Abstract
Amyloid β (Aβ) fibrils and amorphous aggregates are found in the brain of patients with Alzheimer’s disease (AD), and are implicated in the etiology of AD. The metal imbalance is also among leading causes of AD, owing to the fact that Aβ aggregation takes place in the synaptic cleft where Aβ, Cu(II) and Fe(III) are found in abnormally high concentrations. Aβ40 and Aβ42 are the main components of plaques found in afflicted brains. Coordination of Cu(II) and Fe(III) ions to Aβ peptides have been linked to Aβ aggregation and production of reactive oxygen species, two key events in the development of AD pathology. Metal chelation was proposed as a therapy for AD on the basis that it might prevent Aβ aggregation. In this work, we first examined the formation of Aβ40 and Aβ42 aggregates in the presence of metal ions, i.e. Fe(III) and Cu(II), which were detected by fluorescence spectroscopy and atomic force microscopy. Second, we studied the ability of the two chelators, ethylenediaminetetraacetic acid and 5-chloro-7-iodo-8-hydroxyquinoline (clioquinol), to investigate their effect on the availability of these metal ions to interact with Aβ and thereby their effect on Aβ accumulation. Our findings show that Fe(III), but not Cu(II), promote aggregation of both Aβ40 and Aβ42. We also found that only clioquinol decreased significantly iron ion-induced aggregation of Aβ42. The presence of ions and/or chelators also affected the morphology of Aβ aggregates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease described firstly by Alois Alzheimer in 1906 (Selkoe and Hardy 2016). AD is characterized by progressive dementia and abnormal extracellular amyloid-beta (Aβ) peptide depositions in the form of senile plaques in the brain (Santos et al. 2016). In the elderly, AD is the most common cause of dementia (Sastre et al. 2015). Aβ appears in two forms, Aβ-40 and Aβ42, and are the main components of the plaques that are found in afflicted brains (Gu et al. 2016). Although the concentration of Aβ40 soluble monomer is more than Aβ42, Aβ42 is the main component of amyloid plaques (Gu et al. 2016). Aβ-40 and Aβ42, differ by two residues at the C-terminus (Cukalevski et al. 2015). These two hydrophobic residues (Ile and Ala), make Aβ42 more aggregation prone than Aβ40 (Cukalevski et al. 2015). The amyloid beta peptides are cleaved from amyloid precursor protein (APP) by two secretase enzymes, called β- and γ-secretases (Wallin et al. 2016). Oligomers, amyloid fibrils, protofibrils and diffuse plaques (non-fibrous aggregates) of amyloid beta peptides such as amorphous aggregates are also found in the brain (Jiang et al. 2012). Aβ oligomers are more cytotoxic than fibrillary aggregates (Jamasbi et al. 2016). Oligomers have a wide molecular weight range (from <10 to >100 kDa) (Sakono and Zako 2010).
Decreasing synapse number, inhibiting long-term potentiation in the hippocampus of rodent and memory loss in rats are the results of Aβ oligomer injections into their healthy brains (Selkoe and Hardy 2016).
Copper and iron ions play pivotal roles in the biological processes in some regions of the brain (Wineman-Fisher et al. 2016). According to previous studies, high concentrations of Cu(II) (400 mM) and Fe(III) (1 mM) are co-localized within the amyloid plaques in the brain and may be involved in their formation (Orvig et al. 2012).
These redox-active metal ions can bind to Aβ and may produce free-radicals or reactive oxygen species (ROS) that are the major source of oxidative stress (Orvig et al. 2012). According to a recent study, they bind to the His13 and His14 residues of Aβ peptides in such a way that the relative strength of the binding of iron to above residues is more than that of copper and zinc (Kong et al. 2015).
Metal ion chelation has been proposed as a novel therapeutic strategy for Alzheimer’s disease (Santos et al. 2016). The field of metal chelation in AD continues to expand rapidly (Santos et al. 2016). Treating AD by chelation therapy should primarily involve a low molecular weight, lipophilic metal chelator to cross the blood–brain barrier (BBB), bind metal ions, and thereby prevent Aβ aggregation (Prachayasittikul et al. 2013). Clioquinol (CQ) (one of the metal chelators that was used in this study) is a small lipophilic antiamoebic drug that binds non-specifically to Cu(II) or Zn(II) in a 2:1 ligand: metal ratio. The affinity constant of CQ for Cu(II) is only one order of magnitude higher than that for Zn(II) (log K = 10 and 9, respectively) (Robert et al. 2015). CQ can bind to iron to a lesser extent than copper (Matlack et al. 2014). It is a bidentate chelating agent that forms 2/1 (ligand/metal ratio) square planar complexes and is able to cross the BBB (Lim et al. 2009). Ethylenediaminetetraacetic acid (EDTA), a hexadentate chelator that does not cross the BBB and forms especially strong complexes with Cu(II) and Fe(III) (also with ions such as Mn(II), Pb(II) and Co(III)), was used to see the effect of using another chelator (without considering its potential therapeutic use) on aggregate formation. In this way we would presumably better ascribe any effect of CQ on Aβ aggregation to its metal ion chelation rather than to its non-chelation chemical interaction with the peptide. Many reports about the effects of metals and chelators on amyloid aggregation have been published (Santos et al. 2016). It is proposed that metal chelators or better to say, metal protein attenuating compounds (MPACs), either might dissolve the deposits in the brain tissue or might prevent amyloid beta aggregation (Santos et al. 2016). It is reported that CQ can inhibit Aβ accumulations and improve memory capacity in Alzheimer’s disease transgenic mice (Robert et al. 2015).
Amyloid fibrils can be detected by fluorescence spectroscopy, with the use of thioflavin T (ThT), a specific dye with specific binding capacity to β-sheets (Faller et al. 2013). We used TFE to prepare relatively hydrophobic Aβ40 and Aβ42 stock solutions (modeling the hydrophobic environment of neuronal membrane) as TFE does not interfere with ThT fluorescence and it does not affect the ThT emission of Aβ fibrils (Tiiman et al. 2015). Chloride salts of copper and iron ions were used because of their relatively easy solubilization in aqueous solutions and also because Cl− anion is abundantly present in biological fluids.
Dysregulation of copper and iron homeostasis is known to occur upon normal aging and in neurodegenerative diseases, specifically in AD (Robert et al. 2015). Consequently, it has been proposed that oxidative stress and release of reactive oxygen species could be the result of their dysregulation. But it is remained to be clarified if copper and iron dysregulation also promote Aβ peptide aggregation? Here, we have tried to investigate this question.
In this work we have examined the effects of copper and iron ions on the aggregation process of Aβ. Herein, we made use of fluorescence spectroscopy to provide evidence whether Cu(II) or Fe(III) can strengthen the ability of Aβ40 and Aβ42 to form ThT-positive structures or not. In addition, with the use of atomic force microscopy (AFM), we tried to visualize the aggregates (oligomers, fibrils, and amorphous aggregates) that were formed in the presence of beta-amyloid peptides, alone, or in the presence of Fe(III) or Cu (II) ions. We also examined the presence of metal chelators (CQ and EDTA) in the reaction mixtures containing beta amyloid peptides and metal ions to investigate their effect on beta-amyloid aggregate formation. Selection of CQ was mainly because it can pass through BBB (contrary to EDTA) and consequently because of its therapeutic potential in AD.
Materials and methods
Chemicals
Aβ40 and Aβ42 peptides were purchased from Sigma and their stock solutions were prepared in TFE. All samples were prepared by diluting proper volumes of peptide stocks in 0.05 M Tris buffer solution, pH 7.4. Chloride salt solutions of Fe(III) and Cu(II), were purchased from Aldrich. ThT was from Sigma and its stock solution was prepared in distilled water. CQ was purchased from Fluka and ethanol was used to prepare its stock solution. The EDTA stock solution was prepared in distilled water.
ThT fluorescence assay
In all experiments, the concentrations of Aβ40 and Aβ42 were 1 µM. The final concentrations of Cu(II) and Fe(III) in the sample mixtures were held at 1 µM (equimolar metal: peptide ratio), and were added before the start of incubation (25 °C for 24 h). The concentrations of EDTA and CQ in sample mixtures were 20 µM and were also added at the beginning of the incubation. The β-sheet-rich content of amyloid aggregates was detected by ThT fluorescence spectroscopy. Experiments were triplicated and the data points were obtained by averaging. The fluorescence intensity of each sample was measured by excitation and emission at 440 and 480 nm (slit width 5 nm), respectively, with the use of a Cary Eclips VARIAN fluorescence spectrophotometer. To eliminate the interference effects of CQ and EDTA in chelator-containing samples during fluorescence measurements, we dialyzed the incubated samples for 12 h (10 KDa cut-off) and exchanged the buffer four times.
Atomic force microscopy (AFM)
In order to confirm and visualize the presence of amyloid fibrils and study the morphology of aggregates, AFM technique was employed. 5 µL of each sample was deposited onto a freshly cleaved mica slide, with a dimension of 1 × 1 cm, and incubated for 30 min at room temperature. Samples were rinsed with 50–100 µL deionized water to remove unbound protein and were left to dry at room temperature. The AFM images were obtained using a Veeco AFM instrument with the use of a non-contact mode AFM (nc-AFM). WSXM software was used to measure the size and diameter of aggregates from the Z-heights in AFM images (Horcas et al. 2007).
Statistical analysis
Statistical analyses were carried out with GraphPad Prism version 7.00 software. Two-tailed Student’s t test and two-way ANOVA test were used for differences between two groups. P-value < 0.05 was considered significant statistically. The data are presented as mean ± SD of at least three independent experiments.
Results and discussion
Aggregation of Aβ in the presence of metal ions
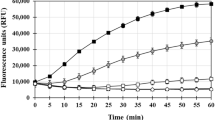
Aβ40 and Aβ42 in the absence of Cu(II) and Fe(III) ions formed ThT-reactive amyloid species. The fluorescence studies of samples containing only Aβ40 or Aβ42 resulted in the same intensities (25 a.u., Fig. 1). In the case of Aβ40, the presence of Cu(II) (Aβ40 + Cu) decreased the intensity (17 a.u., Fig. 1) and that of Fe(III) (Aβ40 + Fe) increased considerably the intensity (37 a.u., Fig. 1). Effect of Cu(II) on Aβ42 (Aβ42 + Cu) was the same as that of Aβ40, i.e. 17 a.u., and the presence of Fe(III) (Aβ42 + Fe) also showed an increase, although the increase was mild in comparing with Aβ40 (29 a.u.). So, it seems that copper ions could not increase ThT-reactive species and iron ions showed increases, for both Aβ40 and Aβ42 peptides.
AFM images of beta amyloid peptide aggregates in the absence metal ions showed the presence of numerous oligomers with a diameter about 40–60 nm (Fig. 2I), although they were more numerous in Aβ42 samples (Fig. 2IB). Since their fluorescence intensities were the same (above paragraph) and since in ThT fluorescence, the beta structures are detected, it seems that amorphous aggregates were more numerous in Aβ42 samples. Some small clusters of individual amyloid fibers and amorphous aggregates were evident in Aβ40 sample (Fig. 2IA). As it was stated above, the presence of iron increased the fluorescence intensities in both Aβ40 and Aβ42 samples. AFM results confirmed this observation by the appearance of a considerable number of fibrillar structures and disperse aggregates with denser fibrillar and prefibrillar aggregates in the samples containing Fe(III). Figure 2III shows the effect Cu(II) on the extent and morphology of aggregates. Aβ40, in the presence of Cu(II) ions, showed more affinity to form amorphous aggregates and oligomers with a diameter of about 40 nm (Fig. 2III). The diameter of Aβ42 amorphous aggregates and oligomers in the presence of Cu(II) ions were more than that of Aβ40 with Cu(II) (Fig. 3IIIA, B). The number of oligomers in the presence of copper ions were significantly higher than Aβ40 and Aβ42 samples with no Cu(II) ions. The fluorescence intensities of Aβ40 and Aβ42 in the presence of copper ions were significantly lower than those without Cu(II), presumably because they could not have formed considerable numbers of ThT-reactive aggregates (Fig. 1).
(I) AFM images of aggregates containing amyloid peptides and chelators: (IA) Aβ40 + EDTA; (IB) Aβ40 + CQ; (IC) Aβ42 + EDTA; (ID) Aβ42 + CQ. (II) AFM images of aggregates containing amyloid peptides, chelators and Cu(II) ions: (IIA) Aβ40 + Cu + EDTA; (IIB) Aβ40 + Cu + CQ; (IIC) Aβ42 + Cu + EDTA; (IID) Aβ42 + Cu + CQ. (III) AFM images of aggregates containing amyloid peptides, chelators and Fe(III) ions: (IIIA) Aβ40 + Fe + EDTA; (IIIB) Aβ40 + Fe + CQ; (IIIC) Aβ42 + Fe + EDTA; (IIID) Aβ42 + Fe + CQ
Aggregation of Aβ in the presence of metal ions and chelators
As it was stated before, there are extensive evidences that link AD pathology to metal dysregulation. It has been suggested that the problems in the homeostasis of metals in AD patients might be related to amyloid and tau pathologies (Santos et al. 2016; Robert et al. 2015). In this regard, it has been consequently suggested that metal MPACs might have therapeutic potentials in AD. It has also been proposed that metal chelators either might dissolve the deposits in the brain tissue or might prevent amyloid beta aggregation (Santos et al. 2016). It has specifically been reported that CQ can inhibit Aβ accumulations and improve memory capacity in Alzheimer’s disease transgenic mice (Robert et al. 2015).
The fluorescence intensities and data from AFM images of Aβ40 and Aβ42 in the presence of CQ or EDTA was not significantly different from those of samples containing only Aβ40 or Aβ42, and non-fibrillar aggregates were dominantly observed in AFM images (Figs. 1, 3I). Also, the intensities of Aβ40 and Aβ42 in the presence of Cu(II) ions and CQ or EDTA was significantly higher than those containing only Aβ40 or Aβ42 and Cu(II) ions, which means that both of the chelators could chelate copper ions and alleviate the reduction in intensities that was observed because of the presence of only copper ions (Fig. 1). AFM images of above samples showed that the oligomer contents were reduced (in the presence of CQ), although dispersed clusters and amorphous aggregates of beta-amyloid peptides were also evident (Fig. 3II).
The fluorescence intensities of Aβ40 and Aβ42, in the presence of Fe(III) ions containing EDTA, was not significantly different from the sample of amyloids and Fe(III) without EDTA (Fig. 1). AFM images of samples that contained Aβ40 and Fe(III) ions resulted in the appearance of dense fibrillary aggregates (Fig. 2IIA), changed to the appearance of dispersed fibrillary aggregates after inclusion of EDTA (Fig. 3IIIA). The fluorescence intensities of the Aβ40 or Aβ42 in the presence of iron and CQ (26 and 22 a.u., respectively) was considerably lower than that of only iron ion (37 and 29 a.u., respectively) (Fig. 1). From this observation it can be concluded that CQ is a more effective chelator in reducing the appearance of ThT-reactive species in the presence of iron ion than EDTA.
AFM images of samples containing Aβ40 or Aβ42, in the presence of Fe(III) ions and chelators, are presented in Fig. 3III. The presence of EDTA in Aβ40 + Fe(III) samples resulted in the appearance of numerous fibrillar species (Fig. 3IIIA), which could also be seen in samples containing only Aβ40 + Fe(III) (Fig. 2IIA) and was also the case for Aβ42 (compare Figs. 2IIB, 3IIIC). How about the observed reductive effect of CQ on the intensities obtained in the presence of either Aβ40 or Aβ42 and Fe(III)? Comparison between Fig. 2IIA, B (Aβ40 and Aβ42, respectively, in the presence of iron) and Fig. 3IIIB (Aβ40 + Fe + CQ) and 3IIID (Aβ42 + Fe + CQ) shows that disappearance of fibrillar species in the presence of CQ (Fig. 3IIIB) affirms the related fluorescence results (Fig. 1). In the case of Aβ42 (Fig. 3IIID), the fibrillar species still can be observed (compare with Fig. 2IIB) and is not that much quantitative.
Conclusions
Copper and iron dysregulation is considered as a consequence of aging and there are considerable evidences that is also involved in the pathogenesis of AD as a neurodegenerative disease. The main effect of this metal ion dysregulation is proposed to be the oxidative stress and the consequent release of ROS species. Also, these metal ions have been shown to interact with amyloid peptides and potentially affect protein misfolding and aggregation. Our results show that copper not only has no promotive effect in the fabrication ThT-reactive species (i.e. beta sheet structures) but also has a reductive effect. On the other hand, this work shows a considerable positive effect of Fe(III) ions on the appearance of ThT-reactive species and aggregates. Both of these ions were chelated by both of the chelators that were used in this study (EDTA and CQ) and the consequent effect of chelating these ions was observed. In the case of copper, its chelation resulted in an increase in its aggregative properties (copper itself reduced the aggregation), and in the case of iron, only CQ could decrease its positive aggregative effect on both peptides. So according to this study, CQ, which is able to pass the BBB, only shows its potential anti-aggregative effect on Fe(III) ions and this anti-aggregative effect is relevant in the case of Cu(II) ions.
References
Cukalevski R, Yang X, Meisl G, Weininger U, Bernfur K, Frohm B, Knowles TP, Linse S (2015) The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary mixtures but cross-react during primary nucleation. Chem Sci 6:4215–4233
Faller P, Hureau C, Berthoumieu O (2013) Role of metal ions in the self-assembly of the Alzheimer’s amyloid Aβ peptide. Inorg Chem 52:12193–12206
Gu L, Tran J, Jiang L, Guo Z (2016) A new structural model of Alzheimer’s Aβ42 fibrils based on electron paramagnetic resonance data and Rosetta modeling. J Struct Biol 194:61–67
Horcas I, Fernández R, Gomez-Rodriguez JM, Colchero J, Gómez-Herrero J, Baro AM (2007) WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev Sci Instrum 78:013705
Jamasbi E, Wade DJ, Separovic F, Mohammed AH (2016) Amyloid beta (aβ) peptide and factors that play important roles in Alzheimer’s disease. Curr Med Chem 23:884–892
Jiang D, Rauda I, Han S, Chen S, Zhou F (2012) Aggregation pathways of the amyloid β(1−42) peptide depend on its colloidal stability and ordered β-sheet stacking. Langmuir 28:12711–12721
Kong X, Zhao Z, Lei X, Zhang B, Dai D, Jiang L (2015) Interaction of metal ions with the his13-his14 sequence relevant to Alzheimer’s disease. J Phys Chem A 119:3528–3534
Lim MH, Mancino AM, Hindo SS, Kochi A (2009) Effects of clioquinol on metal-triggered amyloid-β aggregation revisited. Inorg Chem 48:9596–9598
Matlack KES, Tardiff DF, Narayan P, Hamamichi S, Caldwell KA, Caldwell GA, Lindquist S (2014) Clioquinol promotes the degradation of metal-dependent amyloid-β (aβ) oligomers to restore endocytosis and ameliorate aβ toxicity. Proc Nat Acad Sci USA 111:4013–4018
Orvig C, Rodríguez-Rodríguez C, Telpoukhovskaia M (2012) The art of building multifunctional metal-binding agents from basic molecular scaffolds for the potential application in neurodegenerative diseases. Coord Chem Rev 256:2308–2332
Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2013) 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug Des Devel Ther 7:1157–1178
Robert A, Liu Y, Nguyen M, Meunier B (2015) Regulation of copper and iron homeostasis by metal chelators: a possible chemotherapy for Alzheimer’s disease. Acc Chem Res 48:1332–1339
Sakono M, Zako T (2010) Amyloid oligomers: formation and toxicity of Aβ oligomers. FEBS J 277:1348–1358
Santos MA, Chand K, Chaves S (2016) Recent progress in multifunctional metal chelators as potential drugs for Alzheimer’s disease. Coord Chem Rev 327:287–303
Sastre M, Ritchie CW, Hajji N (2015) Metal ions in Alzheimer’s disease brain. JSM Alzheimer’s Dis Rel Dement 2:1014–1019
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608
Tiiman A, Krishtal J, Palumaa P, Tõugu V (2015) In vitro fibrillization of Alzheimer’s amyloid-β peptide (1–42). AIP Adv 5:092401
Wallin C, Kulkarni YS, Abelein A, Jarvet J, Liao Q, Strodel B, Olsson L, Luo J, Abrahams JP, Sholts SB, Roos PM (2016) Characterization of Mn(II) ion binding to the amyloid-β peptide in Alzheimer’s disease. J Trace Elem Med Bio 38:183–193
Wineman-Fisher V, Bloch DN, Miller Y (2016) Challenges in studying the structures of metal-amyloid oligomers related to type 2 diabetes, Parkinson’s disease, and Alzheimer’s disease. Coord Chem Rev 327:20–26
Acknowledgements
We acknowledge the support of the research council of the Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, Iran, without which this work could not be possible to start and finish. We also thank the Department of Chemistry at IASBS for their generous cooperation in providing us the opportunity to make use of their fluorescence spectroscopy facility. Also, we thank the Department of Physics at IASBS for their kind permission to use their AFM facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tahmasebinia, F., Emadi, S. Effect of metal chelators on the aggregation of beta-amyloid peptides in the presence of copper and iron. Biometals 30, 285–293 (2017). https://doi.org/10.1007/s10534-017-0005-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-017-0005-2