Abstract
Legionella pneumophila is a waterborne pathogen that can cause Legionnaires’ disease, a fatal pneumonia, or Pontiac fever, a mild form of disease. Copper is an antimicrobial material used for thousands of years. Its incorporation in several surface materials to control the transmission of pathogens has been gaining importance in the past decade. In this work, the ability of copper to control the survival of L. pneumophila in biofilms was studied. For that, the incorporation of L. pneumophila in polymicrobial drinking water biofilms formed on copper, PVC and PEX, and L. pneumophila mono-species biofilms formed on copper and uPVC were studied by comparing cultivable and total numbers (quantified by peptide nucleic acid (PNA) hybridisation). L. pneumophila was never recovered by culture from heterotrophic biofilms; however, PNA-positive numbers were slightly higher in biofilms formed on copper (5.9 × 105 cells cm−2) than on PVC (2.8 × 105 cells cm−2) and PEX (1.7 × 105 cells cm−2). L. pneumophila mono-species biofilms grown on copper gave 6.9 × 105 cells cm−2 for PNA-positive cells and 4.8 × 105 CFU cm−2 for cultivable numbers, showing that copper is not directly effective in killing L. pneumophila. Therefore previous published studies showing inactivation of L. pneumophila by copper surfaces in potable water polymicrobial species biofilms must be carefully interpreted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper as an antimicrobial substance was first used several thousand years ago in Egypt to disinfect water and wounds (Borkow and Gabbay 2009). Copper has broad spectrum antimicrobial properties against bacteria, viruses and fungi; in recent years the mechanism of copper’s antimicrobial action against Gram-negative and Gram-positive bacteria and norovirus has been described (Bleichert et al. 2014; Warnes et al. 2012, 2014; Warnes and Keevil 2013). Consequently, use of copper alloys as a contact surface material has gained recognition, with particular application in food industries and hospitals. A recent study in a hospital in Birmingham (UK) demonstrated that the isolation of several pathogens, such as Clostridium difficile, Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA) from surfaces containing copper was significantly lower compared to plastic, chrome-plated and aluminium surfaces when used for toilet seats, door push plates and tap handles (Casey et al. 2010). In another study, conducted in three hospitals in the US, it has been shown that the numbers of total bacteria as well as the numbers of MRSA and other staphylococci, Gram-negative and vancomycin-resistant enterococcus were lower when copper had been introduced in the surface materials (Schmidt et al. 2012b). Of note, these hospitals also showed a 58 % reduction in the rate of infections (Salgado et al. 2013). Numerous studies have also been conducted in recent years concerning the application of copper to control the formation of drinking water biofilms as well as the inclusion of waterborne pathogens, such as Pseudomonas aeruginosa and Legionella pneumophila, into these biofilms (Moritz et al. 2010; Rogers et al. 1994a, b; van der Kooij et al. 2005). Here, the bacteria grow and proliferate while they are constantly exposed to the metal. By contrast, the antimicrobial efficacy and mode of action of metallic copper described above concerned non-growing microbial cells exposed to dry metallic surfaces. These were non-growing cells that did not form biofilms during the course of the experiments. It is therefore important to understand the antimicrobial effects of copper in wet growing systems where biofilm formation is important.
Legionella pneumophila is an ubiquitous pathogen which can pass through water treatment facilities and contaminate drinking water systems (Atlas 1999; Keevil 2002). Infections caused by the inhalation of contaminated aerosols can lead to a mild type of illness, Pontiac fever, giving flu-like symptoms. However, in susceptible people this waterborne pathogen can be fatal if not detected in time, causing Legionnaire’s disease, a type of pneumonia (McDade et al. 1977; Pasculle 2000). The ecology of L. pneumophila has been widely studied, including the survival strategies this pathogen can adopt in low nutrient environments such as drinking water. The incorporation into heterotrophic biofilms is known to support the survival of this pathogen, where it can persist for long periods (Declerck 2010; Gião et al. 2009b; Murga et al. 2001; Surman et al. 2002). Therefore, the potential use of copper as a material for pipelines has gained more interest. The effect of copper on L. pneumophila when incorporated into drinking water biofilms appears to be controversial, although there is no doubt that copper ions have an inhibitory effect on this pathogen (Landeen et al. 1989; Lin et al. 2002). Early studies by Rogers et al. (1994b) found that no cultivable L. pneumophila were recovered in polymicrobial species biofilms formed in potable water on copper at 20, 50 and 60 °C, only at 40 °C. Later, Moritz et al. (2010) published a study also showing reduced incorporation of cultivable L. pneumophila into drinking water biofilms formed on copper coupons compared to plastic materials; however, numbers appeared higher using DNA-based fluorescence in situ hybridisation (FISH) although viability could not be confirmed. Conversely, a study of a model drinking water system, published by van der Kooij and colleagues (2005) showed that copper pipes had little effect on the incorporation of the cultivable pathogen into heterotrophic biofilms formed in a high carbonate water which may have masked the copper surface. Buse et al. (2014) demonstrated using qPCR that biofilms grown on copper accumulated L. pneumophila and released a higher concentration of this pathogen to the effluent than biofilms grown on unplasticised polyvinylchloride (uPVC). Clearly, it is important to differentiate colonisation based on detectable cultivable numbers and molecular detection using FISH or qPCR which are not necessarily indicative of viability. It is becoming increasingly apparent that the presence of many pathogens can be underestimated when they respond to stressful environments by becoming viable but non cultivable (VBNC) i.e. are not able to grow on agar medium but are still viable and can recover cultivability when in favourable conditions (Gião et al. 2009a; Hussong et al. 1987). The use of peptide nucleic acid (PNA) probes has gained importance over recent years and can overcome the non detection of VBNC, as they provide more sensitive detection of pathogens in complex environments, such as heterotrophic biofilms, than DNA probes (Azevedo et al. 2003; Lehtola et al. 2006). In particular, the use of the specific L. pneumophila PNA probe PLPNE620 has been proved successful in detecting this pathogen in drinking water polymicrobial biofilms and showing a high rRNA content indicative of viability (Gião et al. 2009b, c; Wilks and Keevil 2006).
The aim of this work was to determine whether copper surfaces directly or indirectly affect the cultivability, VBNC formation and proliferation of L. pneumophila in monospecies and polymicrobial heterotrophic biofilms formed in potable water using culture recovery and PNA-FISH techniques.
Materials and methods
Inoculum preparation
Legionella pneumophila NCTC 12821 was kept on protect vials at −80 °C. To recover cells, a bead was plated onto a buffered charcoal yeast extract (BCYE) agar plate (Oxoid, UK) and incubated at 30 °C for 48 h. Cultures were subcultured once for 24 h prior the beginning of the experiment. A loop of cells was removed from the BCYE agar plates and resuspended in filter-sterilised and dechlorinated tap water.
Treatment of coupons for the two-stage chemostats
Copper (Cu), polyvinylchloride (PVC) and cross-linked polyethylene (PEX) coupons, obtained from pipes and cut into 1 cm2 squares, were used as a support for biofilm growth. Coupons were immersed in water and detergent (Guard Professional, UK) for 5 min, washed with a bottle brusher, rinsed twice in distilled water and air-dried. Subsequently, they were washed in 70 % (v/v) ethanol to remove any organic compounds, attached to the end of a titanium wire and autoclaved at 121 °C for 15 min (Keevil 2001).
Legionella pneumophila in heterotrophic biofilms
To study the influence of copper on L. pneumophila when embedded in drinking water biofilms a two-stage chemostat model system was used, as described elsewhere (Gião et al. 2009c). To summarise, a microbial consortium collected from Southampton tap water by filtration through a 0.2 μm pore size Nylon filter (Pall Gelman, UK) was used to inoculate the first stage (seed) vessel filled with 1-litre of filter-sterilised (0.2 μm pore size Nylon filter) and dechlorinated tap water. The seed vessel was maintained in batch mode for 2 days to promote microbial growth and then changed into a continuous mode, being fed with fresh medium (filter-sterilised and dechlorinated tap water) at a flow rate of 50 ml h−1. The seed vessel exit flow was then used to inoculate the second stage, which consisted of three vessels (biofilm-growing vessels) working in parallel. Each biofilm-growing vessel was also fed with fresh medium at a flow rate maintaining a dilution rate of 0.2 h−1, in order to avoid planktonic microbial growth. Temperature was controlled at 30 °C by a proportional integral derivative unit system (Brighton Systems, UK) and the system was stirred at 300 rpm to promote homogeneity of oxygen and nutrients. After 10 days, conditions in the biofilm-growing vessels were stable and sterile Cu, PVC and PEX coupons were introduced (day 0), a different vessel for each substratum. Preliminary experiments had shown that there was a low concentration of autochthonous L. pneumophila in the chemostats. Therefore the biofilm growing vessels were spiked with L. pneumophila NCTC 12821 immediately before the immersion of the coupons, to give a final concentration of approx. 107 cells ml−1. Coupons were removed after 1, 2, 4, 8, 16 and 32 days, gently rinsed in filtered tap water to remove planktonic cells, and scraped to quantify sessile cells.
Legionella pneumophila in monospecies biofilms
To generate L. pneumophila monospecies biofilms a static system was used. Cu and uPVC coupons were prepared as described previously and placed in six-well microtiter plates. A 5 ml inoculum of 107 cells ml−1 L. pneumophila NCTC 12821, prepared as described before, was added to each well and the plates were incubated at 30 °C for 1, 2, 4, 8, 16 and 32 days. After this time one coupon of each material were removed from the wells, gently rinsed in tap water and biofilm scraped to quantify sessile cells.
Quantification of planktonic cells from two-stage chemostats
When coupons were removed from the biofilm-growing vessels a water sample was also taken for planktonic cell quantification. Cells were quantified for total cells, heterotrophic plate counts (HPC) and cultivable L. pneumophila. Total cells were quantified using SYTO 9 (Molecular Probes, Invitrogen, UK) by mixing 1 ml of an appropriate dilution with 0.5 μl of SYTO 9. This suspension was incubated in the dark for 15 min, filtered through a 0.2 μm pore size polycarbonate black Nucleopore® membrane (Whatman, UK) and allowed to air-dry. A drop of non-fluorescence immersion oil (Fluka, UK) was added and a coverslip placed on top of the membrane. Membranes were observed under oil using a Nikon Eclipse E800 episcopic differential interference contrast/epifluorescence (EDIC/EF) microscope (Best Scientific, UK) (Keevil 2003). As the cells were homogenously distributed, 10 fields of view were randomly chosen from each membrane and cells counted. HPC were quantified by plating onto R2A medium (Oxoid, UK) and incubated at 22 °C for 7 days and cultivable L. pneumophila were quantified by plating onto BCYE agar plates and incubating at 30 °C for up to 14 days.
Quantification of sessile cells from two-stage chemostats
At each time point, one coupon of each material was removed from each biofilm-growing vessel and gently rinsed in filter-sterilised tap water to remove planktonic cells loosely adhered to the biofilm. The coupon was vortexed with glass beads in sterile tap water to remove all the biofilm from the surface and homogenise the suspension, as described elsewhere (Gião et al. 2009c, 2011). Total cells, HPC and cultivable L. pneumophila were quantified using the methods described above. In addition, L. pneumophila were quantified using the specific PNA probe PLPNE620 (5′-CTG ACC GTC CCA GGT-3′) (Eurogentec, Belgium) in a FISH assay (Wilks and Keevil 2006). Briefly, 1 ml of an appropriate dilution was filtered through a 0.2 μm Anodisc membrane (Whatman, UK), and left to air dry. The membrane was covered with 90 % (v/v) ethanol to fix the cells and again air dried. The hybridisation, washing and microscopy observation method was performed as described by Wilks and Keevil (2006).
Quantification of planktonic cells from six-well plates
The planktonic cells of the water in the six well-plates assay were quantified for total cells by SYTO 9 and cultivable L. pneumophila by plating onto BCYE agar as described previously in the“Quantification of planktonic cells from two-stage chemostats” section.
Quantification of sessile cells from six-well plates
At each time point one Cu and one uPVC coupon were removed from a well and gently rinsed in sterile tap water. The biofilm was scraped as described previously and cells quantified. To quantify the number of live and dead cells, a known dilution of the scraped biofilm was stained with the LIVE/DEAD® BacLight™ Bacterial Viability kit (Molecular Probes, Invitrogen, UK). A 3 μl volume of an equal proportion of SYTO 9/propidium iodide (PI) mixture was added to 1 ml of a known dilution of sample and incubated in the dark, at room temperature for 15 min followed by filtration through a black polycarbonate membrane. Subsequently, the membranes were air dried, mounted onto glass slides with non-fluorescence immersion oil and a cover slip. The slides were examined using the EDIC/EF microscope. Cultivable L. pneumophila cells and PNA-positive cells were quantified as described above.
Detection of poly-3-hydroxybutyrate deposits
To understand the influence of poly-3-hydroxyburate (PHB) reserves on the cultivability of L. pneumophila biofilms, monospecies biofilms were formed on Cu and uPVC surfaces on six-well microtiter plates as described above. Every week up to 17 weeks, two coupons of each material were removed and gently rinsed with sterile tap water. One coupon was scraped to quantify cultivable numbers by plating onto BCYE agar. The other coupon was stained in situ with Nile Red to detect PHB reserves in the cells, as described by James et al. (1999) with some modifications. For that, the coupons covered with the biofilm were left to air dry and covered with 90 % ethanol for 10 min to fix the cells. The excess of ethanol was then removed and after the coupons had dried off the biofilm was covered with a 50 μM Nile Red solution and incubated in the dark at room temperature for 30 min. The coupons were then rinsed with distilled water and left to air dry. Biofilms were observed under an EDIC/EF microscope.
Statistical analysis
The homogeneity of variances of total number of cells, PNA-positive L. pneumophila, HPC and cultivable L. pneumophila was checked using the Levene test for equality of variances using a statistical package (SPSS Inc., Chicago IL, USA). Differences were subsequently compared by a one-way ANOVA followed by a Bonferroni post hoc test. Differences were considered significant if p < 0.05.
Results
Two-stage chemostats: planktonic cells
To study the influence of copper on the survival of L. pneumophila in drinking water biofilms, a two-stage chemostat was used. The first stage consisted of a seed vessel, where an inoculum collected from Southampton tap water was left to grow, at a low flow rate. After stabilisation the seed vessel had, in general, a constant number of total cells and HPC (p > 0.05), which were on average, 3.24 × 106 cells ml−1 and 1.69 × 106 CFU ml−1, respectively.
The second stage of the chemostat system consisted of three vessels working in parallel. Preliminary experiments using the PNA probe revealed that the concentration of autochthonous L. pneumophila was lower than 104 cells ml−1 (data not shown) and therefore the biofilm growing vessels were spiked with a type collection culture of this pathogen prior to the immersion of the coupons, as described in the “Materials and methods” section. During the experiment there were some significant fluctuations (p < 0.05) in the numbers of planktonic cells, both total and HPC numbers, in the three biofilm growing vessels, possibly due to exchanges with biofilm biomass. The average of the log cell numbers for all the vessels, as well as the standard deviation, is presented in Table 1. The numbers of total cells and HPC present in the bulk water were not significantly different (p > 0.05) when comparing the PVC and PEX vessels (on average, total cell counts were 3.11 × 106 cells ml−1 and HPC were 1.11 × 106 CFU ml−1) however those numbers were significantly lower (p < 0.05) in the bulk water of the copper containing vessel (1.99 × 106 cells ml−1 for total numbers and 6.07 × 105 CFU ml−1 for HPC). Cultivable L. pneumophila was never recovered from the planktonic phase from any of the vessels, although overgrowth of other microorganisms was observed.
Two stage chemostats: sessile cells
In Fig. 1 the variation of total cells, PNA-positive L. pneumophila and HPC numbers is shown for biofilms formed on the different coupon materials with time. There were significant variations in the numbers of total and HPC cells during the 32 days of the experiment (p < 0.05), however the number of L. pneumophila in the biofilms decreased substantially during the first 4 days (p < 0.05) followed by a stabilisation of the numbers (p > 0.05). There was a slightly higher amount of total biofilm (Fig. 1a) on copper and PVC compared to PEX (p > 0.05) while the numbers of HPC (Fig. 1c) were significantly higher in biofilms formed on copper surfaces (in general, p < 0.05). HPC numbers on copper accounted for about 2.5 % of the total cells while this percentage was reduced to 1.5 % on PVC and 1.9 % on PEX. Moreover while the numbers of L. pneumophila in biofilms (Fig. 1b) formed on the plastic materials were similar (on average, 2.83 × 105 cells cm−2 for PVC and 1.74 × 105 cells cm−2 for PEX, p > 0.05) there were significantly more cells (p < 0.05) of this pathogen in biofilms formed on copper (on average, 5.86 × 105 cells cm−2) when detected using PNA-FISH. L. pneumophila was approximately 2.0 % of total biofilm formed on copper but only 1.2 % of biofilms formed on the plastic coupons. In all experiments it was not possible to recover cultivable L. pneumophila on BCYE plates, although overgrowth of other microorganisms was observed which might have obscured low numbers of cultivable legionellae.
The morphology of the colonies obtained was similar to previous studies where bacterial identification has been published (Gião et al. 2009c). On R2A colonies of Acidovorax spp. and Shingobium yanoikuyae were always seen and on BCYE colonies formed by Sphingomonas spp., Stenotrophomonas spp., Mycobacterium chelonae and Variovorax paradoxus were commonly present.
Biofilms formed in six-well plates
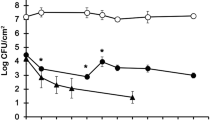
The results obtained for the heterotrophic biofilm studies showed the presence of L. pneumophila on biofilms formed on copper surfaces using the PNA probe, although cells were not detected by culture methods. As L. pneumophila were embedded in heterotrophic biofilms it was not possible to determine whether these non cultivable cells were due to loss of cultivability or if cells were still cultivable but their growth was masked by the overgrowth of other microorganisms. To understand the role of copper on the cultivability and survival of this pathogen in the biofilm state of life, L. pneumophila mono-species biofilms were grown on copper, and compared to uPVC substratum as a control. Biofilms were sampled at the same time points as biofilms formed under dynamic conditions (in the chemostats) and total and cultivable L. pneumophila cells numbers were quantified in the planktonic stage while live/dead, cultivable and PNA-positive L. pneumophila cells were quantified for the sessile phase. In Table 2 the average numbers for total and cultivable cells obtained for both materials in the planktonic phase are shown. There were no significant changes in the numbers of total and cultivable cells with time for the bulk water where copper and uPVC have been immersed (p > 0.05). Moreover, the numbers were similar for both materials (p > 0.05). Live and dead sessile cells were quantified using the Live/Dead kit and it was observed that these numbers remained constant with time (p > 0.05). Furthermore, dead cells were in general almost 1-log lower than live cells and therefore the number of live and total cells were very similar. For this reason it was decided to present results in terms of total cells. Sessile cells were also quantified for PNA-positive and cultivable L. pneumophila. Both numbers were constant with time (p > 0.05). PNA and cultivable L. pneumophila cell numbers were also similar in biofilms formed on copper compared to biofilms formed on uPVC coupons (p > 0.05). Biofilms formed on copper had, on average, 4.36 × 106 cells cm−2 for total numbers, 6.85 × 105 cells cm−2 for L. pneumophila quantified using the PNA probe and 4.82 × 105 CFU cm−2 of cultivable L. pneumophila. For biofilms formed on the uPVC substratum these numbers were 5.41 × 106 cells cm−2, 7.47 × 105 cells cm−2 and 5.40 × 105 CFU cm−2, respectively. In Fig. 2 it is possible to observe the variation of total, PNA-positive and cultivable L. pneumophila in biofilms formed on the copper substratum. It is also possible to observe that the numbers of PNA-positive cells were higher than the numbers of cultivable cells but lower than the total cell numbers. These differences were also verified in the average numbers presented for both materials.
PHB reserves in monospecies Legionella pneumophila biofilms
It was observed that in pure cultures L. pneumophila was able to retain cultivability for at least 32 days. Since tap water is a low nutrient medium and is not able to support the growth of this pathogen, the possibility of cultivability being maintained by using the PHB reserves was studied. For that, biofilms were formed for 17 weeks, checked for presence of PHB by staining them with Nile Red and compared to cultivability results. On both materials, cultivability was lost between week 11 and 17, however the presence of PHB reserves in the cells were still observed after 17 weeks of incubation (results not shown). This shows that PHB was insufficient in supporting L. pneumophila growth in the absence of nutrients and therefore loss of cultivability was not due to the lack of nutrients.
Discussion
Several studies have demonstrated that copper can inactivate various pathogens in contact with its surface (Noyce et al. 2006; Warnes et al. 2012). It has also been demonstrated that copper can also be effective to prevent and control biofilm (Gould et al. 2009; Moritz et al. 2010). Copper can not only negatively affect cells adhered to its surface but also can leach cooper ions into the bulk water affecting microorganisms in suspension (Rogers et al. 1994b). The results presented in this work support those findings since after the immersion of the copper coupons the bulk water had significantly less total and HPC cells compared to the bulk water where uPVC and PEX coupons were immersed. Either before or after the immersion of the coupons no positive results for cultivable L. pneumophila were ever obtained. This could have been due to either loss of cultivability or to the overgrowth of other microorganisms on BCYE plates as it has been observed in previous studies (Gião et al. 2009b, c). Another explanation is the fact that the biofilm growth vessels were spiked with L. pneumophila prior the immersion of the coupons, and washout of the inoculum occurred in less than 24 h.
The use of the PNA probe revealed the presence of L. pneumophila in the biofilm, although cultivable sessile cells were never recovered on agar plates. The detection of the strong PNA signal is connected to the detection of intact rRNA and is associated with the detection of viable cells. It was observed that the numbers of PNA-positive L. pneumophila decreased between day 1 and 4 and remained practically constant at subsequent time points. This has been observed in another study where biofilms were spiked at the beginning of the experiment with the pathogen Helicobacter pylori (Gião et al. 2008). The results obtained were a consequence of cells adhering to the top layers of the biofilm which were then removed due to sloughing and not replaced since the planktonic cells of the inoculated pathogen had been washed out from the system. The same appears to have happened in the current work. Furthermore, it was also observed that the copper substratum supported more PNA-L. pneumophila cells than PVC and PEX, suggesting that copper can enhance the presence of VBNC L. pneumophila. Previous studies have investigated incorporation of L. pneumophila into drinking water biofilms formed on copper materials (Buse et al. 2014; Moritz et al. 2010; Rogers et al. 1994b; Türetgen and Cotuk 2007; van der Kooij et al. 2005). Rogers et al. (1994b) demonstrated that biofilms formed at different temperatures always had less cultivable heterotrophic cells on copper than on PVC and polybutylene. Moreover, they only recovered cultivable L. pneumophila from biofilms formed on copper at 40 °C, while cultivable L. pneumophila was always found in biofilms formed on PVC and polybutylene at 20, 40 and 50 °C. A study published by van der Kooij (2005) revealed the presence of less HPC and cultivable L. pneumophila on copper pipes compared to biofilms formed on stainless steel and PEX. Türetgen and Cotuk (2007) published a report showing that biofilms formed on copper for 60 days supported less cultivable L. pneumophila than the majority of the materials tested, although plastic materials showed less cultivable L. pneumophila in 120 and 180 days-old biofilms. Moritz and colleagues (2010) demonstrated that Legionella can incorporate and persist in biofilms for long periods, independent of the material used, although copper appeared to support less biofilm and less cultivable Legionella. The results presented in those studies are contrary to the findings of the present work, which shows the presence of higher numbers of L. pneumophila on copper surfaces. The difference might result from the fact that in the current work L. pneumophila cells were quantified by the PNA hybridisation method while in other studies cells were detected by culture, which is well known to underestimate and not identify VBNC cells. In fact, Moritz et al. (2010) also obtained higher numbers of L. pneumophila with FISH than with culture, and the number of Legionella cells detected by FISH analysis were similar on copper and the other materials. Another interesting study has been published recently (Buse et al. 2014). The authors showed that although the concentration of L. pneumophila (detected by qPCR) in biofilms formed on copper was in general lower compared to PVC surfaces, the concentration of this pathogen in the effluent from the Cu-vessel was higher. Interestingly they also showed that even when this pathogen was undetected in the biofilm (possibly due a low concentration, below the limit of detection) it was still detected in the effluent indicating that biofilms can be a niche to concentrate this pathogen in drinking water. Lu and colleagues also reported recently that copper supports L. pneumophila in drinking water biofilms possibly due to changes in the heterotrophic community of those biofilms (Lu and Clarke 2005). Moreover, Walker et al. (Walker et al. 1993) utilised GC–MS signatures of L. pneumophila in drinking water consortia similar to Rogers et al. (1994b) to show that the ratios of colonization were a 1:3 ratio on copper and polyethylene, respectively; whereas the recovery of cultivable legionellae yielded a 50-fold difference between copper and polyethylene. The authors suggested that a greater proportion of the L. pneumophila on copper are either non-viable or non-cultivable in comparison to polyethylene. Hence, it is clear that even if L. pneumophila is not detected by culture it does not mean it is not present at all. It has been proved that L. pneumophila cells can enter a VBNC state after being exposed to starvation or other stress conditions, being able to be recovered when cells are in favourable conditions (Gião et al. 2009a; Hussong et al. 1987). Therefore the reports mentioned above, which suggest that the incorporation of L. pneumophila in drinking water biofilms can be effectively controlled by the use of copper materials should be carefully considered as this present work demonstrates the presence of more viable L. pneumophila cells compared to the other plastic materials.
It is not possible to conclude if the lack of cultivability was due to the overgrowth of other microorganisms or due to cells entering into the VBNC state, although the former is unlikely since PNA-FISH showed L. pneumophila being 2 % of the total population. To study the direct effect of copper on the cultivability of L. pneumophila sessile and planktonic cells, monospecies biofilms were formed on copper, using uPVC as control. Results clearly demonstrated that copper per se is not able to kill L. pneumophila or even decrease cultivability during the 32 days of the experiment. Moreover it was observed that cultivability of sessile cells was not maintained using the PHB reserves as previously demonstrated for planktonic L. pneumophila starved in water (James et al. 1999). This shows that sessile L. pneumophila adopt different strategies to survive in low nutrient media but copper has no direct negative effect on L. pneumophila.
Comparing the results obtained here to the results obtained in other studies it seems that copper might however have an indirect effect on L. pneumophila. It is important to mention that temperature, water composition and microbial flora were different in this present work, and this can influence the incorporation and survival of L. pneumophila in water, e.g. copper ions have been shown to have their L. pneumophila biocidal effect reduced if pH is increased to 9 (Lin et al. 2002). It is known that some microorganisms, such as Flavobacterium spp. and Methylobacterium spp., can have a synergistic effect on L. pneumophila cells while others can have a negative effect (Gião et al. 2011; Guerrieri et al. 2008; Surman et al. 1994; Wadowsky and Yee 1983). It is also known that different microorganisms are indeed susceptible to a potential biocidal effect of copper (Gould et al. 2009; Warnes et al. 2012), including Flavobacterium spp. (Nieto et al. 1989) and Methylobacterium spp. (Schmidt et al. 2012a). The difference in the microbial community from different studies could explain the lack of cultivability of L. pneumophila, especially in the presence of copper, although the pathogen would have possibly been detected if molecular methods were used.
Legionella pneumophila is a waterborne pathogen that can, in particular conditions, be fatal. There is no evidence of any case of person-to-person transmission, with the only well-documented route of transmission being the inhalation of contaminated aerosols. Therefore, the role of drinking water in the spread of this pathogen is of high importance, and good detection methods and adequate control measures to limit this pathogen in water are crucial. This is the first work that demonstrates that the use of copper surfaces is not effective in directly controlling L. pneumophila. It suggests that other studies might have underestimated the presence of VBNC cells by relying on cultivable methods only and brings new concerns about the biocidal effect of copper on L. pneumophila. Nevertheless, it is possible that copper has an important indirect effect in the control of this pathogen by affecting key species that support or adversely affect its growth. Clearly a better understanding of the ecology of L. pneumophila and its interaction with drinking water communities is required, in particular when different compositions of species form biofilms on copper or other antimicrobial materials.
References
Atlas RM (1999) Legionella: from environmental habitats to disease pathology, detection and control. Environ Microbiol 1:283–293
Azevedo NF, Vieira MJ, Keevil CW (2003) Development of peptide nucleic acid probes to detect Helicobacter pylori in diverse species potable water biofilms. In: McBain A, Allison C, Brading M, Rickard A, Verran J, Walker J (eds) Biofilm communities: order from chaos? Bioline, Cardiff, pp 231–239
Bleichert P, Espírito Santo C, Hanczaruk M, Meyer H, Grass G (2014) Inactivation of bacterial and viral biothreat agents on metallic copper surfaces. Biometals 27:1179–1189
Borkow G, Gabbay J (2009) Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr Chem Biol 3:272–278
Buse HY, Lu J, Struewing IT, Ashbolt NJ (2014) Preferential colonization and release of Legionella pneumophila from mature drinking water biofilms grown on copper versus unplasticized polyvinylchloride coupons. Int J Hyg Environ Health 217:219–225
Casey AL, Adams D, Karpanen TJ et al (2010) Role of copper in reducing hospital environment contamination. J Hosp Infect 74:72–77
Declerck P (2010) Biofilms: the environmental playground of Legionella pneumophila. Environ Microbiol 12:557–566
Gião MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW (2008) Persistence of Helicobacter pylori in heterotrophic drinking water biofilms. Appl Environ Microbiol 74:5898–5904
Gião M, Wilks S, Azevedo N, Vieira M, Keevil C (2009a) Validation of SYTO 9/propidium iodide uptake for rapid detection of viable but noncultivable Legionella pneumophila. Microb Ecol 58:56–62
Gião MS, Wilks S, Azevedo NF, Vieira MJ, Keevil CW (2009b) Incorporation of natural uncultivable Legionella pneumophila into potable water biofilms provides a protective niche against chlorination stress. Biofouling 25:345–351
Gião MS, Wilks SA, Azevedo NF, Vieira MJ, Keevil CW (2009c) Comparison between standard culture and peptide nucleic acid 16S rRNA hybridization quantification to study the influence of physico-chemical parameters on Legionella pneumophila survival in drinking water biofilms. Biofouling 25:335–343
Gião MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW (2011) Interaction of Legionella pneumophila and Helicobacter pylori with bacterial species isolated from drinking water biofilms. BMC Microbiol 11:57
Gould S, Fielder M, Kelly A, Morgan M, Kenny J, Naughton D (2009) The antimicrobial properties of copper surfaces against a range of important nosocomial pathogens. Ann Microbiol 59:151–156
Guerrieri E, Bondi M, Sabia C, de Niederhäusern S, Borella P, Messi P (2008) Effect of bacterial interference on biofilm development by Legionella pneumophila. Curr Microbiol 57:532–536
Hussong D, Colwell RR, O’Brien M, Weiss E, Pearson AD, Weiner RM, Burge WD (1987) Viable Legionella pneumophila not detectable by culture on agar media. Nat Biotechnol 5:947–950
James BW, Mauchline WS, Dennis PJ, Keevil CW, Wait R (1999) Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low nutrient environments. Appl Environ Microbiol 65:822–827
Keevil CW (2001) Continuous culture models to study pathogens in biofilms. Method Enzymol 337:104–122
Keevil CW (2002) Pathogens in environmental biofilms. In: Bitton G (ed) The encyclopedia of environmental microbiology. Wiley, New York, pp 2339–2356
Keevil CW (2003) Rapid detection of biofilms and adherent pathogens using scanning confocal laser microscopy and episcopic differential interference contrast microscopy. Water Sci Technol 47:105–116
Landeen LK, Yahya MT, Gerba CP (1989) Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl Environ Microbiol 55:3045–3050
Lehtola MJ, Torvinen E, Miettinen LT, Keevil CW (2006) Fluorescence in situ hybridization using peptide nucleic acid probes for rapid detection of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. paratuberculosis in potable water biofilms. Appl Environ Microbiol 72:848–853
Lin YSE, Vidic RD, Stout JE, Yu VL (2002) Negative effect of high pH on biocidal efficacy of copper and silver ions in controlling Legionella pneumophila. Appl Environ Microbiol 68:2711–2715
Lu H, Clarke M (2005) Dynamic properties of Legionella containing phagosomes in Dictyostelium amoebae. Cell Microbiol 7:995–1007
McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, Dowdle WR (1977) Legionnaires’ disease—isolation of a bacterium and demonstration of its role in other respiratory disease. New Engl J Med 297:1197–1203
Moritz MM, Flemming H-C, Wingender J (2010) Integration of Pseudomonas aeruginosa and Legionella pneumophila in drinking water biofilms grown on domestic plumbing materials. Int J Hyg Environ Health 213:190–197
Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM (2001) Role of biofilms in the survival of Legionella pneumophila in a model potable water system. Microbiol 147:3121–3126
Nieto JJ, Fernández-Castillo R, Márquez MC, Ventosa A, Quesada E, Ruiz-Berraquero F (1989) Survey of metal tolerance in moderately halophilic eubacteria. Appl Environ Microbiol 55:2385–2390
Noyce JO, Michels H, Keevil CW (2006) Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl Environ Microbiol 72:4239–4244
Pasculle W (2000) Update on Legionella. Clin Microbiol Newsl 22:97–101
Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW (1994a) Influence of plumbing materials on biofilm formation and growth of Legionella pneumophila in potable water systems. Appl Environ Microbiol 60:1842–1851
Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW (1994b) Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl Environ Microbiol 60:1585–1592
Salgado CDMD, Sepkowitz KAMD, John JFMD et al (2013) Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol 34:479–486
Schmidt M, Attaway H, Terzieva S et al (2012a) Characterization and control of the microbial community affiliated with copper or aluminum heat exchangers of HVAC systems. Curr Microbiol 65:141–149
Schmidt MG, Attaway HH, Sharpe PA et al (2012b) Sustained reduction of microbial burden on common hospital surfaces through introduction of copper. J Clin Microbiol 50:2217–2223
Surman SB, Morton LHG, Keevil CW (1994) The dependence of Legionella pneumophila on other aquatic bacteria for survival on R2A medium. Int Biodeterior Biodegrad 13:223–236
Surman S, Morton G, Keevil B, Fitzgeorge R (2002) Legionella pneumophila proliferation is not dependent on intracellular replication. In: Marre R et al (eds) Legionella. ASM Press, Washingtom DC, pp 86–89
Türetgen I, Cotuk A (2007) Monitoring of biofilm-associated Legionella pneumophila on different substrata in model cooling tower system. Environ Monit Assess 125:271–279
van der Kooij D, Veenendaal HR, Scheffer WJH (2005) Biofilm formation and multiplication of Legionella in a model warm water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res 39:2789–2798
Wadowsky RM, Yee RB (1983) Satellite growth of Legionella pneumophila with an environmental isolate of Flavobacterium breve. Appl Environ Microbiol 46:1447–1449
Walker JT, Sonesson A, Keevil CW, White DC (1993) Detection of Legionella pneumophila in biofilms containing a complex microbial consortium by gas chromatography-mass spectrometry analysis of genus-specific hydroxy fatty acids. FEMS Microbiol Lett 113:139–144
Warnes SL, Keevil CW (2013) Inactivation of norovirus on dry copper alloy surfaces. PLoS One 8:e75017. doi:10.1371/journal.pone.0075017
Warnes SL, Caves V, Keevil CW (2012) Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for gram-positive bacteria. Environ Microbiol 14:1730–1743
Warnes SL, Summersgill EN, Keevil CW (2014) Inactivation of murine norovirus on a range of copper alloy surfaces is accompanied by loss of capsid integrity. Appl Environ Microbiol 81(3):1085–1091
Wilks SA, Keevil CW (2006) Targeting species-specific low-affinity 16S rRNA binding sites by using peptide nucleic acids for detection of legionellae in biofilms. Appl Environ Microbiol 72:5453–5462
Acknowledgments
This research was supported by the Copper Development Association, New York, NY, and the International Copper Association, New York, NY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gião, M.S., Wilks, S.A. & Keevil, C.W. Influence of copper surfaces on biofilm formation by Legionella pneumophila in potable water. Biometals 28, 329–339 (2015). https://doi.org/10.1007/s10534-015-9835-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9835-y