Abstract
Zinc trafficking in pancreatic beta cells is tightly regulated by zinc transporting (ZNTs) proteins. The role of different ZNTs in the beta cells is currently being clarified. ZNT8 transports zinc into insulin granules and is critical for a correct insulin crystallization and storage in the granules whereas ZNT3 knockout negatively affects beta cell function and survival. Here, we describe for the first time the sub-cellular localization of ZNT3 by immuno-gold electron microscopy and supplement previous data from knockout experiments with investigations of the effect of ZNT3 in a pancreatic beta cell line, INS-1E overexpressing ZNT3. In INS-1E cells, we found that ZNT3 was abundant in insulin containing granules located close to the plasma membrane. The level of ZNT8 mRNA was significantly decreased upon over-expression of ZNT3 at different glucose concentrations (5, 11 and 21 mM glucose). ZNT3 over-expression decreased insulin content and insulin secretion whereas ZNT3 over-expression improved the cell survival after 24 h at varying glucose concentrations (5, 11 and 21 mM). Our data suggest that ZNT3 and ZNT8 (known to regulate insulin secretion) have opposite effects on insulin synthesis and secretion possibly by a transcriptional co-regulation since mRNA expression of ZNT3 was inversely correlated to ZNT8 and ZNT3 over-expression reduced insulin synthesis and secretion in INS-1E cells. ZNT3 over-expression improved cell survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes gradually progresses, evolving from a stage of primary insulin resistance in peripheral tissues and accelerated insulin production in beta cells to a state of insulin-deficiency partly triggered by apoptotic beta cell death (Nolan et al. 2011). Maintaining beta cell function and avoiding apoptosis in beta cells are thus one of the targets in preventing the development of type 2 diabetes. Zinc has gained some attention since 1938 when it was first described that patients with diabetes have half the usual amount of zinc in the pancreas when compared to non-diabetic individuals (Scott and Fisher 1938).
Zinc is required by more than 300 enzymes and 2000 transcription factors (Prasad 2012) and maintaining a strict zinc balance is mandatory for several basic cellular functions (Plum et al. 2010). Thus, meticulous regulation of the intracellular zinc homeostasis may prevent cell death (Bozym et al. 2010). In beta cells, the supply of zinc is particularly important since insulin processing, storing and secretion depend on the presence of zinc (Boquist et al. 1968; Emdin et al. 1980; Gold and Grodsky 1984; Wijesekara et al. 2010; Rungby 2010). Zinc is transported through membranes by two families of zinc transporting proteins. SLC39As or ZIPs import zinc to the cytoplasm from the extracellular matrix or from cellular organelles. SLC30As or ZNTs transport zinc the opposite direction that will say ZnTs export zinc from the cytoplasm to the extracellular matrix or to cellular organelles (Cousins et al. 2006).
Studies of zinc transport mechanisms of SLC30As reveal both homo- (ZNT7) and hetero (ZNT5/ZNT6) dimerization as a requirement for zinc transport through membranes (Suzuki et al. 2005; Fukunaka et al. 2009). Furthermore, ZNT3 dimerization and oligomerization are pivotal for ZNT3 targeting to synaptic-like microvesicles, zinc transport capacity and viability in PC12 cells (Salazar et al. 2009). Several studies link dysfunction or altered regulation of ZNTs and ZIPs to a number of chronic diseases such as obesity (ZIP14), atherosclerosis (ZNT3 and ZNT10), Alzheimer’s disease (ZNT3) and diabetes (ZNT3, ZNT7 and ZNT8) (Sladek et al. 2007; Smidt et al. 2009; Nicolson et al. 2009; Adlard et al. 2010; Patrushev et al. 2012; Huang et al. 2012). Sladek et al. (2007) found that a single nucleotide polymorphism, rs13266634, in the SLC30A8 gene gives rise to a R325-W substitution in the final ZNT8 protein and more seriously it gives rise to an increased risk of developing type 2 diabetes. Subsequently, the importance of ZNT8 in insulin processing, storage and clearance process has been thoroughly investigated. In humans, one study has investigated the role of ZNT8 on insulin clearance and showing that individuals harboring R325-allele have an enhanced insulin clearance by the liver compared to humans with the W325-allele (Tamaki et al. 2013). On the contrary, the results obtained in mice are equivocal (Nicolson et al. 2009; Lemaire et al. 2009; Wijesekara et al. 2010). ZNT8 whole-body knockout mice have shown unaffected insulin processing and normal glucose-induced insulin release (Lemaire et al. 2009) whereas another study suggest that genetic background, age, sex and diet have profound effect on the effect of ZNT8 knockout (Nicolson et al. 2009). Beta cell specific ZNT8 knockout mice causes decreased insulin secretion due to diminished zinc supplies to the granules and thereby a defect in the insulin crystallization (Wijesekara et al. 2010). We have previously demonstrated that knock-down of ZNT8 increases cell death in INS-1E cells at 16.7 mM glucose (Petersen et al. 2011). Similarly, we found that knock-down of ZNT3 may increase cell death and decreases glucose-induced insulin secretion (Petersen et al. 2011) and whole-body knockout of ZNT3 renders KO mice more prone to stress-induced beta cell failure compared to wild type mice (Smidt et al. 2009). In addition, a protective role of ZNT3 has also been demonstrated after traumatic brain injury where ZNT3 KO mice have a higher number of apoptotic neurons compared to wild type mice (Doering et al. 2010). In vitro studies in INS-1E cells reveal a low basal ZNT3 mRNA level with a transcriptionally up-regulation of ZNT3 under stressful conditions like zinc chelation, zinc supplementation, and glucotoxicity (Smidt et al. 2009; Petersen et al. 2011; Nygaard et al. 2014, 2015).
The aim of this study was to further investigate the effect of ZNT3 on beta cell function including insulin secretion and beta cell viability, testing the hypothesis that ZNT3 over-expression has opposite effects to the ones seen in ZNT3 knockdown experiments, thus supporting a potential role of ZNT3 in cell protection. Further, we aimed to provide evidence for the localization of ZNT3 in INS-1E cells as a model system for beta cells.
Methods
Cell cultures
INS-1E cells were cultured in a 5 % CO2 atmosphere in complete RPMI 1640 medium supplemented with 11 mM glucose, 10 % (v/v) heat-inactivated fetal bovine serum, 50 μM β-mercaptoethanol, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. For stimulation assays INS-1E cells were plated (1,400,000 cells/well) into six-well plates (NUNC) at 11 mM glucose and cultured for 24 h. Then the cell medium was changed and cells were stimulated for 24 h with fresh medium containing 5, 11 or 21 mM glucose, respectively. For each glucose concentration, replicas of five were used for RNA extraction and replicas of four used for cell death measurements. For insulin measurement 200,000 cells/well were plated into 24-well plates (NUNC) in a cell medium supplied with 11 mM glucose. Twenty-four hours later, media was changed to Krebs–Ringer bicarbonate HEPES (KRBH) buffer (further described below) with 5, 11, 16, and 21 mM glucose and the cells incubated for 2 h in replicas of four. Cells used for immunoblot analysis were cultured at 11 mM glucose and cells used for immunohistochemistry and electron microscopy (EM) were cultured at 5 and 11 mM glucose.
Plasmid constructs
The EST clone HsCD00040424 (PlasmID, Harvard Medical School, US) was used for PCR amplification of the coding region of the human SCL30A3 (hZNT3) (NM_003459.4). The hZNT3 cDNA was cloned into the HindIII/Not1 sites in the mammalian expression vector pcDNA3 (Invitrogen, Life Technology, Denmark), and the final construct was verified by sequencing.
Transfection
INS-1E stable cell lines were established by transfecting cells with Lipofectamine™ LTX and PLUS™ reagent (Invitrogen, Life Technology, Denmark) following the protocol from the supplier (Invitrogen, Life Technology, Denmark). The stable cell lines were selected and maintained by culturing the cells in medium containing G418 (200 μg/ml), Geneticin® (Gibco®, Invitrogen, Life Technology, Denmark). The presence of hZNT3 was confirmed by reverse transcription (RT)-PCR and immunoblot analysis. G418 was omitted 24 h prior start in all experiments using stable transfected hZNT3 (hZNT3-INS-1E) cells and empty vector transfected control (EV-INS-1E) cells.
RNA extraction and cDNA synthesis
INS-1E total RNA was extracted using Qiagen RNeasy mini kit (VWR, Denmark) and treated with DNase (VWR, Denmark). The RNA quality was controlled on a 1 % (v/v) agarose gel stained with ethidium-bromide and 500 ng total RNA was reversely transcribed using ImProm-II™ RT System (Promega, Denmark) and oligo dT18 primers (TAC, Copenhagen). The cDNA was checked for genomic DNA contamination by PCR analysis using Qiagen HotStarTaq master mix kit (VWR, Denmark) with an intron-spanning primer-set of β-actin (TAC, Copenhagen). The PCR product was analyzed by ethidium bromide staining after electrophoresis in a 1 % agarose gel (Smidt et al. 2009).
Real-time PCR
Quantitative real-time PCR was performed as previously described (Smidt et al. 2009). For all reactions a melting curve was included. The results were analyzed with iQ™ 5 Optical System Software, Version 2.0. Starting quantities were calculated from a standard curve. Values were normalized to the geomean of two housekeeping genes; β-actin and heat shock protein class b member (HSPcb) (Vandesompele et al. 2002; Smidt et al. 2006). The expression of these housekeeping genes was analyzed to assure stable expression and glucose treatment seemed not to change the expression of these genes (data not shown). Primers used for real-time PCR were rat-β-actin forward: 5′-CTA CAA TGA GCT GCG TGT GGC-3′, rat β-actin reverse: 5′-ATC CAG ACG CAG GAT GGC ATG-3′, rat HSPcb forward: 5′-GAT TGA CAT CAT CCC CAA CC-3′, rat HSPcb reverse: 5′-CTG CTC ATC ATC GTT GTG CT-3′, human ZNT-3 forward: 5′-CTC CTG TAC CTG GCC TTC GTC-3′, human ZNT-3 reverse: 5′-CGA TGC TGG CGG TCA G-3′, rat ZNT-5 forward: 5′-GCA CAG CTC TCA GTC GGT CCC-3′, rat ZNT-5 reverse: 5′-CCC ATG ACC AAG GCG GAG CA-3′, rat ZNT-8 forward: 5′-GGT GGA CAT GTT GCT GGG AG-3′, rat ZNT-8 reverse: 5′-CAC CAG TCA CCA CCC AGA TG-3′. Primers design was performed using Primer-BLAST at www.ncbi.nlm.nih.gov. There was 85 and 81 % homology with the endogenous rat mRNA and the human ZNT3 forward and reverse primers, respectively.
Immunoblot analysis
Cells prepared for lysis were harvested in a lysis buffer [20 mmol/l Tris/HCl, 5 mmol/l EGTA, 150 mmol/l NaCl, 20 mmol/l glycerophosphate, 10 mmol/l NaF, 1 % Triton X-100, 0.1 % Tween-20 with the addition of 0.01 % halt protease and phosphatase inhibitor cocktail (Life Technology, Denmark)] prior to use. Aliquots of 5 µg protein extracts from INS-1E, hZNT3-INS-1E, and EV-INS-1E were resolved by Criterion™ TGX™ gels (26 wells) (Bio-Rad, Denmark), for 25 min at 250 V. Running buffer consisted of DDT including SDS leading to denaturing conditions. The gel was activated by ultraviolet exposure for 5 min using a Bio-Rad ChemiDoc MP imager. Proteins were transferred to PVDF by semi dry transfer for 30 min, 1 A and 25 V, followed by a wash in Tris-buffered saline Tween-20 (10 mM Tris, 150 mM NaCl, pH 8.0, and 0.1 % Tween-20, TBS-T). After protein transfer, membranes were imaged for stain-free staining and total protein (a loading control) was quantified using ImageLab 4.1 software (Bio-Rad) (Gilda and Gomes 2013).
The membrane was blocked with 1 % bovine serum albumin (BSA) in TBS-T for 2 h following incubation with a ZNT3 antibody (197 002, SySy.com, Germany) (1:5000) in 1 % BSA in TBS-T at 4 °C O/N. The membrane was washed three times in TBS-T and then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:10,000) (Santa Cruz, Life Technology, Denmark) as secondary antibody for 2 h at room temperature. The membrane was washed three times in TBS-T, incubated with Clarity™ western ECL blotting substrate (Bio-Rad), and imaged on the ChemiDoc MP. Protein extracts were analyzed in triplicates from three different wells. For the blocking experiment 15 μg control ZNT3 (197-0P. SySy.com) was mixed with 15 ml ZNT3 antibody (1:5000) for 2 h at 4 °C prior incubating the membrane O/N. INS-1E, EV-INS-1E and hZNT3-INS-1E samples were pooled respectively from the original study (Fig. 1b). A control experiment for the presence of ZNT3 in INS-1E cells was performed using 20 μg protein from non-transfected INS-1E cells and analyzed as above described (results not shown). For the negative control experiment the membrane was incubated with 1 % BSA alone without ZNT3 antibody (result not shown).
Establishment of stable expressing hZNT3 INS-1E cells and empty vector INS-1E cells. a RT-PCR analysis of relative hZNT3 mRNA levels in transfected and non-transfected INS-1E cells. hZnT3-INS-1E cells (black), EV-INS-1E cells (grey) and INS-1E cells (white) treated for 24 h with 5, 11 or 21 mM glucose. b Immunoblot analysis of hZNT3 protein extracts from INS-1E cells (lanes 2–4), EV-INS-1E cells (lanes 5–7) and hZNT3-INS-1E cells (lanes 8–10) cultured in 11 mM glucose. The arrow at 40 kDa indicates the position of the hZNT3. c, d Control immunoblot experiment of ZNT3 antibody specificity. c INS-1E (lane 2), EV-INS-1E (lane 3) and hZNT3-INS-1E (lane 4) with ZNT3 antibody. d INS-1E lane (lane 1), EV-INS-1E (lane 2) and hZNT3-INS-1E (lane 3) with ZNT3 antibody blocked with ZNT3 protein. The arrow at 40 kDa indicates the position of the hZNT3
Insulin measurement
24 h prior to analysis hZNT3-INS-1E and control EV-INS-1E cells were plated in 24-well plates and maintained in complete RPMI medium with 11 mM glucose. Hereafter the cells were incubated for 1 h in a KRBH buffer (at pH 7.4) containing 115 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 2.6 mM CaCl2, 1.2 mM KH2PO4, 20 mM HEPES, 5 mM NaHCO3, 0.1 % (v/v) human serum albumin (Sigma, Denmark) and 0 mM glucose. The cells were then incubated for 2 h in KRBH and one of the concentrations 5, 11, 16, or 21 mM glucose for insulin release determination. The medium was collected for insulin analysis. Insulin content and secretion were measured using an ultra-sensitive rat insulin ELISA kit from DRG Diagnostics (VWR) as described previously (Smidt et al. 2009; Petersen et al. 2011).
Cell death measurement
Fragmentation of histone-associated-DNA after cell death induced by glucose was determined by photometric enzyme immunoassay (Cell Death Detection ELISAPLUS, Roche Applied Science) according to manufactures instructions as described previously (Smidt et al. 2009; Petersen et al. 2011). In short, hZNT3-INS-1E and EV-INS-1E cells were plated on a 24-well plate with 200,000 cells per well. After 24 h cells were treated with 5, 11 or 21 mM glucose for 24 h. Experiments were performed in quadruplicates. Cells were scraped off with a rubber policeman and centrifuged at 200×g for 10 min. Supernatants containing the necrotic fraction, i.e., DNA from necrotic cells were removed and stored at 4 °C for further analysis. Cell pellets containing DNA fragments were lysed and centrifuged at 200×g for 10 min. According to the manufactures instructions, the two supernatants, i.e., the supernatant containing the cytoplasmatic fraction and the previously obtained supernatant containing the necrotic fraction were transferred to the streptavidin coated microtiter plate in duplicate and incubated with anti-histone-biotin and anti-DNA-peroxidase. The amount of fragmented DNA bound to anti-DNA-peroxidase was measured by 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) with 405 and 490 nm as reference wavelengths.
Double labeling immune-electron microscopy of ZNT3 and insulin
Cells were fixed for 15 min in 4 % paraformaldehyde in 0.01 M PBS. Afterwards cells were incubated in 12 % gelatin at 37 °C and placed on ice for at least 10 min for stiffening of the gelatin. Square sections (2 mm × 2 mm) were cut and infiltrated in 2.3 M sucrose in PBS overnight followed by incubation in a fresh solution of 2.3 M sucrose in PBS for 1 h at room temperature and finally sections were frozen in liquid nitrogen. Semi-thin sections (700–800 nm) were cut using an ultra-cryomicrotome (Reichert Ultracut S, Leica, Wetzlar, Germany) and mounted on glass slides.
Ultrathin cryosections (50–80 nm) were cut from the square sections described above on the ultra-cryomicrotome, mounted onto grids and pre-incubated in PBS with 0.1 % skimmed milk and 50 mM glycine. The sections were incubated overnight at 4 °C with the first primary antibody (rabbit anti-ZNT3) (197 002, SySy.com, Germany) diluted in PBS containing 0.1 % skimmed milk. The first primary antibody was visualized by incubation for 1 h at room temperature with the secondary antibody (goat anti-rabbit IgG) conjugated to 10 nm colloidal gold particles (GAR.EM10 Bio-Cell Research Laboratories, Cardiff, UK) diluted 1:50 in PBS with 0.1 % skimmed milk, 1 % fish gelatin and 0.06 % polyethyleneglycol.
The sections were then blocked with normal rabbit serum 1:10 for 10 min followed by blocking with Fab fragments 1:10 for 10 min in PBS. The sections were incubated overnight at 4 °C with the second primary antibody (guinea pig anti-insulin) (ab-7842, Abcam, Denmark) diluted in PBS containing 0.1 % skimmed milk. The second primary antibody was visualized by incubation for 1 h at room temperature with the secondary antibody (goat anti-guinea pig IgG) conjugated to 5 nm colloidal gold particles (GAG.EM5 Bio-Cell Research Laboratories, Cardiff, UK) diluted 1:50 in PBS with 0.1 % skimmed milk, 1 % fish gelatin and 0.06 % polyethyleneglycol. After rinsing, sections were counter stained and embedded in 1.8 % methylcellulose containing 0.4 % uranyl acetate for 5 min.
Confocal immunofluorescent microscopy
Cryosections placed on Super Frost slides and incubated with primary antibodies guinea pig anti-insulin (ab-7842, Abcam, Denmark) 1:100 recognizing both pro-insulin and insulin, rabbit anti-ZNT3 (197 002, SySy.com, Germany) 1:1000 in 0.1 % skim milk powder, 0.3 % Triton X-100 in PBS overnight at 4 °C, and rinsed in 0.1 % skim milk powder, 0.2 % gelatin, and 0.05 % saponin in PBS. The appropriate Alexa488 (1:4000) and Alexa555 (1:4000) secondary antibodies were applied, and coverslips were mounted with a hydrophilic mounting medium containing antifading reagent (Dako, glycergel). Fluorescence imaging was performed on a Leica DM IRE2 inverted confocal microscope using a Leica TCS SP2 laser module and an HCX PC APO CS 63x/1.32 NA oil objective. Images were acquired using the following settings: eight bit image depth, 1024 × 1024 pixel resolution, and an image averaging of six frames.
Electron microscopy
Electron micrographs of ZNT3 immuno-strained sections were acquired on a FEI Morgagni 268 electron microscope (Philips Electron Optics, Netherlands).
Statistical analysis
Cell cultures: mean ± SEM are shown. Comparisons between groups in RT-PCR experiments, analysis of cell death and insulin content and secretion were made by two-way ANOVA followed by Bonferroni post test. Comparisons between different glucose concentration in the cell death experiment were performed by an un-paired t test with Welch’s correction when means were different. Differences were considered significant at p < 0.05. Statistical analyses were performed using GraphPad Prism 5.02 Software (GraphPad Software, Inc.).
Results
Over-expression of hZNT3 in cultured cells
We transfected INS-1E cells with either an hZNT3 encoding plasmid or an empty vector (EV) pcDNA3 plasmid (control) and two stable INS-1E cell lines, hZNT3-INS-1E and EV-INS-1E (control) were established. The presence of hZNT3 over-expression was verified by RT-PCR and immunoblot analysis (Fig. 1a, b). Endogenous expression of ZNT3 mRNA was present but negligible compared to exogenous hZNT3 mRNA expression (25–40-fold higher mRNA level in transfected cells) (results not shown). As previously described (Smidt et al. 2009; Nygaard et al. 2015) 16 mM glucose stimulates the expression of ZNT3 mRNA although 21 mM glucose decreases the mRNA expression of hZNT3 (Fig. 1a) (Smidt et al. 2009; Nygaard et al. 2015). At the protein level we detected a band at 40 kDa corresponding to ZNT3 (Fig. 1b). The specificity of the ZNT3 antibody was proved by blocking the ZNT3 antibody with a ZNT3 protein prior incubating the membrane (Fig. 1c, d). The blocking totally removed the ZNT3 signal at 40 kDa (Fig. 1d).
hZNT3 over-expression leads to decreased ZNT8 expression
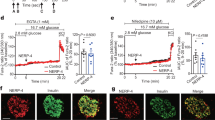
We have previously investigated the relationship between ZNT3 knock-down and the zinc transporters localized in insulin containing granules (ZNT5 and ZNT8) showing unchanged ZNT5 mRNA levels and increased ZNT8 mRNA levels after ZNT3 knock-down (Kambe et al. 2002; Chimienti et al. 2004; Petersen et al. 2011). In order to investigate the reverse situation hZNT3-INS-1E and EV-INS-1E cells were treated for 24 h with different glucose concentrations (5, 11 and 21 mM) followed by RT-PCR analysis of ZNT5 and ZNT8. The ZNT8 mRNA levels were reduced at all glucose concentrations tested (5 and 11 mM glucose with p < 0.01 and 21 mM glucose with p < 0.001) in the hZNT3-INS-1E cells compared with EV-INS-1E control cells (Fig. 2a). Similarly, hZNT3 over-expression resulted in a reduced mRNA expression of ZNT5 but only following exposure to a high glucose environment (21 mM glucose with p < 0.05; Fig. 2b).
The effect of hZNT3 over-expression on the transcription of the zinc transporters ZNT5 and ZNT8 localized to insulin granules. RT-PCR analysis of ZNT5 and ZNT8 mRNA levels in hZNT3-INS-1E (black) and EV-INS-1E (grey) cells treated with different glucose concentrations (5, 11 or 21 mM) for 24 h. a Relative mRNA level of ZNT8. b Relative mRNA level of ZNT5. Error bars represent mean ± SEM, *p < 0.05, **p < 0.01 and ***p < 0.001
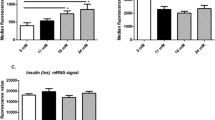
Decreased insulin content in hZNT3-INS-1E cells
ZNT3 knock-down leads to a down-regulation of glucose-stimulated insulin secretion in INS-1E cells compared to mock transfected cells (Petersen et al. 2011). To further clarify the potential role of ZNT3 in INS-1E cells insulin content and insulin secretion were examined, calculating the ratio between insulin content and secretion in hZNT3-INS-1E and EV-INS-1E cells. Both cell types, EV-INS-1E and hZNT3-INS-1E, showed a decrease in insulin content at high glucose concentrations 16 and 24 mM compared to 5 mM glucose concentration. Interestingly, comparing the two cell lines showed that hZnT3 over-expression reduced the insulin content at 5 mM (p < 0.01), 16 and 24 mM glucose concentrations (p < 0.05) compared to vector transfected INS-1E cells (Fig. 3a). This limiting effect of hZnT3 over-expression was further affecting insulin secretion with a substantial reduction in the insulin secretion in relation to insulin content (insulin secretin/insulin content ratio) in hZNT3 over-expressing INS-1E cells at 11 mM (p < 0.01), 16 mM (p < 0.01) and 24 mM glucose concentrations (p < 0.05) compared to vector transfected INS-1E cells (Fig. 3b). Analyzing insulin secretion with two-way ANOVA without relating to insulin content furthermore showed that hZNT3 overexpression significantly decreased insulin secretion (data not shown).
The effect of hZNT3 over-expression on insulin content and secretion. ELISA analysis of insulin content and insulin secretion after 2 h of glucose stimulation with different concentrations (5, 11, 16 and 24 mM) in hZNT3-INS-1E (black) and EV-INS-1E (grey) cells. a Intracellular insulin content (in relation to total amount of protein) and b insulin secretion/insulin content ratio. Error bars represent mean ± SEM, *p < 0.05, **p < 0.01 and ***p < 0.001
Improved cell survival after hZNT3 over-expression
Another effect of ZNT3 knock-down has proven to be an increase in apoptosis in INS-1E cells (Petersen et al. 2011). We investigated the effect of hZNT3 over-expression on cell survival. hZNT3-INS-1E and EV-INS-1E cells were treated for 24 h with different glucose concentrations (5, 11 and 21 mM) followed by an ELISA analysis of the level of histone-associated-DNA as a measurement for cell death. Fragmented DNA as a marker for apoptosis was measured in whole cells whereas supernatant concentrations of fragmented DNA were used as indicators of necrosis. We found that hZNT3 over-expression improved cell survival by reducing both apoptotic (5 and 21 mM glucose with p < 0.001 and 11 mM glucose with p < 0.05; Fig. 4a) and necrotic cell death (p < 0.05; Fig. 4b). The cells are routinely cultured at 11 mM glucose and changing the culture conditions seemed to stress the EV-INS-1E cells leading to an increase of apoptosis (Fig. 4a) at 5 and 21 mM glucose compared to 11 mM glucose (5 mM glucose with p < 0.05 and 21 mM with p < 0.007 compared to 11 mM glucose). This glucose induced stress condition was not seen in the hZNT3-INS-1E cells.
The effect of hZNT3 over-expression on INS-1E cell death. ELISA analysis of histone-associated-DNA-fragments in whole cell lysate (apoptosis) and cell media supernatant (necrosis) from hZNT3-INS-1E (black) and EV-INS-1E (grey) cells stimulated with different glucose concentrations (5, 11 and 21 mM) for 24 h. a The percentage of apoptosis and b necrosis normalized to EV-INS-1E treated with 5 mM glucose. Error bars represent mean ± SEM, *p < 0.05 and ***p < 0.001
ZNT3 localization in INS-1E beta cells
Immunocytochemistry and confocal microscopy were employed to localize ZNT3 in the cells. In INS-1E cells, ZNT3 co-localized partly with insulin both at 5 mM glucose (Fig. 5a–c) and 11 mM glucose (Fig. 5c–e). The subcellular localization of ZNT3 in INS-1E cells was investigated by EM, revealing ZNT3 as localized primarily in insulin containing granules, especially those close to the plasma membrane corresponding to the ready releasable pool (Fig. 6a, b). We found no difference in cellular localization in cells treated with 5 mM glucose compared to cells treated with 11 mM glucose.
Discussion
We demonstrate here that ZNT3 may have a beta cell protective role, at least in vitro. The present study demonstrates an improved survival after ZNT3 over-expression regardless of the present glucose concentration. This improvement of cell survival correlates with our previous findings in INS-1E cells, describing that ZNT3 knock-down increases cells death compared to control cells (Petersen et al. 2011). Furthermore, this present study confirms previous findings of an inverse correlation between ZNT3 and ZNT8 (Petersen et al. 2011). Recently, Gerber et al. (2014) found that hypoxia did not affect cell death in islets from young (10–15 weeks) ZNT8 −/− mice and explained their findings by the fact that metallothioneins 1 and 2 contributed to maintain normal cell function although these two genes were transcriptionally reduced by 50 % during hypoxia. Based on our previous and present findings we suggest that the results from Gerber et al. in islets from ZNT8 −/− mice can be additionally explained by an up-regulation of ZNT3 and thereby a cell death protection by ZNT3 (Petersen et al. 2011).
The inverse transcriptional correlation between ZNT3 and ZNT8 found in this study is in line with previous findings. Opposite transcriptional effects of ZNT3 and ZNT8 have been found after chelation of zinc by DEDTC and glucose treatment in INS-1E beta cells (Smidt et al. 2009) and a transcriptional up-regulation of ZNT8 in ZNT3 knock-down cells and vice versa has been described in INS-1E (Petersen et al. 2011). Recently, Flannick et al. (2014) genotyped 150,000 individuals and found loss of function mutations in the SLC30A8 gene leading to a protection against development of type 2 diabetes. The specific effect of the loss of function mutation has not been investigated. We hypothesize that the loss of function mutation in the SLC30A8 gene could affect different genes among these also the SLC30A3 gene.
The expression of ZNT3 varies with age. The impact of this variation has been studied in the cerebral cortex. Here, the expression of ZNT3 decreases with age followed by a loss of transsynaptic Zn2+ and ultimately cognitive losses (Adlard et al. 2010). Age-dependent gene expression of ZNT3 also exists in the pancreas as previously reviewed (Smidt and Rungby 2012). The age-dependent gene expression of ZNT3 may explain the findings by Gerber et al. (2014) showing increased cell death in islets from old ZNT8 −/− mouse compared to young ZNT8 −/− mouse. This difference could be explained by an age-declined ZNT3 expression and thereby an age-declined cell death protection. We have previously found that ZNT3 gene expression is up-regulated due to treatment with common substances such as glucose, zinc and the antabuse metabolite, DEDTC, in semi-toxic and toxic concentrations (Smidt et al. 2009; Nygaard et al. 2014). These findings all together suggest that ZNT3 has a role in cell death pathways. The present findings demonstrated an isolated effect of ZNT3 up-regulation suggesting that the effects of glucose- and zinc manipulations may be secondary to ZNT3 up- or down regulation.
The decreased level of intracellular insulin seen after ZNT3 up-regulation may be due to the decreased ZNT8 expression correlating with the findings by Fu et al. describing that ZNT8 knock-down in INS-1 cells causes a reduction in insulin content (Fu et al. 2009). However, contradicting results dispute the effect of ZNT8 −/− and ZNT8 over-expression. In vitro studies of insulin content in INS-1E and Min-6 cells after ZNT8 knock-down have demonstrated both increased and decreased insulin content (Souza et al. 2008; Fu et al. 2009; Petersen et al. 2011). In addition, contradicting results are found in regard to the effect of ZNT3 on insulin processing and secretion since ZNT3 knock-down has been shown to decrease the insulin content and insulin secretion in INS-1E cells (Smidt et al. 2009; Petersen et al. 2011). Although, the results in this present study indicate that the insulin production (either at the transcription or processing level) may be reduced by hZNT3 over-expression. This cannot be said conclusively and need further investigation.
We have previously used INS-1E cells as a model for the function of isolated beta cells still recognizing the limitations of a cell line versus isolated islets (Søndergaard et al. 2005; Smidt et al. 2009; Petersen et al. 2011; Nygaard et al. 2014, 2015). INS-1E has previously proven particularly useful for a specific characterization of ZNT8 and its beta cell function by other researchers (Chimienti et al. 2006). Our present results may be translated to other model systems, e.g., PC12 cells in which an increase in ZNT3 dimerization and oligomerization improves cell viability at semi-toxic and toxic conditions (Salazar et al. 2009).
Salazar et al. (2009) describe a redox-regulated homo-oligomerisation of ZNT3. The western blot from this present study showed additional un-specific bands besides the 40 kDa ZNT3 (not shown). Co-incubating the ZNT3 antibody with a control ZNT3 protein totally blocked the antibody signal at 40 kDa but we were not able to block the un-specific bands at 80 and 160 kDa which lead to the conclusion that ZnT3 was not part of the bands at 80 and 160 kDa in our non-redox experimental setup with various glucose concentrations. We have previously described the presence of ZNT3 in INS-1E cells in isolated islets from mice (Smidt et al. 2009) and would have expected to find ZNT3 in EV-INS-1E and INS-1E cells in this present study. In this present study a low amount of loaded protein (5 μg) lead to a lack of signal in INS-1E and EV-INS-1E cells. In previous studies of ZNT3 immunoblot assays using 20 μg of protein we found the presence of ZNT3 in INS-1E (Smidt et al. 2009).
The presence of ZNT3 in synaptic vesicles in neurons is well-established (Palmiter et al. 1996; Salazar et al. 2004). We have previously demonstrated the presence of ZNT3 in pancreatic islets from mice (Smidt et al. 2009). This present study managed to localize ZNT3 to the INS-1E cells (Fig. 5a–f) and, furthermore, the intracellular localization of ZNT3 in INS-1E cells was described at the ultra structural level (Fig. 6). We found that ZNT3 is localized primarily in insulin containing granules close to the plasma membrane leading to the assumption that ZNT3 is localized in the ready releasable pool of insulin secretory vesicles (Fig. 6). Until now, only ZNT5 and ZNT8 have been localized to the insulin containing granules (Kambe et al. 2002; Salazar et al. 2009) and with our new findings a close interplay between the different zinc transporters may exist. Further studies will help to establish the precise interplay between the zinc transporters.
In conclusion, we find ZNT3 up-regulation to cause improved cell survival in INS-1E suggesting ZNT3 up-regulation as a possible therapeutic strategy in type 2 diabetes prevention. The localization of ZNT3 in secretory vesicles suggests a role for ZNT3 in maintaining intravesicular and cytoplasmic zinc homeostasis.
References
Adlard PA, Parncutt JM, Finkelstein DI, Bush AI (2010) Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J Neurosci 30:1631–1636
Boquist L, Falkmer S, Havu N, Pihl E (1968) Insulin biosynthesis, storage and secretion. Pancreatic-islet tissue and heavy metals—some ultrastructural and experimental observations. Lakartidningen 65:3603–3607
Bozym RA, Chimienti F, Giblin LJ et al (2010) Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp Biol Med (Maywood) 235:741–750
Chimienti F, Devergnas S, Favier A, Seve M (2004) Identification and cloning of a beta-cell specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53:2330–2337
Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, Seve M (2006) In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 119:4199–4206
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281:24085–24089
Doering P, Stoltenberg M, Penkowa M, Rungby J, Larsen A, Danscher G (2010) Chemical blocking of zinc ions in CNS increases neuronal damage following traumatic brain injury (TBI) in mice. PLoS One 5:e10131
Emdin SO, Dodson GG, Cutfield JM, Cutfield SM (1980) Role of zinc in insulin biosynthesis. Some possible zinc–insulin interactions in the pancreatic B-cell. Diabetologia 19:174–182
Flannick J, Thorleifsson G, Beer NL et al (2014) Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet 46:357–363
Fu Y, Tian W, Pratt EB, Dirling LB, Shyng SL, Meshul CK, Cohen DM (2009) Down-regulation of ZnT8 expression in INS-1 rat pancreatic beta cells reduces insulin content and glucose-inducible insulin secretion. PLoS One 4:e5679
Fukunaka A, Suzuki T, Kurokawa Y, Yamazaki T, Fujiwara N, Ishihara K, Migaki H, Okumura K, Masuda S, Yamaguchi-Iwai Y, Nagao M, Kambe T (2009) Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J Biol Chem 284:30798–30806
Gerber PA, Bellomo EA, Hodson DJ, Meur G, Solomou A, Mitchell RK, Hollinshead M, Chimienti F, Bosco D, Hughes SJ, Johnson PR, Rutter GA (2014) Hypoxia lowers SLC30A8/ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells. Diabetologia 57:1635–1644
Gilda JE, Gomes AV (2013) Stain-free total protein staining is a superior loading control to beta-actin for western blots. Anal Biochem 440:186–188
Gold G, Grodsky GM (1984) Kinetic aspects of compartmental storage and secretion of insulin and zinc. Experientia 40:1105–1114
Huang L, Kirschke CP, Lay Y-AE, Levy LB, Lamirande DE, Zhang PH (2012) Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J Biol Chem 287:33883–33896
Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M (2002) Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem 277:19049–19055
Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA, Gilon P, in’t Veld PA, Schuit FC (2009) Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA 106:14872–14877
Nicolson TJ, Bellomo EA, Wijesekara N et al (2009) Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58:2070–2083
Nolan CJ, Damm P, Prentki M (2011) Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 378:169–181
Nygaard SB, Larsen A, Knuhtsen A, Rungby J, Smidt K (2014) Effects of zinc supplementation and zinc chelation on in vitro β-cell function in INS-1E cells. BMC Res Notes 7:84
Nygaard SB, Lund NS, Larsen A, Pedersen N, Rungby J, Smidt K (2015) Exogenous metallothionein potentiates the insulin response at normal glucose concentrations in INS-1E beta-cells without disturbing intracellular ZnT8 expression. Basic Clin Pharmacol Toxicol 116:173–177
Palmiter RD, Cole TB, Quaife CJ, Findley SD (1996) ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci USA 93:14934–14939
Patrushev N, Seidel-Rogol B, Salazar G (2012) Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells. PLoS One 7:e33211
Petersen AB, Smidt K, Magnusson NE, Moore F, Egefjord L, Rungby J (2011) siRNA-mediated knock-down of ZnT3 and ZnT8 affects production and secretion of insulin and apoptosis in INS-1E cells. APMIS 119:93–102
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7:1342–1365
Prasad AS (2012) Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol 26:66–69
Rungby J (2010) Zinc, zinc transporters and diabetes. Diabetologia 53:1549–1551
Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, Faundez V (2004) The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol Biol Cell 15:575–587
Salazar G, Falcon-Perez JM, Harrison R, Faundez V (2009) SLC30A3 (ZnT3) oligomerization by dityrosine bonds regulates its subcellular localization and metal transport capacity. PLoS One 4:e5896
Scott DA, Fisher AM (1938) The insulin and the zinc content of normal and diabetic pancreas. J Clin Investig 17:725–728
Sladek R, Rocheleau G, Rung J et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885
Smidt K, Rungby J (2012) ZnT3: a zinc transporter active in several organs. Biometals 25:1–8
Smidt K, Wogensen L, Brock B, Schmitz O, Rungby J (2006) Real-time PCR: housekeeping genes in the INS-1E beta-cell line. Horm Metab Res 38:8–11
Smidt K, Jessen N, Petersen AB et al (2009) SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS One 4:e5684
Smidt K, Sørensen K, Larsen A, Prætorius J, Martensen PM, Rungby J (2013) Insulin secretion and ZnT8 gene expression are decreased by ZnT3 overexpression. Diabetologia 56(Suppl 1):A478 (Abstract)
Søndergaard LG, Brock B, Stoltenberg M, Flyvbjerg A, Schmitz O, Smidt K, Danscher G, Rungby J (2005) Zinc fluxes during acute and chronic exposure of INS-1E cells to increasing glucose levels. Horm Metab Res 37:133–139
Souza SC, Qiu L, Inouye KE, Roix JJ, Chen H (2008) Zinc transporter ZnT-8 regulates insulin and glucagon secretion in Min6 and aTC1-9 pancreatic cell lines. Diabetologia 51(Suppl 1):A501 (Abstract)
Suzuki T, Ishihara K, Migaki H, Ishihara K, Nagao M, Yamaguchi-Iwai Y, Kambe T (2005) Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J Biol Chem 280:30956–30962
Tamaki M, Fujitani Y, Hara A et al (2013) The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Investig 123:4513–4524
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY, Rutter GA, Wheeler MB (2010) Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 53:1656–1668
Acknowledgments
INS-1E cells were kindly provided by Professor Emeritus Claes Wollheim and Professor Pierre Maechler, University of Geneva, Switzerland. We want to thank laboratory technician Elin Carstensen for help with the insulin assay, Else-Merete Løcke for assistance in electron microscopy and immunofluorescence, and laboratory technician Helle Zibrandtsen for help with the immunoblotting analysis. The study was supported by AP Møller and wife Chastine Mc-Kinney Møller Foundation and Desirée and Niels Ydes Foundation. Preliminary reports of these findings have previously been published in abstract form (Smidt et al. 2013) and reported at the 49th EASD Annual Meeting in Barcelona in 2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Smidt, K., Larsen, A., Brønden, A. et al. The zinc transporter ZNT3 co-localizes with insulin in INS-1E pancreatic beta cells and influences cell survival, insulin secretion capacity, and ZNT8 expression. Biometals 29, 287–298 (2016). https://doi.org/10.1007/s10534-016-9915-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9915-7