards of medical care in diabetes-2019. Diabetes Care 42:S1–S2
Berridge MJ (2015) Vitamin D cell signalling in health and Disease. Biochem Biophys Res Commun 460:53–71
Berridge MJ (2017) Vitamin D deficiency and Diabetes. Biochem J 474:1321–1332
Bertoccini L, Bailetti D, Study S et al (2018) Variability in genes regulating vitamin D metabolism is associated with vitamin D levels in type 2 diabetes. Oncotarget 9:34911–34918
Bilous R, Donnelly R, Excerpt #10 (2010) Handbook of Diabetes, 4th Edition, Management of Type 2 Diabetes. http://www.diabetesincontrol.com/handbook-of-diabetes-4th-edition-excerpt-10-management-of-type-2-diabetes/
Brandão-Lima PN, De Carvalho GB, Santos RKF et al (2018) Intakes of Zinc, Potassium, Calcium, and Magnesium of individuals with type 2 Diabetes Mellitus and the relationship with glycemic control. Nutrients 10:1–12. https://doi.org/10.3390/nu10121948
Castro JT, Santos EC, Santos WPC et al (2009) A critical evaluation of digestion procedures for coffee samples using diluted nitric acid in closed vessels for inductively coupled plasma optical emission spectrometry. Talanta 78:1378–1382. https://doi.org/10.1016/j.talanta.2009.02.030
Chausmer AB (1998) Zinc, insulin and Diabetes. J Am Coll Nutr 17:109–115. https://doi.org/10.1080/07315724.1998.10718735
Christakos S, Veldurthy V, Patel N, Wei R (2017) Intestinal regulation of calcium: vitamin D and bone physiology. Understanding the Gut-bone signaling axis: mechanisms and therapeutic implications. Springer, New York, pp 3–12
Chutia H, Lynrah KG (2015) Association of Serum Magnesium Deficiency with Insulin Resistance in type 2 Diabetes Mellitus. J Lab Physicians 7:075–078. https://doi.org/10.4103/0974-2727.163131
Coregliano-Ring L, Goia-Nishide K, Rangel ÉB (2022) Hypokalemia in diabetes mellitus setting. Medicina 58:431
Cunha DB, de Almeida RMVR, Pereira RA (2010) A comparison of three statistical methods applied in the identification of eating patterns. Cad Saude Publica 26:2138–2148. https://doi.org/10.1590/S0102-311X2010001100015
Deng B, Zhu P, Wang Y et al (2008) Determination of free calcium and calcium-containing species in human plasma by capillary electrophoresis-inductively coupled plasma optical emission spectrometry. Anal Chem 80:5721–5726. https://doi.org/10.1021/ac800715c
Ekmekcioglu C, Elmadfa I, Meyer AL, Moeslinger T (2016) The role of dietary potassium in Hypertension and Diabetes. J Physiol Biochem 72:93–106
Elsayed NA, Aleppo G, Aroda VR et al (2023) 6. Glycemic targets: standards of Care in Diabetes—2023. Diabetes Care 46:S97–S110. https://doi.org/10.2337/dc23-S006
Farooq D, Alamri A, Alwhahabi B et al (2020) The status of zinc in type 2 diabetic patients and its association with glycemic control. J Family Community Med 27:29–36. https://doi.org/10.4103/jfcm.JFCM_113_19
Geloneze B, Vasques ACJ, Stabe CFC et al (2009) Índices HOMA1-IR e HOMA2-IR para identificação De resistência à insulina e síndrome metabólica - estudo Brasileiro De Síndrome Metabólica (BRAMS). Arq Bras Endocrinol Metabol 53:281–287. https://doi.org/10.1590/S0004-27302009000200020
GIBSON RS (2005) Principles of Nutritional Assessment, 2nd edn. OXFORD USA II, New York
Grudet C, Wolkowitz OM, Mellon SH et al (2020) Vitamin D and inflammation in major depressive disorder. J Affect Disord 267:33–41. https://doi.org/10.1016/j.jad.2020.01.168
Hasanato RMW (2020) Trace elements in type 2 Diabetes Mellitus and their association with glycemic control. Afr Health Sci 20:287–293. https://doi.org/10.4314/ahs.v20i1.34
Hassan SAElr, Elsheikh WAR, Rahman NIA, ElBagir NM (2016) Serum calcium levels in correlation with glycated hemoglobin in type 2 Diabetic Sudanese patients. Adv Diabetes Metabolism 4:59–64. https://doi.org/10.13189/adm.2016.040401
Holick MF, Binkley NC, Bischoff-Ferrari HA et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930. https://doi.org/10.1210/jc.2011-0385
Hosmer DW, Lemesbow S (1980) Goodness of fit tests for the multiple logistic regression model. Commun Stat Theory Methods 9:1043–1069. https://doi.org/10.1080/03610928008827941
Huang L (2014) Zinc and its transporters, pancreatic β-cells, and insulin metabolism. Vitamins and Hormones. Academic Press Inc., London, pp 365–390
Jomova K, Makova M, Alomar SY et al (2022) Essential metals in health and disease. Chem Biol Interact 367:110173
Kaiser HF (1974) An index of factorial simplicity. Psychometrika 39:31–36. https://doi.org/10.1007/BF02291575
Khundmiri SJ, Murray RD, Lederer E (2016) PTH and Vitamin D. Comprehensive Physiology. Wiley, Hoboken, pp 561–601
Kim HW, Lee DH, Lee SA, Koh G (2015) A relationship between serum potassium concentration and insulin resistance in patients with type 2 Diabetes Mellitus. Int Urol Nephrol 47:991–999. https://doi.org/10.1007/s11255-015-1001-5
Kolmogorov A, Smirnov N (1933) Sulla determinazione Empirica Di Una Legge Di distribuzione. Giornale dell’Istituto Italiano Degli Attuari 4:83–91
Kratz A, Ferraro M, Sluss PM, Lewandrowski KB (2004) Laboratory reference values. N Engl J Med 351:1548–1563
Kreyszig E, Kreyszig H, Norminton EJ (2011) Advanced Engineering Mathematics. 10 ed. Wiley India Pvt. Limited. 1244p
Ledesma RD, Valero-Mora P, Macbeth G (2015) The Scree Test and the number of factors: a dynamic Graphics Approach. Span J Psychol 18:E11. https://doi.org/10.1017/sjp.2015.13
Levy JC, Matthews DR, Hermans MP (1998) Correct Homeostasis Model Assessment (HOMA) evaluation uses the computer program. Diabetes Care 21:2191–2192. https://doi.org/10.2337/diacare.21.12.2191
Li X, Liu Y, Zheng Y et al (2018) The effect of vitamin D supplementation on Glycemic Control in type 2 Diabetes patients: a systematic review and Meta-analysis. Nutrients 10:375. https://doi.org/10.3390/nu10030375
Liu M, Dudley SC (2020) Magnesium, oxidative stress, inflammation, and Cardiovascular Disease. Antioxidants 9:1–31
Mauricio D, Palomer X, Gonza JM (2008) Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab 10:185–197. https://doi.org/10.1111/j.1463-1326.2007.00710.x
Medalle R, Waterhouse C, Hahn TJ (1976) Vitamin D resistance in magnesium deficiency. Am J Clin Nutr 29:854–858. https://doi.org/10.1093/ajcn/29.8.854
Morakinyo AO, Samuel TA, Adekunbi DA (2018) Magnesium upregulates insulin receptor and glucose transporter-4 in streptozotocin-nicotinamide-induced type-2 diabetic rats. Endocr Regul 52:6–16. https://doi.org/10.2478/enr-2018-0002
Morró M, Vilà L, Franckhauser S et al (2020) Vitamin D receptor overexpression in β-Cells ameliorates Diabetes in mice. Diabetes 69:927–939. https://doi.org/10.2337/db19-0757
Morte ES, da Barbosa B, dos Santos I et al (2012) Axial view inductively coupled plasma optical emission spectrometry for monitoring tin concentration in canned tomato sauce samples. Food Chem 131:348–352. https://doi.org/10.1016/j.foodchem.2011.08.015
Neelankal John A, Jiang FX (2018) An overview of type 2 Diabetes and importance of vitamin D3-vitamin D receptor interaction in pancreatic β-cells. J Diabetes Complications 32:429–443
Organization WH (2000) Obesity: preventing and managing the global epidemic Report of a WHO Consultation (WHO Technical Report Series 894)
Ozcaliskan Ilkay H, Sahin H, Tanriverdi F, Samur G (2019) Association between Magnesium Status, Dietary Magnesium Intake, and Metabolic Control in patients with type 2 Diabetes Mellitus. J Am Coll Nutr 38:31–39. https://doi.org/10.1080/07315724.2018.1476194
Palacios C, Pérez CM, González-Sepúlveda L et al (2020) Vitamin D, calcium, Magnesium, and potassium consumption and markers of glucose metabolism in the Hispanic Community Health Study/Study of Latinos. J Am Coll Nutr. https://doi.org/10.1080/07315724.2020.1833790
Pfeffer PE, Lu H, Mann EH et al (2018) Effects of Vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLOS ONE 13:e0200040. https://doi.org/10.1371/journal.pone.0200040
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) Review: the role of vitamin D and calcium in type 2 Diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029
Prasad AS (2009) Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 12:646–652
Reddy P, Edwards LR (2017) Magnesium supplementation in Vitamin D deficiency. Am J Ther 9:1–9
Rosanoff A, West C, Elin RJ et al (2022) Recommendation on an updated standardization of serum magnesium reference ranges. Eur J Nutr. https://doi.org/10.1007/s00394-022-02916-w
Saad MJA (2002) Vias de Sinalização da Insulina. Arquivos Brasileiros de Endocrinologia & Metabologia 46:419–425
Santos RKF, Brandão-Lima PN, Tete RMDD et al (2018) Vitamin D ratio and glycaemic control in individuals with type 2 Diabetes Mellitus: a systematic review. Diabetes Metab Res Rev 34:1–11. https://doi.org/10.1002/dmrr.2969
Santos SH, Costa SSL, Souza SO et al (2021) Development of analytical method for determination of inorganic constituents in powder refreshment using dilute mineral acids and detection by ICP OES. J Braz Chem Soc 32:1361–1372. https://doi.org/10.21577/0103-5053.20210034
Silva FV, Trevizan LC, Silva CS et al (2002) Evaluation of inductively coupled plasma optical emission spectrometers with axially and radially viewed configurations. Spectrochim Acta B 57:1905
Skoog FV, Holler FJ, Nieman Ta (2002) Princípios de Análise Instrumental, 5th edn. Bookman, Porto Alegre
Szymczak-Pajor I, Drzewoski J, Śliwińska A (2020) The molecular mechanisms by which vitamin d prevents insulin resistance and associated disorders. Int J Mol Sci 21:1–34
Lohman TG (1992) Advances in body composition assessment. Human Kinetics Publishers, Champaign, Ill
Vardatsikos G, Pandey NR, Srivastava AK (2013) Insulino-mimetic and anti-diabetic effects of zinc. J Inorg Biochem 120:8–17
Vondra K, Hampl R (2021) Vitamin D and new insights into pathophysiology of type 2 Diabetes. Horm Mol Biol Clin Investig 42:203–208
Ward LC (2019) Bioelectrical impedance analysis for body composition assessment: reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr 73:194–199
World Health Organization (2011) Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation: Ginebra, 8–11, 2011. 8–11
Yeung SM, Oosterwijk MM, Poelstra M et al (2022) Low urinary potassium excretion is Associated with higher risk of all-cause mortality in patients with type 2 Diabetes: results of the Dutch Diabetes and Lifestyle Cohort Twente (DIALECT). J Nutr 152:2856–2864. https://doi.org/10.1093/jn/nxac215
Zahra H, Berriche O, Mizouri R et al (2021) Plasmatic magnesium deficiency in 101 outpatients living with type 2 Diabetes Mellitus. Clin Pract 11:791–800. https://doi.org/10.3390/clinpract11040095
Zeitz U, Weber K, Soegiarto DW et al (2003) Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 17:509–511. https://doi.org/10.1096/fj.02-0424fje
Zelko IN, Mariani TJ, Folz RJ (2002) Superoxide Dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33(3):337–349. https://doi.org/10.1016/s0891-5849(02)00905-x
Acknowledgements
The authors would like to thank the National Council for Scientific and Technological Development (CNPq) for the financial support (Process: 455117/2014-4 and 432298/2018-5), to the Foundation for the Support of Research and Technological Innovation of the State of Sergipe (Call CAPES/FAPITEC - PROMOB Process: 88881.157967/2017-01), and to the Coordination for the Improvement of Higher Education Personnel (CAPES - Finance Code 001).
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq) (Process: 455117/2014-4 and 432298/2018-5), the Foundation for the Support of Research and Technological Innovation of the State of Sergipe (Call CAPES/FAPITEC - PROMOB Process: 88881.157967/2017-01), and to the Coordination for the Improvement of Higher Education Personnel (CAPES - Finance Code 001).
Author information
Authors and Affiliations
Contributions
The study was conceptualized by RKFS and LVP All authors contributed to data analysis, interpretation, and manuscript drafting. Each author made a significant contribution to the project and has approved the final version. Study supervision was provided by LVP and AMOS, with funding acquisition managed by LVP.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, R.K.F., Costa, S.S.L.d., Santos, S.H.d. et al. Association between circulating micronutrient pattern, glycemic control, and insulin resistance in type 2 diabetes mellitus. Biometals 37, 527–537 (2024). https://doi.org/10.1007/s10534-023-00568-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-023-00568-9