Abstract
Russula atropurpurea can accumulate remarkably high concentrations of Zn in its sporocarps. We have previously demonstrated that 40 % of the intracellular Zn in this species is sequestered by MT-like RaZBP peptides. To see what other mechanisms for the handling of the accumulated Zn are available to R. atropurpurea, we searched its transcriptome for cDNAs coding for transporters of the cation diffusion facilitator (CDF) family. The transcriptome search enabled us to identify RaCDF1 and RaCDF2, which were further subjected to functional studies in metal sensitive Saccharomyces cerevisiae. The expression of RaCDF1 and its translational fusion with green fluorescent protein (GFP) protected the yeasts against Zn and Co, but not Cd or Mn, toxicity and led to increased Zn accumulation in the cells. The GFP fluorescence, observed in the RaCDF1::GFP-expressing yeasts on tonoplasts, indicated that the RaCDF1-mediated Zn and Co tolerance was a result of vacuolar sequestration of the metals. The expression of RaCDF2 supported Zn, but not Mn, tolerance in the yeasts and reduced the cellular uptake of Zn, which is congruent with the proposed idea of the Zn-efflux function of RaCDF2, supported by the localization of GFP-derived fluorescence on the plasma membrane of the yeasts expressing functional RaCDF2::GFP. Contrarily, RaCDF2 increased the sensitivity to Co and Cd in the yeasts and significantly promoted Cd uptake, which suggested that it can act as a bidirectional metal transporter. The notion that RaCDF1 and RaCDF2 are genuine CDF transporters in R. atropurputrea was further reinforced by the fact that the RaCDF-associated metal tolerance and uptake phenotypes were lost upon the replacement of histidyl (in RaCDF1) and aspartyl (in RaCDF2), which are highly conserved in the second transmembrane domain and known to be essential for the function of CDF proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is an essential catalytic and structural component of many proteins. However, an uncontrolled access of proteins to Zn under a transient or permanent (in Zn accumulators) metal overload would result in an aberrant binding of Zn ions to cysteinyl thiols or other functional groups, rendering proteins dysfunctional. In an extensive study, scoring the Zn contents of basidiomycetous fungi, Vetter et al. (1997) have identified Russula atropurpurea, an ectomycorrhizal (EM) fungus common in the northern temperate ecosystems, as a species with metal concentrations in the sporocarp tissue of up to 1067 mg Zn kg−1 dry weight (dwt). This is markedly exceeding the Zn levels (median of 98.6 mg Zn kg−1 dwt) reported in the sporocarps of other 86 EM species, including 22 Russula spp., collected from unpolluted areas (Borovička and Řanda 2007); the authors likewise noted high Zn concentrations in R. atropurpurea, ranging from 745 to 1062 mg kg−1 dwt.
We have recently documented that 40 % of the R. atropurpurea sporocarp Zn binds with isomorphic, 53-amino acid (AA) RaZBP1 peptides, which are only distantly related to metallothioneins (MTs) (Leonhardt et al. 2014). MTs are cytosolic, metal-chelating peptides, which constitute a part of the metal-handling system in cells, both for the storage of biologically important metals (Zn and Cu in particular) and sequestration of toxic metal species (Palacios et al. 2011). Saccharomyces cerevisiae and Schizosaccharomyces pombe possess MTs Crs5 (Pagani et al. 2007) and Zym1 (Borrelly et al. 2002), respectively, which are implicated in the handling of the physiological cytosolic Zn pools under normal conditions, while excess metal is compartmentalized. The compartmentalization of Zn2+ into vacuoles plays a dominant role in the elimination of excess Zn in S. cerevisiae (Bleackley and MacGillivray 2011) and is mediated by the Zrc1 and Cot1 transporters of the cation diffusion facilitator (CDF) family (MacDiarmid et al. 2000, 2002, 2003), the later recognizing also Co2+ for the transport and safe storage in the vacuole (Conklin et al. 1992). Sequestration of Zn in the endoplasmic reticulum (ER) via a CDF transporter Zhf1 has been shown to confer Zn tolerance to S. pombe (Borrelly et al. 2002; Clemens et al. 2002), indicating that different compartments can be employed as a sink for Zn in different species.
The members of the CDF family are found at all phylogenetic levels with a key function in the transport of transition metal cations (as Zn2+, Co2+, Fe2+, Cd2+, Ni2+ or Mn2+/H+ or K+ antiporters) from the cytoplasm out of the cell or into the subcellular compartments (Gaither and Eide 2001; Kolaj-Robin et al. 2015; Montanini et al. 2007). Based on the comprehensive phylogenetic analysis of putative CDF proteins and the knowledge about the selectivity or preferences for the major metal substrate of functionally characterized ones, Montanini et al. (2007) sorted the transporters into three major clusters, Zn-CDF, Fe/Zn-CDF, and Mn-CDF.
The three members of the CDF family characterized so far from mycorrhizal fungi belong to the Zn-CDF cluster. Similar to Zhf1 of S. pombe, the HcZnT1 protein of EM Hebeloma cylindrosporum (Blaudez and Chalot 2011) and OmZnT1 of ericoid mycorrhizal Oidiodendron maius (Khouja et al. 2013) localized in model yeasts to ER, conferring increased tolerance to Zn2+ (HcZnT1) and Zn2+ and Co2+ (OmZnT1), but not Cd2+ or Mn2+. Also the Zn-inducible GintZnT1 from arbuscular mycorrhizal (AM) Rhizophagus intraradices localized to non-vacuolar endomembranes in S. cerevisiae but did not contribute to its Zn tolerance (Gonzáles-Guerrero et al. 2005). Studies in H. cylindrosporum mycelium further revealed the targeting of excess Zn into small, non-vacuolar vesicles (Blaudez and Chalot 2011); these were observed also in Hebeloma mesophaeum and the sequestration of Zn in its sporocarps appeared, unlike in R. atropurpurea, dominated by compartmentalization (Sácký et al. 2014).
Here we describe two distinct functional CDF transporters of R. atropurpurea, RaCDF1 and RaCDF2, which cluster with Zn-CDF and Mn-CDF proteins, respectively. The expression of RaCDF1 in S. cerevisiae revealed vacuolar localization of the encoded protein, which could be correlated with its ability to confer Zn and Co tolerance. We further document that RaCDF2 in yeasts localizes to the plasma membrane where it acts as a Zn exporter, supporting the detoxification of Zn, and apparently transports also Cd and Co, but in the opposite direction.
Materials and methods
R. atropurpurea and general procedures
The sporocarp of R. atropurpurea isolate PRM 858109 (Leonhardt et al. 2014) fixed by freeze-drying upon harvest and stored at −80 °C was used as a source of RNA and genomic DNA. The sequence information of the population of shotgun sequenced sporocarp transcripts was available from the same isolate, and it was searched using characterized CDF proteins of fungal, animal and plant origin as queries in translated nucleotide tBLASTn analysis (Altschul et al. 1990). Routine DNA manipulations and RNA work were performed according to the standard protocols. Double stranded cDNAs and genomic clones were produced by PCR that used (if not stated otherwise) Q5 High-Fidelity DNA polymerase (New England Biolabs) and gene-specific primers of sequences shown in Table S1; primary amplicons were maintained in pGEM-T Easy vector (Promega). Recombinant DNAs propagated in E. coli DH5α were subjected to custom DNA sequencing on both strands with the vector-specific primers.
Amplification of cDNA and genomic RaCDF clones and sequence analysis
Total cellular RNA was extracted from 50 mg of the freeze-dried fungal tissues ground with a mortar and pestle using an RNeasy Plant Mini Kit with the RLT buffer and RNase free DNase Set (Qiagen) according to manufacturer’s instructions. The mRNA contained in 1 μg of total RNA was reverse transcribed in 20 µl reactions using a Transcriptor First Strand cDNA Synthesis Kit (Roche). The primer pairs used to amplify the coding sequences of RaCDF1 and RaCDF2 in PCR with 1 µl aliquot of the first-strand cDNAs were eCDF1-F plus eCDF1-R and eCDF2-F plus eCDF2-R, respectively. To obtain the genomic sequence data, the chromosomal DNA was isolated using a NucleoSpin Plant II Kit (Macherey–Nagel). The DNA fragments harboring the exons and introns were amplified using the primer pairs designed to match the untranslated 5´ end and 3´ end of cDNA (agCDF1-F and -R for RaCDF1 and agCDF2-F and -R for RaCDF2). The adjacent 5´ DNA sequences were obtained using a GenomeWalker Universal Kit (Clontech) following the manufacturer’s directions. DNA libraries were prepared by using the blunt-end-cutting restriction enzymes EcoRV, PvuII, StuI, and DraI and adaptors provided with the kit. The PCR amplification procedure used the adaptor-specific primers and the gene-specific primary (gw1CDF) and secondary (gw2CDF) nested PCR primers hybridizing within the first or second gene exons. The longest resulting amplicons—899 bp for RaCDF1 and 1149 bp for RaCDF2 clone from StuI and DraI library, respectively—were subjected to DNA sequencing.
The protein sequences deduced from the cDNAs were confirmed by a reciprocal BLASTP analysis (Altschul et al. 1990) against UniProtKB/Swiss-Prot entries and subjected to a transmembrane domain prediction at a Phobius webserver (Käll et al. 2007). A Molecular Evolutionary Genetics Analysis (MEGA) 6.0 package (Tamura et al. 2013) was used to align amino acid sequences of functionally characterized CDFs by MUSCLE (Edgar 2004) and construct the corresponding unrooted phylogenetic trees using the Neighbor-Joining method with Poisson correction model and 10,000 bootstrap replications. The 5´-regions of RaCDF genes were analyzed for the presence of potential transcription factor-binding sites by the scanning against the JASPAR CORE database (Mathelier et al. 2013). The RaCDF1 and RaCDF2 gene sequences were deposited in GenBank: KU311684 and KU311685, respectively.
Construction of expression plasmids
To express RaCDF1 and RaCDF2 in yeasts, the coding sequences amplified from cDNA using the gene-specific primers (eCDF1-F plus eCDF1-R and eCDF2-F plus eCDF2-R, respectively), which introduced BamHI and HindIII sites at the 5´ and 3´ DNA, ends, respectively, were inserted into a BamHI-/HindIII-treated vector p416GPD. This vector contains the low copy yeast origin of replication CEN6/ARS4, uracil selection marker URA3, and constitutive glyceraldehyde-3-phosphate dehydrogenase promoter plus cytochrome-c oxidase gene terminator to control the heterologous gene expression (Mumberg et al. 1995). For the carboxy-terminal green fluorescence protein (GFP)-tagging of RaCDFs, the coding sequence of RaCDF1 (amplified with the primers ifCDF1-F and -R) and RaCDF2 (amplified with the primers ifCDF2-F and -R) without the termination codon were inserted into a BamHI-digested plasmid p416GFP by using an In-Fusion HD Cloning Kit (Clontech Labs.) according to manufacturer’s instructions. The plasmid p416GFP (Hložková et al. 2015) is p416GPD with GFP from plasmid pEGFP-C1 (Clontech) inserted as a BamHI/HindIII DNA fragment. Site-directed mutagenesis of RaCDF- and RaCDF::GFP in p416GPD was performed by the inverse PCR method (Füzik et al. 2014) with Phusion High-Fidelity DNA Polymerase (Thermo Scientific) and the overlapping primers mCDF1-D38A-F and -R or mCDF1-H41A-F and -R for RaCDF1 variants and mCDF2-D163A-F and -R for RaCDF2 variants.
Yeast complementation and metal uptake assays
The yeast strains used for the heterologous expression of RaCDF variants were zrc1Δcot1Δ strain CM137 (MATα can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52 zrc1::His3 cot1::KanR; MacDiarmid et al. 2000), ycf1Δ strain DTY168 (MATα his6 leu2-3,-112 ura3-52 ycf1::hisG; Szczypka et al. 1994), and BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ) and its pmr1Δ derivative (pmr1::kanMX4) obtained from Euroscarf (http://web.uni-frankfurt.de/fb15/mikro/euroscarf/index.html). Transformed yeasts were grown at 30 °C on URA + selective SD agar medium containing (w/v) 0.7 % yeast nitrogen base (Difco), 0.005 % adenine hemisulfate, 2 % glucose, and 0.003 % of each of the essential amino acids (Sigma-Aldrich).
For the metal tolerance plate assays, the mid-log cultures of S. cerevisiae transformants were adjusted to an optical density at 590 nm (OD590) of 0.05, and 5 μl of serial dilutions were spotted on SD medium plates without metal addition or supplemented with Cd, Co, Mn or Zn chlorides. To determine the 50 % inhibitory concentration (IC50metal, the metal concentration that reduced the culture turbidity by half), the growth of the transformants was initiated by inoculation of a metal-containing SD medium with mid-log cultures to a turbidity of 0.4 McFarland units (equivalent to the OD590 of 0.1). Culture growth was monitored with a model DEN-1 densitometer (Bioscan) as cell suspension turbidity T after 24 h (ycf1Δ and zrc1Δcot1Δ cells) or 48 h (cup1Δ cells) of cultivation. The data were adapted to the formula T(c) = T(0)/{1 + exp[c-IC50]/b]}, where c is the added metal concentration, T(0) is the culture density with no added metal and b is the slope of a sigmoidal dose-dependent curve (Anton et al. 2004; Hložková et al. 2015).
Metal uptake assays in SD medium were initiated by adding the metal to 40 ml of the cultures of transformed yeasts that reached OD590 of 2. The cultures were further agitated for 60 min at 30 °C, and the cells were harvested by centrifugation at 4000×g and 25 °C for 1 min. To determine the concentration of the accumulated metal, the surface-bound metal was removed by incubating the cells twice with 5 ml of SD containing 5 mM EDTA for 5 min and washing with 5 ml of 50 mM HEPES (pH 6.0). The separated cells were then digested with 0.75 ml of 65 % nitric acid for 16 h and the sample volume was brought to 5 ml with distilled water. The metal content of the supernatant resulting from a 20 min centrifugation at 20,000×g to remove any cell debris was analyzed by atomic absorption spectrometry (AAS; model Spectr AA300, Varian).
Fluorescence microscopy
The fluorescence microscopy was performed by using a BioSystems Imaging station Cell^R with a MT20 illumination and DSU semiconfocal unit on an IX-81 microscope (Olympus BioSystems) equipped with the model C9100 EM-CCD camera (Hamamatsu Photonix). The yeasts expressing RaCDF::GFP were grown to OD590 of 2.5 in YPD medium (1 % [w/v] yeast extract, 2 % [w/v] peptone and 2 % [w/v] glucose), separated at 5000×g and 25 °C for 1 min and washed with fresh YPD medium. Vacuoles were labeled for 2 h in YPD medium with 30 µg ml−1 FM4-64 (Molecular Probes, Invitrogen, Calsbad, CA), a lipophilic dye staining specifically in yeasts the vacuolar membrane (Hickey et al. 2004). A GFP-deriving fluorescence was then observed with a U-DM-DA-FI-Tx2 FITC filter (excitation band: 495/15 nm, emission band: 530/30 nm) and vacuoles were observed with a U-DM-Cy5 filter (excitation band: 590–650 nm, emission band: 665–740 nm). The recorded black and white images were processed using the ImageJ software (http://imagej.nih.gov/ij/).
Results
Identification of RaCDF cDNAs and genomic RaCDF clones
The BLAST searches of the population of cDNAs from R. atropurpurea PRM 858109 using various CDF proteins as queries enabled the identification of two transcripts designated RaCDF1 and RaCDF2. Predicted coding sequences were confirmed by sequencing of the corresponding amplicons obtained from an independent PCR with the reverse-transcribed sporocarp RNA. Alignment of the deduced 487-AA RaCDF1 and 417-AA RaCDF2 showed that RaCDFs encode unrelated proteins; only a single 45-AA homology region (29 % identity and 45 % similarity) was identified between residues 324–368 of RaCDF1 and 282–325 of RaCDF2 (not shown). The predicted RaCDF1 showed a substantial identity to the vacuolar Zrc1 and Cot1 from S. cerevisiae (Fig. 1a; 41 and 39 % identity was observed along the best-matching 373- and 390-AA protein fragments, respectively). As further indicated in the Neighbor-Joining tree in Fig. 2, RaCDF1 belongs to the Zn-CDFs according to Montanini et al. (2007) and clustered with fungal Zn-tolerance CDFs, including the H. cylindrosporum ZnT1 (Blaudez and Chalot 2011) and O. maitus OmZnT1 (Khouja et al. 2013). The best BLASTP hits obtained for putative RaCDF2 were the Golgi and/or pre-vacuolar Mn2+-transporting AtMTP11 from Arabidopsis thaliana (Delhaize et al. 2007; Peiter et al. 2007) and plasma membrane Mn2+ and Cd2+ exporter CsMTP9 from Cucumis sativus (Migocka et al. 2015) with which a highly homologous, 324-AA part of RaCDF2 shares 38 and 36 % identity, respectively (Fig. 1b). Like the plant homologues, RaCDF2 clustered to the Mn-CDF clade (Fig. 2) and appeared also related to the E. coli Fe-, Zn, and Cd-transporting YiiP (Grass et al. 2005; Wei and Fu 2006) from the Fe/Zn-CDF clade. Both RaCDFs contain the features of CDF proteins described in other organisms (Gaither and Eide 2001; Kolaj-Robin et al. 2015). These include six predicted transmembrane domains (TMDs) with conserved H-X3-D (in RaCDF1) and D-X3-D (in RaCDF2) motifs (X represents any amino acid) requisite in TMD2 and TMD5 for the metal transport, and C-terminal histidyl and aspartyl-containing domain (CTD) implicated in specific sensing of the divalent metal and delivering it to the transmembrane part of the protein. Like in its homologues, in RaCDF1 are TMD4 and TMD5 separated by a histidyl-rich sequence characteristic of CDFs transporting Zn2+ as the major substrate and thought to act as a second cytosolic metal-binding domain in addition to CTD (Kolaj-Robin et al. 2015).
Alignment of predicted RaCDF1 (a) and RaCDF2 (b) with functionally characterized Cation Diffusion Facilitator (CDF) proteins, and the schematic representation of RaCDF genes (c). The positions of predicted transmembrane domains (TMDs) are indicated and conserved residues of TMD2 and TMD5 are boxed. Asterisks indicate identities and dots indicate residues conserved in two of three aligned sequences. The accession numbers for ScZrc1, ScCot1, CsMTP9, and ATMTP11 are as follows: UniProt: P20107, P32798, I1ZI48, and O80632, respectively. The sequence features indicated in the RaCDF genes are the initiation ATG and termination TAG or TGA codons, introns (In), the animal-type metal responsive element (MRE) and the S. cerevisiae-like zinc-responsive elements (ZRE)
An unrooted, Neighbor-Joining-based tree of CDFs characterized from different species. The UniProt accession numbers and substrate metals of the transporters from Zn-, Fe/Zn- and Mn-CDF classes according to Montanini et al. (2007) are indicated. The tree was generated using MEGA 6.0 after the sequence alignment by using MUSCLE. Bootstrap values (%; 10000 replicates) are indicated. Branch lengths are proportional to phylogenetic distances
In order to obtain genomic sequence information, the RaCDF genes along with the sequences upstream from their initiation codons (Fig. 1c) were isolated by using the 5´-genome walking strategy. The mRNA-to-genomic sequence alignments revealed that both coding sequences were interrupted by small, 47 to 58 bp introns flanked by conserved |GT-AG| junctions; RaCDF1 contained nine exons and RaCDF2 contained 14 exons of which exons 6 and 12 were only 7 and 9 bp long, respectively. The screening of potential cis-acting metal-responsive elements from JASPAR CORE databases indicated that with a single exception of the metal response element (MRE; 5´-TGCRCNC-3´, R: A or G, N: any nucleotide), the promoter regions did not contain the metalloregulatory features corresponding to the consensus sequences known from other organisms. Putative MRE (TGCGCGC) was identified at position −540 relative to the initiation codon of RaCDF1. In animals, MREs are typically present in multiple copies in the promoters of metal-responsive genes and recruit the activating MRE-binding transcription factor 1 (MTF-1) metallated with Zn (Andrews 2001; Günther et al. 2012). It is worth noting, however, that the sequences that resemble, but do not fully match the 11 bp zinc-responsive element (ZRE; general consensus 5′-ACCTTNAAGGT-3′) of S. cerevisiae were observed at positions -217 bp (tCCTTCAAGcT) and −279 bp (ACCTgCAgGaT) in RaCDF1 and −605 bp (AaCTgGAgGGT) in RaCDF2 clones (non-consensus nucleotides are indicated by lower case). In the yeast, ZRE is recognized by transcriptional factor Zap1, which, among other genes, activates the expression of ZRC1 (Bleackley and MacGillivray 2011; Eide 2009; MacDiarmid et al. 2003).
Metal tolerance in yeasts expressing RaCDF1 and RaCDF2
To obtain information about the capability of RaCDFs to protect the cells from metal toxicity, the corresponding coding sequences were inserted into the centromeric vector p416GPD for the constitutive expression in the metal-sensitive S. cerevisiae strains, and the RaCDF-transformed and control p416GPD-transformed yeasts were grown on solid and in liquid SD medium with or without metal supplements. To better compare the performance of RaCDF1 and RaCDF2, the concentrations of metal impairing the growth of the yeast in liquid medium by 50 % (IC50metal) were determined from the dose-dependent inhibition curves as described in “Materials and Methods” section. When expressed in the zrc1Δcot1Δ cells, RaCDF1, but not RaCDF2, complemented the Zn- and Co-sensitive phenotype of the double mutant on agar media supplemented with 10 mM Zn2+ and 500 µM Co2+, respectively (Fig. 3a). Noteworthy, compared to the controls, the expression of RaCDF2 appeared detrimental to the cell growth on media supplemented with 100 µM Co2+. The ability of RaCDF2 to complement Zn sensitivity in yeast, albeit with much lower efficiency than RaCDF1, was seen in liquid medium (Fig. 3b) in which the expression of RaCDF1 increased IC50Zn of zrc1Δcot1Δ cells by two orders of magnitude and the expression of RaCDF2 2.3-fold (Table 1). The liquid medium growth assays further confirmed that RaCDF1 has certain protective effect against Co2+ toxicity (twofold increase in IC50Co). The opposite was seen with RaCDF2 (IC50Co value decreased by 35 % compared to the controls), which also impaired the growth of yeasts in the presence of Cd2+. While RaCDF1 had no effect on the Cd tolerance in ycf1Δ yeasts that showed an IC50Cd value of 19 μM, the expression of RaCDF2, further added to the Cd sensitivity of this strain and resulted in nearly fourfold decrease in IC50Cd (Table 1). Although the elimination of ABC-type Ycf1 transporter involved in the vacuolar sequestration of Cd renders the ycf1Δ mutant highly sensitive towards Cd2+ (Szczypka et al. 1994), the mutant was still able to proliferate in the presence of 10 µM Cd2+ that did not allow the RaCDF2-expressing cells to grow both on the agar medium (Fig. 3a) and in liquid medium (Fig. 3b). Neither RaCDF1 nor RaCDF2 conferred increased Mn2+ tolerance upon the pmr1Δ strain that is unable to grow under Mn replete conditions due to the lack of Ca2+, Mn2+-ATPase for the efflux of the cytosolic metal into Golgi (Baelen et al. 2004; Bleackley and MacGillivray 2011); the RaCDF cells and the controls showed the same growth pattern up to the Mn2+ concentration of 2 mM that fully inhibited the growth of all transformants (not shown). Considering that Mn-transporting CDFs, such RaCDF2 homologues AtMTP11 (Delhaize et al. 2007; Peiter et al. 2007) or CsMTP9 (Migocka et al. 2015), can rescue Mn-sensitivity in pmr1Δ, the absence of RaCDF-associated phenotype in this strain suggested that RaCDFs cannot recognize Mn2+ for transport.
Functional expression of RaCDFs in Saccharomyces cerevisiae strains. a Zn- and Co-sensitive zrc1Δcot1Δ and Cd-sensitive Δyap1 strains were transformed with an empty p416GPD vector or with the same expression vector containing indicated RaCDF variants. The p416GPD-transformed BY4741 strain was used as wild-type control. Diluted transformant cultures (5 µl; OD590 of 5.10−3 and 5.10−4) were spotted on SD medium with or without a metal supplement as indicated. b The p416GPD and RaCDF1 and RaCDF2 transformants were grown in SD media with different concentrations of Zn2+, Co2+ and Cd2+ and the turbidity of the culture was measured after 24 h. Values plotted represent an average of at least three biological replicates ± standard deviation of the mean
The data obtained with the yeast mutants implied a Zn and Co transport function for RaCDF1 and Zn, Cd and Co transport function for RaCDF2, with a potential Cd and Co transport activity manifested by the quite unexpected gain-of-sensitivity phenotype. Considering the importance of the transmembrane H/D-X3-D motifs for the CDF-mediated transport, the mutant RaCDFs variants were constructed in which the codons for the TMD2 His (H41 in RaCDF1) or first Asp (D163 in RaCDF2) were changed to encode Ala residues. The observation that the expression of the resulting RaCDF1 H41A did not confer increased Zn and Co tolerance, and that RaCDF2 D163A cell did not show phenotype associated with the expression of non-mutant variant (Fig. 3a; Table 1) provided further support to the notion that the phenotypes associated in yeasts with wild-type RaCDF were due to the metal transport abilities of RaCDFs. Since the aspartyl 38 proximal to H-X3-D in TMD2 of RaCDF1 (Fig. 1a) appears well conserved in Zn-CDFs (extended consensus motif D-X2-H-X3-D; Montanini et al. 2007), the corresponding mutant was also constructed. The D38A mutation in the coding sequence impaired, but not fully inhibited the ability of RaCDF1 to complement Zn sensitivity in yeasts; compared to the controls, the zrc1Δcot1Δ cells expressing RaCDF1 D38A were still able to grow well on medium supplemented with 1 mM Zn2+ (Fig. 3a) and showed 40-fold higher IC50Zn. The Co tolerance of RaCDF1 D38A-expressing yeasts was, however, undistinguishable from that of the controls.
Targetting of RaCDFs in S. cerevisiae
To explore the localization of RaCDFs in yeasts using direct fluorescence microscopy, the RaCDF1::GFP, RaCDF1 H41A::GFP, RaCDF1 D38A::GFP, RaCDF2::GFP and RaCDF D163A::GFP fusions coding for RaCDFs and their mutant variants extended at their C-termini with GFP were constructed in the p416GPD vector and expressed in the zrc1Δcot1Δ or ycf1Δ cells. Complementation assays revealed that the levels of metal tolerance conferred by the fusions upon the yeasts were close to those observed with the corresponding mere RaCDF variants (as judged from IC50metal values in Table 1 and growth pattern in Fig. 3a), signifying that the tagging with GFP did not impair the performance of the RaCDF proteins.
The microscopy of the yeasts expressing RaCDF1::GFP detected clear GFP signals colocalizing with the fluorescence of vacuole-specific fluorophore FM4-64 (Fig. 4a). The same fluorescence pattern was observed with the RaCDF1 H41A::GFP- and RaCDF1 D38A::GFP-expressing cells, implying that the mutations did not affect the localization of the fusion to the vacuolar tonoplast. The strong, continuous GFP fluorescence detected at the cell periphery of the RaCDF2::GFP- and RaCDF1 D163A::GFP-expressing zrc1Δcot1Δ, with only a weak, yet detectable signal at the tonoplast (Fig. 4b), indicated that both the wild-type and the mutant RaCDF2 fusions were preferentially localized to the plasma membrane in yeasts (the same targeting of the fusions was observed also in ycf1Δ cells; not shown).
Visualization of Saccharomyces cerevisiae zrc1Δcot1Δ expressing RaCDF::GFP variants by fluorescence microscopy. a Yeasts expressing RaCDF1::GFP and its H41A and D38A mutant variants. b Yeasts expressing RaCDF2::GFP and its D163A mutant variant. The vacuoles of cells grown in YPD medium were stained with FM4-64; displayed panels from left to right: bright field micrograph, green GFP fluorescence, red FM4-64 fluorescence, and GFP/FM4-64 merged image with yellow/orange GFP and FM4-64 signals overlap. In panel b, the best achieved visualization of tonoplast-localizing GFP fluorescence in RaCDF2::GFP-expressing cells is also shown (PM, plasma membrane, VM, tonoplast). (For the interpretation of the references to colors in this figure legend, the reader is referred to the web version of this article)
Metal uptake in RaCDF-expressing yeasts
To obtain additional link between the transporter localization and RaCDF-associated metal tolerance phenotypes, the accumulation of the metals was investigated in the metal-sensitive yeasts incubated for 1 h in SD medium supplemented with potential metal substrates for the RaCDF-mediated transport. It was reasonable to assume that if the functional RaCDF1 funneled the excess Zn into the vacuoles in the zrc1Δcot1Δ strain, raising markedly its Zn tolerance, it would enable the cells to accumulate Zn in high concentrations. Indeed, the RaCDF1- and RaCDF1::GFP-expressing cells, but not those expressing the mutant RaCDF1 H41A or RaCDF1 H41A::GFP, accumulated significantly more Zn compared to the p416GPD-transformed controls (Fig. 5a; 2.8 and 3.2 times in the presence of 100 µM Zn2+). The increase in Zn concentrations in yeasts expressing RaCDF1 D38A and RaCDF1 D38A::GFP under the same conditions was less pronounced (1.6 and 1.8-fold, respectively), which is consistent with the lower level of Zn tolerance RaCDF1 D38A conferred to the zrc1Δcot1Δ strain. The expression of RaCDF1 alone or fused with GFP also contributed to the uptake of Zn from plain SD medium (45 % increase), which itself contains 2.5 µM Zn2+.
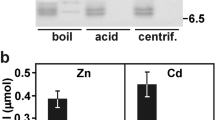
Accumulation of metals in RaCDF-expressing yeasts. The Zn uptake rates were assessed in the zrc1Δcot1Δ cells expressing the indicated RaCDF1 (a) and RaCDF2 (b) variants. c Cd accumulation in ycf1Δ yeasts expressing RaCDF2 variants. The metal content of the RaCDF-expressing and control p416GPD-transformed cells propagated in SD medium was measured following 1-h incubation in the presence of the indicated concentrations of metals as described in Materials and Methods. The plotted values represent the average of at least three biological replicates ± standard deviation of the mean, and significant differences (p < 0.05, ANOVA followed by Turkey’s test) in a particular treatment are indicated by different letters above the bars
Congruent with the predominant localization of GFP-tagged RaCDF2 to the plasma membrane, the expression of RaCDF2 and RaCDF2::GFP reduced the concentrations of Zn in zrc1Δcot1Δ yeasts exposed to 100 or 250 µM Zn by 40–50 %, suggesting the Zn export function for RaCDF2 (Fig. 5b). Reduced Zn concentrations (10–25 % decrease) were also seen in the cells harboring mutant RaCDF2 D163A or RaCDF2 D163A::GFP. This suggests that D163A replacement variants may partially retain the Zn2+ export function, which was seemingly not sufficient to substantially increase the Zn tolerance in the yeast. By contrast, the RaCDF2 D163A variants did not affect Cd uptake in ycf1Δ yeasts in which RaCDF2 and RaCDF2::GFP stimulated accumulation of Cd (Fig. 5c). The accumulation of Cd upon the exposure to 0.5 µM and 1 µM Cd in ycf1Δ expressing RaCDF2 (2.2- 1.7-fold increase, respectively) and RaCDF2::GFP (1.8 and 1.6-fold increase, respectively) suggested that the reason for the increased Cd-sensitivity in these cells lies in the RaCDF2-mediated Cd uptake.
Irrespective of the expressed RaCDF1 or RaCDF2 variant, the concentrations of Co in the yeasts incubated in the presence of 100 and 250 µM Co2+ were essentially the same as in the controls, which accumulated 0.34 ± 0.02 and 0.66 ± 0.04 nmol Co mg−1 cell dwt, respectively. This may be attributed to the relatively small differences (compared to those seen with Zn) between Co tolerance in the zrc1Δcot1Δ yeasts expressing RaCDF1 or RaCDF2 and p416GPD-transformed control cells.
Discussion
At the cellular level in eukaryotes, CDF family proteins serve in cleaning up the cytoplasm from excess heavy metal ions and in specific targeting of physiologically relevant metals into organelles to ensure their correct function. The results obtained in this study strongly suggest that the two, in the terms of primary sequence and gene structure unrelated, RaCDF proteins may serve in increasing the Zn tolerance of R. atropurpurea cells, specifically via the cytoplasm Zn cleanup. Along with the high homology to the Zn-CDFs Zrc1 and Cot1 of S. cerevisiae, several lines of experimental evidence implicate that RaCDF1 is specifically transporting Zn2+ and Co2+ into the vacuole: the expression of RaCDF1 protected yeasts from Zn and Co, but not Cd or Mn toxicity; the remarkably high level of Zn tolerance conferred by RaCDF1 allowed the yeasts to safely accumulate high concentrations of Zn; the functional GFP-tagged protein localized exclusively to the vacuolar membrane; and the replacement of the histidyl known from other CDFs as involved in the metal transport (Anton et al. 2004; Hoch et al. 2012; Montanini et al. 2007) with alanyl (H41A in D-X2-H-X3-D motif in TMD2) abolished the RaCDF1-associated Zn and Co tolerance phenotypes. The first aspartyl in the TMD2 motif, was shown to be essential for the Zn transport function of CDF proteins from diverse species—the Populus trichocarpa × deltoides PtdMTP1 (Blaudez et al. 2003) and E. coli ZitB (Anton et al. 2004). The corresponding D38A substitution in RaCDF1 D38A abolished the contribution of RaCDF1 to the Co tolerance, but it only impaired and did not fully inhibit the Zn tolerance and accumulation in zrc1Δcot1Δ yeasts [2.5-fold in terms of IC50Zn (Table 1) and by half (Fig. 5a), respectively], thereby indicating that the protein can, to a certain extent, at this position tolerate the absence of the residue that has a metal coordination ability.
The knowledge that RaCDF1 was expressed in R. atropurpurea sporocarps makes it reasonable to assume that the fungus has the high-efficacy means to deposit overaccumulated Zn in the vacuoles. The Zn partitioning analysis in previous study (Leonhardt et al. 2014) revealed that 40 % of the total sporocarp Zn is bound with MT-like RaZBP peptides, while only a minor portion (6 %) corresponded to presumably compartmentalized Zn (free or bound with low-molecular mass, ~1 kDa ligands) and the remaining metal appeared insoluble. Considering the insoluble Zn compounds in mycorrhizal fungi, it is worth noting that X-ray microanalyses in several species, including AM R. intraradices (González-Guerrero et al. 2008), an unspecified EM mycobiont of Pterium aquilinum (Turnau et al. 1993) and EM Suillus bovinus (Bücking and Heyser 1999; Olsson et al. 2011), determined the vacuolar Zn to be associated with polyphosphate bodies, and that crystalline, seemingly carboxylate-bound Zn intracellular species were identified in EM Rhizopogon rubescens (Fomina et al. 2007). Although the possibility that Zn can be in the sporocarps of R. atropurpurea immobilized extracellularly (e.g. precipitated with excreted oxalate; Gadd et al. 2012) or in the cytoplasm could not be ruled out, it is tempting to speculate that insoluble Zn species are formed in the vacuoles from the RaCDF1-imported Zn2+ ions.
The predicted RaCDF2 phylogenetically grouped with Mn-CDF proteins, which instead of the H-X3-D motif characteristic of Zn-CDFs conserve in their TMD2 and TMD5 the functional aspartyls of the D-X3-D motif (Kolaj-Robin et al. 2015; Montanini et al. 2007); these are in RaCDF2 D163 plus D168 and D261 plus D265, respectively. Despite the shared characteristics, RaCDF2 did not prove efficient in affecting the Mn tolerance of pmr1Δ yeasts, suggesting that Mn2+ unlike Zn2+ and Cd2+, is not a substrate for RaCDF2-mediated transport. It is worth noting in this context that the D-X3-D motif for the binding of the substrate metal ion is also present in the Zn-, Cd- and Fe-transporting Yiip from E. coli (Wei and Fu 2006), which clustered closely to RaCDF2, and that the substitution of the H-X3-D histidyl in TMD2 of the human Zn-CDFs ZnT5 and ZnT8 with aspartyl enabled them to transport Cd2+ in addition to Zn2+ (Hoch et al. 2012); furthermore, the D- or H-to-A substitutions rendered both the bacterial and human CDFs inactive. The lack of RaCDF2-associated phenotypes observed in the complementation and metal uptake assays with yeasts expressing RaCDF2 D163A and its fusion with GFP indicated that the corresponding aspartyl is requisite for the transport also in RaCDF2. Considering the preferential localization of GFP-tagged RaCDF2 to the plasma membrane in S. cerevisiae, the increased Zn tolerance and decreased Zn accumulation are attributable to the RaCDF2-mediated export out of the cells and was an expected phenotype of the RaCDF2- and RaCDF2::GFP-expressing zrc1Δcot1Δ cells. However, the promoted accumulation of Cd and decreased Cd tolerance in ycf1Δ strain observed upon the expression of both RaCDF2 and RaCDF2::GFP suggested the opposite direction of Cd2+ transport. The RaCDF2-mediated uptake of Co2+ would also explain the decreased Co tolerance in zrc1Δcot1Δ transformants. Bidirectional CDF-mediated metal transport is not without precedent and was reported for the human plasma membrane ZnT5 transporting Zn2+ in and out of Xenopus levis oocytes, but the physiological consequences of this property remain unclear (Valentine et al. 2007). Concerning the possible roles of RaCDF2 in the handling of Cd and Co in R. atropurpurea, we assume that this transporter is not of a significant importance under natural conditions because the Cd and Co uptake ability of this species, as judged from concentrations in the sporocarps, is rather low. Vetter (1994) reported the Cd contents to be 0.62 ± 0.05 mg kg−1 dry sporocarp weight, which is in good agreement with our unpublished data that show Cd contents well below 1 mg kg−1 dwt. The concentrations of Co were reported ranging from between 0.16 and 0.47 mg kg−1 dwt in the sporocarps, which contained 745–1062 mg Zn kg−1 dwt (Borovička and Řanda 2007).
The GFP-fluorescence pattern in yeasts expressing RaCDF2::GFP indicated the localization of a minor portion of the fusion protein to the vacuolar membrane. Considering this and the high degree of identity with the endomembrane-localizing AtMTP11 (Delhaize et al. 2007; Peiter et al. 2007), the possibility that RaCDF2 localizes in R. atropurpurea to the tonoplast and is somehow involved in supporting the high efficacy RaCDF1 in the vacuolar sequestration of Zn could not be excluded. On the other hand, the second closest characterized RaCDF2 homologue, CsMTP9, transports in C. sativus Mn2+ and Cd2+ across the plasma membrane of root cells into the apoplasm and promotes the translocation of the metal to aerial tissues (Migocka et al. 2015), and thus a similar metal-dispatch role of RaCDF2 in R. atropurpurea could not be excluded either.
The observation that may further support the notion that RaCDF1 and RaCDF2 have important functions in the handling of the accumulated Zn in R. atropurpurea is the presence of potential Zn-related metalloregulatory elements in the promoter regions of their genes—MRE in RaCDF1 and ZRE-like sequences in both RaCDF1 and RaCDF2 (Fig. 1c). While our BLAST searches of the R. atropurpurea transcriptome did not identify a homologue of the MRE-biding MTF-1 protein, we identified putative Zap1, which shared 35 % identity with Zap1 from S. cerevisiae (unpublished data). In the yeast, Zap1 is central to the activation of more than 80 Zn-related genes, including ZRC1 (Eide 2009; MacDiarmid et al. 2003). It is worth noting that the ZREs of several Zap1-activated genes show the deviations from the consensus in the positions observed in the RaCDF elements (Lyons et al. 2000). Further studies are thus needed to ascertain the link between the RaCDF ZRE-like elements and putative Zap1 homologue in R. atropurpurea.
In conclusion, the results obtained in this study place R. atropurpurea on a list of mycorrhizal species from which functional CDF proteins have been characterized. The presented results further suggest that RaCDF1 and RaCDF2 have different roles in the handling/targeting of the excess metal ions than those attributed to the R. intraradices GintZnT1 (González-Guerrero et al. 2005), H. cylindrosporum HcZnT1 (Blaudez and Chalot 2011), and O. maitus OmZnT1 (Khouja et al. 2013), which in yeasts localized to ER or unidentified non-vacuolar vesicles (GintZnT1). Localization to ER and metal detoxification functions of HcZnT1 and OmZnT1 seen in yeasts suggest that certain EM species can buffer cytosolic metal concentrations via the export to ER, which resembles the detoxification of Zn in S. pombe (Borrelly et al. 2002; Clemens et al. 2002). Vacuolar sequestration, in which we propose here a role for RaCDF1, was proven to be an important pathway for metal detoxification in various other EM species, including S. bovinus or S. luteus (Colpaert et al. 2011; Ruytinx et al. 2013) and P. involutus (Blaudez et al. 2000; Ott et al. 2002). It is worth noting that our search of the R. atropurpurea sporocarp transcriptome sequence allowed us to identify partial sequences coding for three additional RaCDF proteins, and the full length coding and genomic sequences were also obtained (GeneBank acession nos. KU311686, KU311687, KU311687), but not described in this study. Our preliminary analyses revealed that these RaCDFs tagged with GFP localized in yeasts to ER, but the corresponding RaCDF cDNAs expressed alone or in various combinations did not affect the metal tolerance of the metal-sensitive strains (unpublished observations). Given the localization of RaCDF2 to the plasma membrane in yeasts, it is worth for further studies to elucidate whether or not RaCDF2 may exert the metal efflux function also in R. atropurpurea. Such efflux function could support the translocation of Zn along the hyphae and/or redistribution of the metal in the sporocarp tissue. However, proving this could be somehow complicated by the fact that Russula belongs to culture-resistant genera and we are still unsuccessful in obtaining a mycelial culture of R. atropurpurea.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. doi:10.1016/S0022-2836(05)80360-2
Andrews GK (2001) Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals 14:223–237. doi:10.1023/A:1012932712483
Anton A, Weltrowski A, Haney CJ, Franke S, Grass G, Rensing C, Nies DH (2004) Characteristics of zinc transport by two bacterial cation diffusion facilitators from Ralstonia metallidurans CH34 and Escherichia coli. J Bacteriol 186:7499–7507. doi:10.1128/JB.186.22.7499-7507.2004
Baelen VK, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F (2004) The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta 1742:103–112. doi:10.1016/j.bbamcr.2004.08.018
Blaudez D, Chalot M (2011) Characterization of the ER-located zinc transporter ZnT1 and identification of a vesicular zinc storage compartment in Hebeloma cylindrosporum. Fungal Genet Biol 48:496–503. doi:10.1016/j.fgb.2010.11.007
Blaudez D, Botton B, Chalot M (2000) Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus. Microbiology 146:1109–1117. doi:10.1099/00221287-146-5-1109
Blaudez D, Kohler A, Martin F, Sanders D, Chalot M (2003) Poplar metal tolerance protein 1 confers zinc tolerance and is an oligomeric vacuolar zinc transporter with an essential leucine zipper motif. Plant Cell 15:2911–2928. doi:10.1105/tpc.017541
Bleackley MR, MacGillivray RTA (2011) Transition metal homeostasis: from yeast to human disease. Biometals 24:785–809. doi:10.1007/s10534-011-9451-4
Borovička J, Řanda Z (2007) Distribution of iron, cobalt, zinc and selenium in macrofungi. Mycol Prog 6:249–259. doi:10.1007/s11557-007-0544-y
Borrelly GPM, Harrison MD, Robinson AK, Cox SG, Robinson NJ, Whitehall SK (2002) Surplus zinc is handled by Zym1 metallothionein and Zhf endoplasmic reticulum transporter in Schizosaccharomyces pombe. J Biol Chem 277:30394–30400. doi:10.1074/jbc.M203145200
Bücking H, Heyser W (1999) Elemental composition and function of polyphosphates inectomycorrhizal fungi–an X-ray microanalytical study. Mycol Res 103:31–39. doi:10.1017/S0953756298006935
Clemens S, Bloss T, Vess C, Neumann D, Nies DH, Zur Nieden U (2002) A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. J Biol Chem 277:18215–18221. doi:10.1074/jbc.M201031200
Colpaert JV, Wevers JHL, Krznaric E, Adriaensen K (2011) How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Ann For Sci 68:17–24. doi:10.1007/s13595-010-0003-9
Conklin DS, McMaster JA, Culbertson MR, Kung C (1992) COT1, a gene involved in cobalt accumulation in Saccharomyces cerevisiae. Mol Cell Biol 12:3678–3688. doi:10.1128/MCB.12.9.3678
Delhaize E, Gruber BD, Pittman JK, White RG, Leung H, Miao Y, Jiang L, Ryan PR, Richardson AE (2007) A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J 51:198–210. doi:10.1111/j.1365-313X.2007.03138.x
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi:10.1093/nar/gkh340
Eide DJ (2009) Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J Biol Chem 284:18565–18569. doi:10.1074/jbc.R900014200
Fomina M, Charnock J, Bowen AD, Gadd GM (2007) X-ray absorption spectroscopy (XAS) of toxic metal mineral transformations by fungi. Environ Microbiol 9:308–321. doi:10.1111/j.1462-2920.2006.01139.x
Füzik T, Ulbrich P, Ruml T (2014) Efficient mutagenesis independent of ligation (EMILI). J Microbiol Meth 106:67–71. doi:10.1016/j.mimet.2014.08.003
Gadd GM, Rhee YJ, Stephenson K, Wei Z (2012) Geomycology: metals, actinides and biominerals. Environ Microbiol Rep 4:270–296. doi:10.1111/j.1758-2229.2011
Gaither LA, Eide DJ (2001) Eukaryotic zinc transporters and their regulation. Biometals 14:251–270. doi:10.1023/A:1012988914300
González-Guerrero M, Azcón-Aguilar C, Mooney M, Valderas A, MacDiarmid CW, Eide DJ, Ferrol N (2005) Characterization of a Glomus intraradices gene encoding a putative Zn transporter of the cation diffusion facilitator family. Fungal Genet Biol 42:130–140. doi:10.1016/j.fgb.2004.10.007
González-Guerrero M, Melville LH, Ferrol N, Lott JN, Azcón-Aguilar C, Peterson RL (2008) Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can J Microbiol 54:103–110. doi:10.1139/w07-119
Grass G, Otto M, Fricke B, Haney CJ, Rensing C, Nies DH, Munkelt D (2005) FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch Microbiol 183:9–18. doi:10.1007/s00203-004-0739-4
Günther V, Lindert U, Schaffner W (2012) The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta 1823:1416–1425. doi:10.1016/j.bbamcr.2012.01.005
Hickey PC, Swift SR, Roca MG, Read ND (2004) Live-cell imaging of filamentous fungi using vital fluorescent dyes and confocal microscopy. Method Microbiol 34:64–87. doi:10.1016/S0580-9517(04)34003-1
Hložková K, Matěnová M, Žáčková P, Strnad H, Hršelová H, Hroudová M, Kotrba P (2015) Characterization of three distinct metallothionein genes of the Ag-hyperaccumulating ectomycorrhizal fungus Amanita strobiliformis. Fungal Biol. doi:10.1016/j.funbio.2015.11.007
Hoch E, Lin W, Chai J, Hershfinkel M, Fu D, Sekler I (2012) Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc Natl Acad Sci USA 109:7202–7207. doi:10.1073/pnas.1200362109
Käll L, Krogh A, Sonnhammer ELL (2007) Advantages of combined transmembrane topology and signal peptide prediction-the Phobius web server. Nucleic Acids Res 35:W429–W432. doi:10.1093/nar/gkm256
Khouja HR, Abbà S, Lacercat-Didier L, Daghino S, Doillon D, Richaud P, Martino E, Vallino M, Perotto S, Chalot M, Blaudez D (2013) OmZnT1 and OmFET, two metal transporters from the metal-tolerant strain Zn of the ericoid mycorrhizal fungus Oidiodendron maius, confer zinc tolerance in yeast. Fungal Genet Biol 52:53–64. doi:10.1016/j.fgb.2012.11.004
Kolaj-Robin O, Russell D, Hayes KA, Pembroke JT, Soulimane T (2015) Cation Diffusion Facilitator family: structure and function. FEBS Lett 589:1283–1295. doi:10.1016/j.febslet.2015.04.007
Leonhardt T, Sácký J, Šimek P, Šantrůček J, Kotrba P (2014) Metallothionein-like peptides involved in sequestration of Zn in the Zn-accumulating ectomycorrhizal fungus Russula atropurpurea. Metallomics 6:1693–1701. doi:10.1039/c4mt00141a
Lyons TJ, Gasch AP, Gaither LA, Botstein D, Brown PO, Eide DJ (2000) Genomewide characterization of the Zap1p zinc-responsive regulon in yeast. Proc Natl Acad Sci USA 97:7957–7962. doi:10.1073/pnas.97.14.7957
MacDiarmid CW, Gaither LA, Eide DJ (2000) Zinc transporters that regulate Vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J 19:2845–2855. doi:10.1093/emboj/19.12.2845
MacDiarmid CW, Milanick MA, Eide DJ (2002) Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J Biol Chem 277:39187–39194. doi:10.1074/jbc.M205052200
MacDiarmid CW, Milanick MA, Eide DJ (2003) Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J Biol Chem 278:15065–15072. doi:10.1074/jbc.M300568200
Mathelier A, Zhao X, Zhang AW, Parcy F, Worsley-Hunt R, Arenillas DJ, Buchman S, Chen CY, Chou A, Ienasescu H, Lim J, Shyr C, Tan G, Zhou M, Lenhard B, Sandelin A, Wasserman WW (2013) JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res 42:D142–D147. doi:10.1093/nar/gkt997
Migocka M, Papierniak A, Kosieradzka A, Posyniak E, Maciaszczyk-Dziubinska E, Biskup R, Garbiec A, Marchew T (2015) Cucumber Metal Transport Protein CsMTP9 is a plasma membrane H+-coupled antiporter involved in the Mn2+ and Cd2+ efflux from root cells. Plant J 84:1045–1058. doi:10.1111/tpj.13056
Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M (2007) Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genom 8:1–16. doi:10.1186/1471-2164-8-107
Mumberg D, Müller R, Funk M (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119–122. doi:10.1016/0378-1119(95)00037-7
Olsson PA, Hammer EC, Pallon J, van Aarle IM, Wallander H (2011) Elemental composition in vesicles of an arbuscular mycorrhizal fungus, as revealed by PIXE analysis. Fungal Biol 115:643–648. doi:10.1016/j.funbio.2011.03.008
Ott T, Fritz E, Polle A, Schützendübel A (2002) Characterisation of antioxidative stress systems in the ectomycorrhiza-building basidiomycete Paxillus involutus (Bartsch) Fr. and its reaction to cadmium. FEMS Microbiol Ecol 42:359–366. doi:10.1111/j.1574-6941.2002.tb01025.x
Pagani A, Villarreal L, Capdevila M, Atrian S (2007) The Saccharomyces cerevisiae Crs5 metallothionein metal-binding abilities and its role in the response to zinc overload. Mol Microbiol 63:256–269. doi:10.1111/j.1365-2958.2006.05510.x
Palacios O, Atrian S, Capdevila M (2011) Zn- and Cu-thioneins: a functional classification for metallothioneins? J Biol Inorg Chem 16:991–1009. doi:10.1007/s00775-011-0827-2
Peiter E, Montanini B, Gobert A, Pedas P, Husted S, Maathuis FJ, Blaudez D, Chalot M, Sanders D (2007) A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc Natl Acad Sci USA 104:8532–8537. doi:10.1073/pnas.0609507104
Ruytinx J, Nguyen H, Van Hees M, Op De Beeck M, Vangronsveld J, Carleer R, Colpaert JV, Adriaensen K (2013) Zinc export results in adaptive zinc tolerance in the ectomycorrhizal basidiomycete Suillus bovinus. Metallomics 5:1225–1233. doi:10.1039/c3mt00061c
Sácký J, Leonhardt T, Borovička J, Gryndler M, Briksí A, Kotrba P (2014) Intracellular sequestration of zinc, cadmium and silver in Hebeloma mesophaeum and characterization of its metallothionein genes. Fungal Genet Biol 67:3–14. doi:10.1016/j.fgb.2014.03.003
Szczypka MS, Wemmie JA, Moye-Rowley WS, Thiele DJ (1994) A yeast metal resistence protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem 269:22853–22858
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis Version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Turnau K, Kottke I, Oberwinkle F (1993) Element localization in mycorrhizal roots of Pteridium aquilinum (L.) Kuhn collected from experimental plots treated with cadmium dust. New Phytol 123:313–324. doi:10.1111/j.1469-8137.1993.tb03741.x
Valentine RA, Jackson KA, Christie GR, Mathers JC, Taylor PM, Ford D (2007) ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J Biol Chem 282:14389–14393. doi:10.1074/jbc.M701752200
Vetter J (1994) Data on arsenic and cadmium contents of some common mushrooms. Toxicon 32:11–15. doi:10.1016/0041-0101(94)90016-7
Vetter J, Siller I, Horvath Z (1997) Zinc content of sporocarps of basidiomycetous fungi. Mycologia 89:481–483. doi:10.2307/3761041
Wei Y, Fu D (2006) Binding and transport of metal ions at the dimer interface of the Escherichia coli metal transporter YiiP. J Biol Chem 281:23492–23502. doi:10.1074/jbc.M602254200
Acknowledgments
We thank Dr. Jan Borovička (Institute of Geology and Nuclear Physics Institute, Academy of Science of the Czech Republic) for the provision of characterized R. atropurpurea sporocarps and helpful discussions, Prof. David Eide (University of Wisconsin-Madison) for the gift of CM137 and Prof. Dennis J. Thiele (Duke University Medical Center) for the gift of DTY168 strains. This work was funded by research project P504-11-0484 from the Czech Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sácký, J., Leonhardt, T. & Kotrba, P. Functional analysis of two genes coding for distinct cation diffusion facilitators of the ectomycorrhizal Zn-accumulating fungus Russula atropurpurea . Biometals 29, 349–363 (2016). https://doi.org/10.1007/s10534-016-9920-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9920-x