Abstract
This study aimed to investigate the short-term effects of three magnesium (Mg) dietary supplements containing mineral immediately available for absorption on Mg biochemical status indices (ionized and total Mg), as well as their effects on electrolytes levels in healthy female young adults (n = 61). After a 10-days intervention period supplementation with powder/granulate containing Mg oxide led to an increase in both ionized Mg concentration and % in total Mg in comparison with the baseline. Supplementation with Mg citrate was associated with the significant increase in % of ionized fraction and decrease in serum total Mg concentration. By contrast, among participants consuming Mg carbonate in the form of effervescent tablets ionized Mg concentration and % in total Mg decreased, without detectable changes in serum total Mg. In conclusion, after the short-term supplementation period, Mg oxide demonstrated superior bioavailability compared to the other examined Mg supplements without affecting other minerals’ levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnesium (Mg) is an essential micronutrient necessary for a broad range of biochemical and physiological functions. It serves as a cofactor of several hundred enzymes systems that regulate numerous key biochemical pathways, including pathways related to macronutrient degradation, DNA and protein synthesis, energy metabolism, bone mineralization, glycemic control, and blood pressure regulation. Furthermore, Mg exerts structural function and plays a significant role in controlling the transport of calcium and potassium ions across cell membranes, a process fundamental for neuromuscular excitability and heart rhythm maintenance (De Baaij et al. 2015; Philipp Schuchardt and Hahn 2017; Veronese et al. 2020).
Recommended intakes are 300 mg/day and 350 mg/day for adult women and men, respectively, in Europe (Dietary Reference Values, DRVs) (EFSA 2015) and 310–320 mg/day and 400–420 mg/day, respectively in the United States (Recommended Daily Allowances, RDA) (Intakes 1997). Mg is found in significant amounts in several types of foods such as whole grains and cereal products, green vegetables, nuts, seeds, and legumes. Regardless, a significant percentage of people, especially in Western countries, do not meet the minimum daily needs for Mg (Newhouse and Finstad 2000). It is estimated that 2.5% to 15% of the world population experiences some form of hypomagnesaemia. Furthermore, Mg deficiency is frequently observed in industrialized countries, including the United States and European countries (Cazzola 2020). Data derived from the National Health and Nutrition Examination Survey (NHANES) 2013–2016 showed that approximately one-half of all American adults have inadequate intake of Mg (Committee 2020). The Nutritional Study in Spanish Pediatric Population (EsNuPI) which analyzed the intake and dietary sources of Mg in Spanish children (aged 1 to 10 years old) has reported that the intake of this mineral was below the adequate intake level in a considerable percentage of children population (Cuadrado-Soto et al. 2020). This could be explained by the fact that typical Western diets, high in refined grains and processed food, may not contain sufficient amounts of Mg (Muñoz-Garach et al. 2020). Furthermore, it has been determined that the content of Mg in soil and, consequently, in food, has decreased significantly over the last fifty years, while as much as 80% of this metal is lost during food processing (Cazzola et al. 2020; De Baaij et al. 2015). Moreover, absorption and elimination of Mg might be easily disturbed by several factors present in food or due to pharmacotherapy (Belluci et al. 2020; Philipp Schuchardt and Hahn 2017).
Mg homeostasis under normal conditions depends on a dynamic and complex interplay between dietary intake, intestinal absorption, and renal excretion. Assessing Mg status is challenging since the serum and plasma Mg, i.e. extracellular Mg accounts for about 1% of the total body content. Determination of Mg in main reservoirs, bones, and muscles, provides a reliable evaluation of Mg status. Nevertheless, being invasive, expensive, and impracticable, these methods are not suitable for the routine clinical setting. In clinical and research practice the measurement of total Mg (tMg) concentration in serum and plasma has been commonly performed for identifying changes in Mg homeostasis (Blancquaert et al. 2019; Gröber et al. 2015). However, approximately 20–30% of tMg in the circulation is bound to plasma proteins and is therefore physiologically inactive. Free or ionized Mg (iMg), which is considered to be a biologically active form of tMg, could be a physiologically more significant marker for the analyses of Mg status. Ionized Mg constitutes approximately 60–70% of circulating tMg in serum and plasma (De Baaij et al. 2015; Gröber et al. 2015; Rooney et al. 2020). Although commercial analyzers, based on ion-selective electrodes for determining levels of Mg ions were utilized for a substantial amount of time(Altura and Altura 1991) it was only recently, when technological advances and specialized equipment allowed the accurate, reliable, and time-optimized determination of iMg concentration in a direct manner, without correction for the concentration of Ca ions (Rooney et al. 2020).
Over the last couple of decades, the consumption of dietary supplements is increasing globally, and according to the recently published data from NHANES 2017–2018 their use is higher among women (63.8%) than men (50.8%) (Dickinson and MacKay 2014; Mishra et al. 2021). Based on a consumer survey performed by the Council for Responsible Nutrition in 2019, Mg supplements were among the ten most popular dietary supplements used in the adult population (Kamiński et al. 2020). Wide-spread consumption and high-level consumer interest in Mg supplements may be attributed to its role in numerous physiological processes relevant to health promotion, disease prevention, and sport performance enhancement (Blancquaert et al. 2019; Tang et al. 2020). Commercially available Mg supplements differ in Mg content, sources of elemental Mg (organic or inorganic compounds), and pharmaceutical dosage form (capsules, tablets, effervescent tablets, powder/granulate). Published research data suggest that the bioavailability of Mg depends on all these factors. Organic compounds have better bioavailability compared to inorganic Mg compounds; dosage forms that are consumed in dissolved manner (effervescent tablets) have better bioavailability compared to tablets and capsules; and the multiple intakes of lower doses throughout the day results in superior bioavailability compared to a single intake of a higher dose (Blancquaert et al. 2019; Philipp Schuchardt and Hahn 2017; Siener et al. 2011). In recent years, one of the most popular pharmaceutical forms of supplements is powder/granulate for direct oral administration, which is significantly more expensive than supplements in the form of capsules and (effervescent) tablets with the same Mg content. Up to now, there is a lack of evidence regarding the pharmacokinetic profile and bioavailability of this pharmaceutical dosage form that could justify higher prices compared to effervescent tablets as a more economically affordable option. Furthermore, data on the effects of short-term Mg supplementation on Mg status, based on measurements of tMg and iMg are scarce in healthy adults, especially in females as more frequent consumers of supplements. There is limited information regarding the effects of Mg dietary supplements on the status of other cations, such as calcium (Ca), sodium (Na) and potassium (K), and anions, such as phosphorus (P) that are in close physiological interplay with Mg.
Taking into account all the above, the aims of this study were as follows: (1) to examine the effects of three different Mg supplements with the same Mg doses, two in the form of a powder/granulate for direct oral use that differ in the source of elemental Mg (organic and inorganic compounds) and the third in the form of effervescent tablets, on iMg and tMg concentrations in healthy female volunteers after ten days supplementation period; (2) to examine the effects of Mg supplementation on Ca, Na, K and P levels.
Materials and methods
Participants
One hundred healthy female students were recruited through the University of Belgrade—Faculty of Pharmacy facilities where flyers about participation in the study were distributed. Recruitment material contained detailed information regarding the purpose of the study, procedures involved as well as rights and expectations of the potential participants. A questionnaire was applied to screen for eligibility or disqualification. The main inclusion criteria were: female sex, age between 18 and 30 years, body mass index (BMI) between 18.5 and 29.9 kg/m2, and willingness to maintain regular dietary habits throughout the study. The exclusion criteria were the intake of other Mg supplements or medications that affect Mg levels at least three months preceding and during the study, and chronic gastrointestinal or renal disorders.
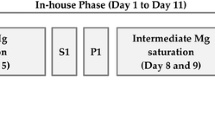
The recruitment process and sample overview are presented in Fig. 1.
Study protocol
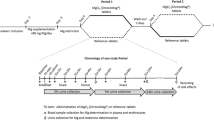
Three Mg supplements providing 375 mg of Mg in daily dose each were tested in the study. An overview of the Mg formulations used in this research is provided in Table 1. After their blood samples and anthropometric measurements were taken, participants were assigned to one of the three groups matched by age, weight, BMI, and body composition. During ten intervention days participants ingested on a daily basis two sachets of Supplement 1 containing 375 mg of elementary Mg as magnesium citrate (Supplement 1 group, n = 20), one sachet of Supplement 2 containing 375 mg of elementary Mg as magnesium oxide (Supplement 2 group, n = 20) or two effervescent tablets containing 375 mg of elementary Mg as magnesium carbonate (Supplement 3 group, n = 21). All supplements were purchased at a local pharmacy. Participants were instructed to consume their supplements once a day, during an evening meal. In order to ensure high protocol adherence and promote participants’ commitment short message service (SMS) reminder system was established. Furthermore, participants were required to refrain from taking supplements and other medications during the study period and to maintain a regular diet regimen. To evaluate their compliance, participants were asked to return the empty sachets (Supplement 1 and Supplement 2 groups) and PVC tubes (Supplement 3 group) at the final testing after the intervention. The primary study outcome was a change in iMg and tMg concentrations. The secondary outcome was the detection of changes in Ca, Na, K and P serum concentrations.
Anthropometry
Anthropometric parameters were measured at the beginning of the study. Height was measured to the nearest 0.1 cm (Perspective Enterprises, Kalamazoo, MI, USA). Body weight and body fat percentage were determined using the bioimpedance method (BC-418MA, Tanita, USA). Body Mass Index (BMI) was calculated as weight (kg)/height2 (m2).
Dietary intake assessment
Dietary intake assessment was based on repeated 24 h recalls. Participants reported complete consumption of food and beverages within in-person structured interviews conducted by trained researchers in accordance with a predefined, standardized protocol. Multiple-pass method, a variety of memory cues and probing questions were applied to guide reporting, enhance respondents’ engagement and improve data accuracy. Dietary data were collected for two consecutive days within baseline evaluation (t0) and follow-up assessment organized after 10 days of using the provided dietary supplements (t2). Respondents were instructed to quantify portion sizes using natural units, household measures and packaging information for commercially-available products. Furthermore, validated, 135-item, Balkan region-specific Food Atlas representing coloured photographs of graduated portion sizes for a variety of simple foods and composite dishes was applied as a supportive portion-size estimation aid (Nikolic et al. 2018). Questionnaires were processed and analyzed via Diet Assess & Plan – an innovative software-based platform for dietary data collection and comprehensive nutritional assessment (Gurinovic et al. 2018). National Serbian Food Composition Database, complying with European Food Composition Resource (EuroFIR AISBL) standards, was employed to convert food consumption information into energy, macro- and micronutrient estimates (Gurinovic et al. 2016). To perform micronutrient adequacy evaluation participants’ recall data was compared against the age and gender-specific nutritional recommendations with DRVs proposed by European Food Safety Agency (EFSA) used as reference standards (Authority 2017; EFSA 2017).
Biochemical assessment
Assessment of serum biochemical parameters was performed before the initiation of the intervention (i.e. baseline analyses—t0), and on the fifth (t1) and eleventh (t2) day of the intervention period. Blood samples were collected by trained medical staff via venipuncture in the morning hours (between 7:00 am and 9:00 am), following 12 h of overnight fasting in the absence of prior vigorous physical activity. Standard operating procedures for blood collection and separation of serum and whole blood for analysis were followed. A closed venipuncture system, Beckton Dickinson (BD) 22 Standard Wire Gauge (SWG), a reusable adapter and tubes were used. Samples used for measurement of tMg, tCa, P, Na and K were collected in serum tube with clot activator (BD Vacutainer® SST™ Tubes) and for measurement of iMg and iCa lithium-heparin spray-coated tube (BD Vacutainer® Heparin Tubes) were used. Total tMg, tCa and P concentrations were measured via spectrophotometric method with commercial reagents, and Na and K concentrations were determined by ion-selective electrodes, on Olympus AU400 biochemical analyzer (Beckman Coulter, Inc., CA, USA). Ionized Mg and iCa concentrations were measured in whole blood approximately up to 60 min after collection using Stat Profile Prime Plus Critical Care blood gas analyzer (Nova Biomedical, USA). Commercial control samples were applied to verify the precision and accuracy of the methods.
Statistical analysis
The normality of the data distribution was analyzed using Shapiro–Wilk test. For data that were not normally distributed, values were log-transformed before analysis. All the data were tested using Multi-Factor ANOVA, General Linear Models for repeated measures. To assess the statistical relationship between the analyzed parameters Pearson’s bivariate correlation coefficients were calculated for both baseline values, and the final-point measurements. A scatter plot was employed to visualize the association between iMg and tMg concentrations. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS version 24 (SPSS Inc., USA).
Ethical approval
This study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki and the study protocols were approved by the Ethics Commission of the University of Belgrade—Faculty of Pharmacy, Belgrade, Serbia (approval number: 188/2, 2020). All subjects went through verbal and written consent processes.
Results
Anthropometric and biochemical data of study participants
One hundred female students were recruited for the study but only 61 participants completed the 11-day experimental protocol. Among them, based on predefined monitoring indicator i.e. empty packaging return rate, the compliance in all groups was > 90%. The subjects’ mean age was 22.8 ± 2.0 years and their mean BMI was 22.7 ± 3.4 kg/m2. According to the anthropometric measurements, 71.7% had BMI within the normal range (BMI = 18.5–24.9 kg/m2), 8.7% of the subjects were classified in the category of underweight (BMI < 18.5 kg/m2), 17.4% in overweight (BMI = 25.0–29.9 kg/m2), while 2.2% were obese (BMI ≥ 30 kg/m2). There were no statistically significant differences between the groups at the baseline with respect to age, weight, BMI, body composition (i.e. total body fat %), blood pressure indices, as well as Mg and Ca status biomarkers (i.e. iMg, tMg, iCa, tCa) (p > 0.05). Furthermore, no significant differences were observed regarding the lifestyle habits (i.e. average physical activity level, smoking, and alcohol consumption prevalence). Detailed study participant characteristics at baseline by treatment group are presented in Table 2.
Dietary intake assessment
Dietary intake
Estimated daily energy, macronutrient (as percentage contributions to total energy intake) and micronutrient intakes assessed by 24 h recalls at baseline evaluation (t0) and after 10 days of using provided dietary supplements (t2) are presented in Table 3.
Food consumption analyses confirmed stable dietary intake during the experimental period since no significant differences were observed in energy, macronutrient, and intake of Mg, Ca, Na, K and P between the baseline evaluation and after 10 days within all three intervention groups (p > 0.05). Furthermore, intergroup variance evaluation revealed no statistically significant difference concerning these parameters.
Dietary intakes of magnesium among study participants
The mean dietary Mg intake in all participants was 232.4 ± 85.3 mg/day. Only 19% of all participants reached magnesium DRV for women (300 mg/day). Cereals (mainly wheat and rice) were the dominant source of dietary Mg. The proportion of total dietary Mg coming from cereals was 29.17% for study participants. Other food groups with significant contribution to Mg intake were milk and dairy products (17.28%) and vegetables (mainly beans and legumes, 13.30%). Table 4 provides an overview of the median values and 5th and 95th percentiles of the daily intake levels of eight food groups and their relative corresponding contributions to the daily Mg intake based on 24 h dietary recalls among study participants.
Mg intake estimates correlated significantly with the consumption of certain food groups. The most noteworthy correlations were those for milk and dairy food group (r = 0.36, p < 0.001), nuts and seeds (r = 0.32, p < 0.001), meat (r = 0.26, p < 0.05) and vegetables (r = 0.22, p < 0.05). Significant correlations were determined between the estimated intake of Mg and total energy (r = 0.48, p < 0.001) and protein intake (r = 0.53, p < 0.001) as well as with P (r = 0.71, p < 0.001) and K intake (r = 0.73, p < 0.001).
Correlation between magnesium intake and magnesium biochemical parameters
The subjects’ mean whole blood iMg and serum tMg concentrations at baseline were 0.59 ± 0.03 and 0.89 ± 0.05 mmol/L, respectively. Data analyses revealed weak correlations between iMg and tMg concentrations and Mg dietary intake estimates. However, when iMg and tMg concentration were presented by quartiles of Mg intake assessed by 24 h recalls at baseline significant decrease of iMg concentrations were identified with an increase of Mg intake (p = 0.026), unlike the tMg (p > 0.05) (Table 5).
Effects of short-term supplementation with different magnesium preparations on whole blood ionized and serum total magnesium concentrations
Changes of whole blood iMg, serum tMg concentrations and iMg/tMg index in female student participants in the middle (day 5) and after the supplementation (day 11) with different Mg preparations are presented in Table 6. In all three experimental groups, whole blood iMg and serum tMg have changed on the 5th and 11th day of intervention but in a different manner.
In Supplement 1 and Supplement 2 groups, in which Mg was in the form of powder for direct oral administration (direct form) but with different sources of elemental Mg (organic vs. inorganic compounds), significant changes were observed in concentrations of iMg, tMg and iMg/tMg in both study test-points (Table 6).
In these groups, on the 5th day of the intervention, tMg concentrations have decreased significantly compared to baseline concentrations (− 0.13 mmol/L, p < 0.001, and 0.09 mmol/L, p < 0.001, respectively). However, iMg concentrations in both groups have increased significantly (+ 0.03 mmol/L, p < 0.05 and + 0.07 mmol/L, p < 0.001, respectively), as well as the percentage of serum Mg that was ionized (+ 14%, p < 0.001 and + 15.9%, p < 0.001, respectively). In Supplement 3 group, in which Mg was in the form of effervescent tablets, at the 5th day of the intervention there were no significant changes in concentration of both, tMg and iMg, in comparison to baseline levels, although both biomarkers increased slightly. In this group decrease of the ionized fraction of tMg was observed, but the decline did not reach statistical significance (p > 0.05).
After 10 days of supplementation in Supplement 1 and Supplement 2 groups, concentrations of tMg increased compared to the 5th day. Nevertheless, in Supplement 1 group (Mg citrate) tMg concentration remained significantly lower in comparison with the baseline (− 0.07 mmol/L, p < 0.001). Conversely, in the Supplement 2 group (Mg oxide) this parameter was significantly higher than the baseline value at the final experimental point (+ 0.02 mmol/L, p < 0.05). On the other hand, iMg concentrations decreased compared to the 5th day in both Supplement 1 and Supplement 2 groups but remained significantly higher in comparison with the baseline concentrations (+ 0.02 mmol/L, p < 0.05 and + 0.04, p < 0.001, respectively). Changes in iMg concentrations were followed by changes in percentages of iMg from tMg and, although these values decreased compared to day 5, they were significantly higher in both groups after ten days of supplementation (+ 6.9%, p < 0.05 and 1.5%, p < 0.05). In the third intervention group (supplement containing Mg bicarbonate), end-point tMg concentration did not change compared to the 5th day of the intervention and was slightly higher compared to the baseline concentration (+ 0.01 mmol/L). Contrariwise, iMg level and ionized fraction of tMg significantly decreased compared to baseline values (− 0.03 mmol/L, p < 0.05 and − 5.2%, p < 0.001, respectively).
Regardless of the type of pharmaceutical preparation that was administered, 10 days of supplementation caused an increase in the percentage of iMg from tMg (Table 7).
Effects of short-term supplementation with different magnesium preparation on serum calcium, potassium and phosphorus concentration
Assessment of biochemical indices of mineral status revealed changes of tCa, iCa, Na, and K levels in the Supplement 1 group at the end of supplementation. Total serum Ca concentration significantly decreased after 5 and 10 days of intervention in comparison with the baseline level (− 0.14 mmol/L, p < 0.005 and − 0.12 mmol/L, p < 0.05, respectively) (Fig. 2A). The same trend was observed for ionized calcium but without statistical significance (5th day: -0.02 mmol/l, p > 0.05 and 10th day: − 0.01 mmol/L, p > 0.05) (Fig. 2B). In this group, at the end of the intervention period, there was a significant decrease in Na concentration (-2.4 mmol/L, p < 0.001) and increase in K concentration (+ 0.55 mmol/l, p < 0,05) (Fig. 2C and D).
Change in analyzed parameters concentrations measured in three points of interest for: A total calcium; B ionized calcium; C sodium; D potassium; E inorganic phosphate. Differences between the fifth day and the eleventh day of supplementation in relation to the baseline (*p < 0.05, **p < 0.001). Differences between the eleventh day and the fifth day of supplementation (†p < 0.05, ††p < 0.001)
In Supplement group 2 (Mg oxide) after 5 days of supplementation a significant increase was determined in K concentration (+ 0.05 mmol/L, p < 0.05) but there was no statistical significance at the end of the intervention compared to the baseline (Fig. 2D).
In Supplement group 3 (Mg carbonate) assessment of biochemical indices of mineral status revealed only slight, but no significant changes in measured parameters (Fig. 2A–E).
There was no statistically significant change in P concentrations in any of the study groups (Fig. 2E).
Discussion
The current study aimed to investigate the short-term effects of three Mg supplements on biochemical status indices, measured as both iMg and tMg, as well as their effects on the levels of Ca, Na, K, and P in healthy female young adults.
Daily dose i.e. 375 mg was determined in accordance with the Nutritive Referent Value (NRV) for Mg. NRVs are the European Union (EU) labeling guidance levels for consumers on the daily amount of vitamin or mineral that the average healthy person needs for deficiency prevention. Furthermore, in the majority of studies that explored the effects of Mg supplementation in healthy populations or among people with preclinical or noncommunicable diseases, Mg is administered in doses ranging between 300 and 450 mg/day (Blancquaert et al. 2019; Dibaba et al. 2017; Nielsen et al. 2010; Rooney et al. 2020). Mg toxicities are rather rare, and toxic hypermagnesemia, characterized with clinical sequelae such as hypotension or muscular weakness, is only seen at oral Mg doses greater than 2500 mg. Mg compounds may exert an osmotic effect in the intestine and cause a transient, reversible laxative effect (Authority 2006). Nevertheless, none of the participants reported adverse effects or any discomfort caused by supplementation.
Given that Mg homeostasis under normal conditions depends, among other factors, on dietary intake of this mineral, food consumption data were collected using repeated 24 h recalls at baseline evaluation and after 10 days of using the provided dietary supplements. Dietary intake assessment confirmed the absence of statistically significant differences in energy, macronutrient, and intake of Mg, Ca, Na, K and P between baseline and end-point evaluation in all three intervention groups (Table 3). Maintaining a stable dietary intake during the experimental period was of crucial importance to detect changes in biochemical status caused by Mg supplementation. Furthermore, intergroup variance evaluation revealed no statistically significant differences between intervention groups with regard to these parameters (Table 3). The average dietary intake of Mg in 81% of all participants was lower than recommended 300 mg/day for female adults (DRV, EFSA). Suboptimal dietary intake of Mg was reported among the USA and European populations in all age groups. US National Health and Nutrition Examination Survey (NHANES) of 2013–2016 reported that 89% of female Americans ages 14–18 and 54% of women ages 19–30 had Mg intake from food and beverages below the respective RDA threshold (Committee 2020; Elmadfa et al. 2009). The main food sources of Mg intake among participants in our study were grains and cereal products with a contribution of almost a third of the total dietary intake of this nutrient (Table 4). These findings are in accordance with previously published data for the European population (Olza et al. 2017; Welch et al. 2009). It is noteworthy, though, that the bioavailability of Mg from grains may be substantially decreased due to high levels of phytic acid and dietary fiber. The main food groups that followed cereals were: milk and dairy products (17.28%) and vegetables (mainly beans and legumes), 13.30%. These food groups were recognized as dominant among the US population (Ford and Mokdad 2003).
Regardless of the suboptimal dietary intake of Mg, mean tMg concentrations at baseline (0.89 ± 0.05 mmol/L) were adequate (Table 2). It has been shown that enteral absorption increases when dietary intake is low (Authority 2006). In healthy individuals, the reference range for tMg in serum is 0.76–1.15 mmol/L although according to some literature data the appropriate lower reference limit should be 0.85 mmol/l, especially for diabetic patients (Costello and Nielsen 2017; Gröber et al. 2015). Our findings concerning iMg distribution were consistent with other studies conducted among young healthy subjects (Newhouse and Finstad 2000; Rooney et al. 2020). Although consensus on the threshold for defining optimal iMg concentration is still lacking, proposed reference intervals for whole blood and plasma iMg concentrations are 0.44–0.59 mmol/L and 0.53–0.67 mmol/L, respectively (Rooney et al. 2020).
Correlation analysis was applied to examine the association between the questionnaire-based estimates of Mg dietary intake and Mg status parameters. Neither whole blood iMg nor serum tMg levels reflected Mg intake (p > 0.05). However, the concentration of whole blood iMg differed significantly across quartiles of Mg intake (p = 0.026) (Table 5). Although the utility of serum tMg as an indicator of Mg intake is questionable due to the fact that serum tMg is under physiological control, previously published data argued that serum tMg may respond to Mg intake (Jacques et al. 1993). Nevertheless, a study that examined the effects of Mg supplementation on Mg status biomarkers in young female volunteers revealed no correlation between Mg intakes based on self-reported diet and iMg concentration (Newhouse and Finstad 2000). Obtained iMg changes relative to Mg intake in our study may be explained by wide variability in the way in which Mg is metabolized and complex mechanisms that affect Mg kinetics.
After 10 days of the intervention period, only supplementation with powder/granulate for direct oral use which contained inorganic compound, Mg oxide (Supplement 2 group), led to an increase in both iMg concentration and %iMg in tMg as well as a slight increase in tMg concentration (Table 6). It is noteworthy, though, that more prominent effects on iMg and %iMg, followed by a marked decrease in tMg, were found at the 5th day of supplementation (+ 0.07 mmol/L, + 12.3%, p < 0.001 and + 15.9%, p < 0.001, respectively), probably as a consequence of the organism's adaptation to supplementation.
Supplementation with Mg citrate (Supplement 1 group) after 10 days resulted with the significant increase in %iMg in tMg (+ 6.9%, p < 0.05). However, unlike the situation in Supplement 2 and Supplement 3 groups, serum tMg concentration decreased significantely (− 0.07 mmol/L, − 7.8.%, p < 0.001).By contrast, in the third group (receiving Mg carbonate in the form of effervescent tablets) statistically significant decrease was found for both concentration of iMg and %iMg in tMg, in both study measurement-points in comparison with baseline values (− 0.03 mmol/L, − 6.6%, p < 0.05 and − 5.2%, p < 0.001, respectively). Nevertheless, no change in serum tMg concentration was detected (Table 6).
According to the literature data, Mg absorption is better from organic sources than from inorganic compounds (Ahmed and Mohammed 2019). In the number of animal and human studies in which bioavailability of Mg citrate and Mg oxide from different supplements were compared with respect to the in vitro solubility, in vivo gastrointestinal absorbability, increase in Mg concentration in serum and red blood cells, as well as to 24-h urinary excretion, Mg citrate exhibited better bioavailability (Kappeler et al. 2017; Lindberg et al. 1990; Walker et al. 2003). Results of the present study, on the contrary, suggested the advantage of Mg oxide on improving iMg concentration, as a biochemical form of more clinical relevance than tMg. Nevertheless, comparative analyses with other studies’ findings are quite challenging due to the differences in research designs, pharmaceutical formulations, variability of intervention periods, applied analytical assays, sample characteristics as well as Mg content in utilized products and the manufacturer specificities. Siener et al. investigated the differences in bioavailability of the inorganic Mg compound (Mg oxide) from two pharmaceutical dosage forms, effervescent tablets and capsules, with the same Mg content using urinary Mg excretion as a marker (Siener et al. 2011). Authors attributed better availability of the Mg in the form of effervescent tablets to this specific pharmaceutical formulation which is characterized by dissolution in water prior to the ingestion as well as to the presence of auxiliary components. These components, such as citric acid and bicarbonate produce CO2 in solution and, at the same time, decrease pH value which results in the complete solubility of Mg oxide and instantaneous availability of ionized Mg.
Regarding the clinical significance of the effects observed in the Supplement group 2 on the increase in iMg concentration, it is important to note that there are only a few studies that have included measurements of iMg and tMg in exploring the response to Mg supplementation (Blancquaert et al. 2019; Rooney et al. 2020; Zhan et al. 2020). In a randomized placebo-controlled study that evaluated the effects of Mg oxide supplementation on both iMg and tMg concentrations in healthy volunteers after 10 weeks intervention period, significant increases of both biomarkers’ concentrations (+ 0.04 mmol/L, + 0.03 mmol/L, respectively) were found compared to the placebo group. Although the increase in iMg and tMg compared to the baseline did not reach statistical significance, it corresponded to approximately 60% of 1 SD of their baseline (Rooney et al. 2020). In our study, the increase in iMg corresponds to approximately 133% of 1 SD of the baseline iMg value, while the increase in tMg corresponds to approximately 30% of 1 SD of the baseline tMg. Despite the similarities with regards to the selection of Mg source and the applied daily dose, differences related to study design, dosage form, and participants’ characteristics (middle-aged subjects of both sexes vs. young females) hamper comparison between studies. Newhouse and Finstag (2000) examined the effects of Mg supplementation on exercise performance in physically active young women using measurement of iMg concentration. The authors found that four weeks of 212 mg Mg oxide supplementation significantly improved resting iMg levels compared to the baseline (+ 0.044 mol/L, 7.82%) (Newhouse and Finstad 2000). This level of increase in iMg concentration corresponds well with our observation in Supplement 2 group (Mg oxide) after a shorter period of intervention. Furthermore, another study conducted among middle-aged adults with poor sleep quality reported the increase in serum tMg concentration after supplementation compared to the baseline only in participants with baseline serum tMg concentrations < 0.75 mmol/L (indication of deficient Mg status). Moreover, %iMg in tMg in these participants after the supplementation was significantly higher in comparison with those with tMg concentrations > 0.75 mmol/L (Nielsen et al. 2010). The tMg values of these participants approached the limits of the reference range, which could indicate the first signs of Mg homeostasis disorders. Even though all students that participated in our study had normal serum tMg levels, we analyzed the baseline differences in the mean %iMg in tMg across tertiles of serum tMg concentrations in the complete study cohort. Analysis revealed that 10 days of supplementation caused an increase of % iMg from tMg regardless of the pre-intervention status and the type of pharmaceutical preparation that was administered (Table 7). Other oral Mg supplementation randomized control trials which included blood measurements of both iMg and tMg concentrations were conducted primarily in populations with comorbidities or have found no effects of Mg supplementation on the biodistribution on circulating iMg and tMg (Barbagallo et al. 2010; Kazaks et al. 2010; Závaczki et al. 2003). Moreover, the studies that incorporated these biomarkers of Mg status were heterogeneous regarding the specimen used for the iMg analysis (plasma, serum, whole blood) and thus are difficult to compare directly.
In all three intervention groups slight changes of Ca, Na, K, and P levels were observed in both test days (i.e. follow up—day 5 and end-point—day 11) (Fig. 2). The most prominent effects were detected in Supplement 1 group: reduced level of tCa both in the fifth and eleventh day (Fig. 2A), decreased level of Na at the end of the supplementation period (Fig. 2C), and significantly increased level of K (Fig. 2D). Nielsen et al. reported no significant changes in serum tCa concentration after 5 and 7 weeks of Mg supplementation in 100 adults older than 51 years with sleeping issues. In addition, the same group of authors noted that Mg-supplemented subjects had a significant increase in Mg urinary excretion, while the increase in Ca excretion remained below the significance threshold (Nielsen et al. 2010). Although detected changes in our study were significant compared to the baseline, concentrations of these minerals remained in physiological concentrations after 10 days supplementation period. Confirmed changes in the levels of Ca, Na, K and P in three examined points indicate that a healthy organism establishes natural balance after additional mineral and electrolytes intake if there is no other deficiency or associated diseases of the organs involved in their metabolic homeostasis. Murray et al. (2006) explained that the mineral homeostatic system is sufficiently flexible to maintain blood Ca, Mg and P levels within the normal range regardless of the wide variations in dietary intake. However, changes in Na and K indicate complex interactions among the various nutrients and the physiological compartments in which these minerals are metabolized (Henkin et al. 2018). In order to determine the clinical significance, further research is warranted on a larger number of subjects over a longer period of supplementation, as well as in specifically targeted population groups that may be affected by subtle changes in ion concentration.
Several study limitations should be acknowledged. Relatively small sample size, inclusion of specific population group (i.e. young healthy women), and lack of control group limit the generalization of the results. The duration of the supplementation may have been too short to explore the physiological adaptation and dynamical changes of the supplements’ effects on mineral biomarkers. In addition, a detailed exploration of the impact of auxiliary components in the analyzed dietary supplements on the bioavailability of Mg was outside the scope of this study.
Finally, twenty-four hours dietary recalls, as self-reported retrospective instruments, have inherent measurement error. Additionally, the absence of nutrient intake data correspondent to interventionmid-point is another limitation of this study. Nevertheless, potential restrictions were minimized by the young age of participants, highly standardized interview protocol with memory cues, and application of nationally-appropriate portion size estimation aid.
Conclusions
To the best of our knowledge, this is the first study comparing the effects of three Mg supplements (Mg oxide, Mg citrate and Mg carbonate) given in the same daily doses (100%NRV for Mg) and in pharmaceutical dosage forms with mineral immediately available for absorption considering both iMg and tMg concentration. Although the effects of Mg supplementation have been widely researched and published, there is still a scarcity of literature regarding the bioavailability of Mg administered in the form of powders/granulates. Contrary to previously published data which indicated that organic Mg salt such as magnesium-citrate were more potent in increasing total Mg level, this study revealed that only short-term supplementation with Mg oxide, provided in the form of powder/granulate for direct oral use, resulted in the significant increase of both iMg concentration and %iMg in tMg. Based on the assessment of selected mineral status biomarkers significant changes of Ca, K and Na levels were found exclusively in Mg citrate intervention group. In conclusion, after a short-term supplementation period, Mg oxide demonstrated superior bioavailability compared to Mg citrate without affecting other minerals’ levels. Controlled clinical trials are warranted to further evaluate the effects of Mg supplementation on biochemical status indices of Mg and other relevant minerals especially during a longer period of supplementation and in populations with conditions that could additionally affect their levels such as intestinal and renal disorders.
Data availability
Data available on request due to restrictions eg privacy or ethical.
Code availability
Not applicable.
References
Ahmed F, Mohammed A (2019) Magnesium: the forgotten electrolyte—a review on hypomagnesemia. Med Sci 7(4):56
Altura BT, Altura BM (1991) Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased human subjects. Magnes Trace Elem 10(2–4):90–98
Authority, European Food Safety (2006) Tolerable upper intake levels for vitamins and minerals. Scientific Committee on Food.
Authority, European Food Safety (2017) Dietary reference values for nutrients Summary report. EFSA Supporting Publications 14(12):e15121E
Barbagallo M et al (2010) Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res 23(3):131–137
Belluci MM et al (2020) Severe magnesium deficiency compromises systemic bone mineral density and aggravates inflammatory bone resorption. J Nutrit Biochem 77:108301
Blancquaert L, Vervaet C, Derave W (2019) Predicting and testing bioavailability of magnesium supplements. Nutrients 11(7):1663
Cazzola R et al (2020) Going to the roots of reduced magnesium dietary intake: a tradeoff between climate changes and sources. Heliyon 6(11):e05390
Committee, Dietary Guidelines Advisory (2020) Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the secretary of agriculture and the secretary of health and human services. Agricultural Research Service.
Costello RB, Nielsen F (2017) Interpreting magnesium status to enhance clinical care-key indicators. Curr Opin Clin Nutr Metab Care 20(6):504
Cuadrado-Soto E et al (2020) Usual dietary intake, nutritional adequacy and food sources of calcium, phosphorus, magnesium and vitamin D of Spanish children aged one to< 10 years. findings from the EsNuPI study. Nutrients 12(6):1787
De Baaij JHF, Hoenderop JGJ, Bindels RJM (2015) Magnesium in man: implications for health and disease. Physiol Rev. https://doi.org/10.1152/physrev.00012.2014
Dibaba DT et al (2017) The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: a meta-analysis of randomized controlled trials. Am J Clin Nutr 106(3):921–929
Dickinson A, MacKay D (2014) Health habits and other characteristics of dietary supplement users: a review. Nutr J 13(1):1–8
Elmadfa I, Meyer AL, Nowak V (2009) Content. Ann Nutr Metab 55(Suppl. 2):1–40
Ford ES, Mokdad AH (2003) Dietary magnesium intake in a national sample of US adults. J Nutr 133(9):2879–2882
Gröber U, Schmidt J, Kisters K (2015) Magnesium in prevention and therapy. Nutrients 7(9):8199–8226
Gurinovic M et al (2018) Development, features and application of DIET ASSESS & PLAN (DAP) software in supporting public health nutrition research in Central Eastern European Countries (CEEC). Food Chem 238:186–194
Gurinovic M et al (2016) Establishment and advances in the online Serbian food and recipe data base harmonized with EuroFIR standards. Food Chem 193:30–38
Intakes, Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference (1997) Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academies Press, US.
Jacques PF et al (1993) Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr 57(2):182–189
Kamiński M, Kręgielska-Narożna M, Bogdański P (2020) Determination of the popularity of dietary supplements using google search rankings. Nutrients 12(4):908
Kappeler D et al (2017) Higher bioavailability of magnesium citrate as compared to magnesium oxide shown by evaluation of urinary excretion and serum levels after single-dose administration in a randomized cross-over study. BMC Nutr 3(1):7
Kazaks AG et al (2010) Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: a randomized placebo controlled trial. J Asthma 47(1):83–92
Lindberg JS et al (1990) Magnesium bioavailability from magnesium citrate and magnesium oxide. J Am Coll Nutr 9(1):48–55
Mishra S et al (2021) Dietary supplement use among adults: United States, 2017–2018.
Muñoz-Garach A, García-Fontana B, Muñoz-Torres M (2020) Nutrients and dietary patterns related to osteoporosis. Nutrients 12(7):196
Newhouse IJ, Finstad EW (2000) The effects of magnesium supplementation on exercise performance. Clin J Sport Med 10(3):195–200
Nielsen FH, Johnson LAK, Zeng H (2010) Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes Res 23:158–168
Nikolic M et al (2018) The development and validation of food atlas for portion size estimation in the Balkan region. Front Nutr 5:78
Olza J et al (2017) Reported dietary intake, disparity between the reported consumption and the level needed for adequacy and food sources of calcium, phosphorus, magnesium and vitamin D in the Spanish population: findings from the ANIBES study. Nutrients 9(2):168
Schuchardt JP, Hahn A (2017) Intestinal absorption and factors influencing bioavailability of magnesium-an update. Curr Nutr Food Sci 13(4):260–278
Rooney MR et al (2020) Circulating ionized magnesium: comparisons with circulating total magnesium and the response to magnesium supplementation in a randomized controlled trial. Nutrients 12(1):263
Siener R, Jahnen A, Hesse A (2011) Bioavailability of magnesium from different pharmaceutical formulations. Urol Res 39(2):123–127
Tang C-F et al (2020) Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol 886:173546
Walker AF et al (2003) Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes Res 16(3):183–191
Welch AA et al (2009) Variation in intakes of calcium, phosphorus, magnesium, iron and potassium in 10 countries in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr 63(4):S101–S121
Závaczki Z et al (2003) Magnesium-orotate supplementation for idiopathic infertile male patients: a randomized, placebo-controlled clinical pilot study. Magnes Res 16(2):131–136
Zhan J et al (2020) Circulating ionized magnesium as a measure of supplement bioavailability: results from a pilot study for randomized clinical trial. Nutrients 12(5):1245
Acknowledgements
The authors wish to express their profound appreciation to Nova Biomedical (Waltham, MA, USA) for providing access to Stat Profile Prime Plus Critical Care blood gas analyzer and reagents' donation. Nevertheless, the sponsor had no role in the study design, data collection and analysis, the decision to publish or preparation of the manuscript. We gratefully thank all the students who volunteered to participate in the study for their cooperation, time and motivation. We particularly thank Agnes Kadvan for IT support and technical assistance.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Conceptualization, NI and NM; methodology, NI, NM, MZ, BR; software, NM; validation, MZ, BDJ, SI, BR; formal analysis, MZ, DK and BDJ; writing—original draft preparation, NI, NM and BR; writing—review and editing, MZ, DK, BDJ and SI; visualization, MZ; supervision, DK; project administration, NI. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Consent for publication
All authors have read and approved the final submitted manuscript. We certify that this manuscript is original and not previously published in any form including on preprint servers, nor is it being considered elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivanovic, N.D., Radosavljevic, B., Zekovic, M. et al. Effects of short-term magnesium supplementation on ionized, total magnesium and other relevant electrolytes levels. Biometals 35, 267–283 (2022). https://doi.org/10.1007/s10534-022-00363-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-022-00363-y