Abstract
Zn is an essential element for plants yet some soils are Zn-deficient and/or have low Zn-bioavailability. This paper addresses the feasibility of using ZnO nanoparticles (NPs) as soil amendments to improve Zn levels in the plant. The effects of soil properties on phytotoxicity and Zn bioavailability from the NPs were studied by using an acidic and a calcareous alkaline soil. In the acid soil, the ZnO NPs caused dose-dependent phytotoxicity, observed as inhibition of elongation of roots of wheat, Triticum aestivum. Phytotoxicity was mitigated in the calcareous alkaline soil although uptake of Zn from the ZnO NPs occurred doubling the Zn level compared to control plants. This increase occurred with a low level of Zn in the soil solution as expected from the interactions of Zn with the soil components at the alkaline pH. Soluble Zn in the acid soil was 200-fold higher and shoot levels were tenfold higher than from the alkaline soil correlating with phytotoxicity. Mitigation of toxicity was not observed in plants grown in sand amended with a commercial preparation of humic acid: growth, shoot uptake and solubility of Zn from the NPs was not altered by the humic acid. Thus, variation in humic acid between soils may not be a major factor influencing plant responses to the NPs. These findings illustrate that formulations of ZnO NPs to be used as a soil amendment would need to be tuned to soil properties to avoid phytotoxicity yet provide increased Zn accumulations in the plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is an essential element for plant growth and human health. The ultimate source of Zn for most plants, and in turn animals, is soil. Crops-especially cereals-grown in soils with low concentrations of total or bioavailable Zn, contain correspondingly low Zn concentrations (Li et al. 2007; Alloway 2009). Consumption of crops deficient in Zn has a negative impact on human growth and health and affects one-third of the human population (Roy et al. 2006; Alloway 2009; Borill et al. 2014). A study highlighting a negative correlation between Zn concentrations in crops and atmospheric CO2 concentrations suggests that the problem of crops low in dietary Zn will be exacerbated if atmospheric levels of CO2 continue to rise (Myers et al. 2014). In other soils Zn occurs at high concentrations either naturally, where there are deposits of Zn-rich minerals (Martínez et al. 2007), or due to contamination from mine spoils, biosolids, or smelting. Phytotoxicity is observed when crops are grown in such elevated Zn- soils (Martínez et al. 2007; Beyer et al. 2013; Boussen et al. 2013).
Our work focuses on how plants grown in soil respond to Zn when applied as ZnO in nanoparticle (NP) form. ZnO is one of the forms of Zn used in commercial fertilizers (McBeath and McLaughlin 2014; Alloway 2009; Cakmak 2008). The use of ZnO may be advantageous in acid soils where leaching of Zn out of the rooting zone readily occurs (McBride 1994). To date most studies have focused on the correlation of ZnO NPs with phytotoxicity as observed in germinating seeds (López-Moreno et al. 2010), as well as in seedlings growing in sand (Dimkpa et al. 2012) and hydroponically (Ma et al. 2010). However, the role of soil properties in affecting Zn bioavailability from ZnO NPs is little addressed. Phytotoxicity was not observed in soybean grown in an organic farm soil where the plants accumulated Zn from ZnO NPs (Priester et al. 2012).
NPs are particles that have one dimension less than 100 nm; they have a greater surface area compared to bulk products. ZnO NPs are already documented to be present in sewage treatment plant effluents and in sludge-treated soils (Ma et al. 2013). Thus, formulations containing ZnO NPs could be applied to agricultural as contaminants, such as sludge-bearing NPs (Ma et al. 2013). However the NPs could also be applied purposely in formulations as a fertilizer. The greater surface area of the NPs and their smaller size could promote Zn bioavailability over that of the bulk product with less chemical application (Milani et al. 2010). This aspect is important because nontarget effects from the large scale applications of fertilizer/pesticides occur in agriculture (Li and Schuster 2014). Another anticipated formulation of ZnO NPs for agricultural applications could be as a pesticide because of their antimicrobial properties (e.g. He et al. 2011; Kairyte et al. 2013; Dimkpa et al. 2013b).
In this paper we compared responses to ZnO NPs of hard red wheat, Triticum aestivum, seedlings raised in two agricultural native field soils differing in pH. Previously using sand as a growth matrix, to limit the extent of chelation, complexation, and sorption of metal ions released from the NPs, we found phytotoxicity manifested as inhibition of root elongation (Dimkpa et al. 2012, 2013a, b). Consequently in this paper we examined the dose–response effects on growth and morphology of the seedlings when raised in the soils with and without challenge from ZnO NPs. Plants growing in the presence of ZnO NPs would be responding to both the NPs and Zn ions released from the NPs. There are debates on the relative importance of the nano-sized particles versus the solubilized ions in eliciting cellular responses (Ma et al. 2013; Fernandez et al. 2013). Previous studies with wheat (Dimkpa et al. 2013a) find that most of the Zn accumulating in the shoots is present as Zn phosphate suggesting that ion release from NPs supplied is important. Indeed Lin and Xing (2008) working with ryegrass demonstrate that ZnO NPs had little upward mobility from the apoplastic spaces of the root endodermis and steele. Thus, factors that affect the solubility of Zn from the NPs could be important in the plant responses. Consequently we compared the levels of Zn accumulation in wheat shoots and the concentrations of soluble Zn in the aqueous fractions prepared from soils after plant harvest.
We also studied the effects of adding a commercial humic acid material (to the sand to determine the role of this type of organic material on the plant responses to ZnO NPs. Humic acid (HA)—like materials are used as commercial soil amendments because they improve plant growth and quality, as reported for wheat (Malik and Azam 1985). Reduced phytotoxicity of heavy metals has been attributed to HAs (Stevenson 1994). In part this is due to changes in cation availability because of interactions with functional groups such as the phenolic and carboxylic acids of HAs (Adani et al. 1998; Kopek 2000; Karakurt et al. 2009; Li et al. 2011; Bian et al. 2011). Studies with NPs show adsorption of HA to ZnO NPs (Yang et al. 2009) and effects on Zn solubility from ZnO NPs at alkaline but not acidic pH (Bian et al. 2011). Work by Omar et al. (2013) reveals that HA form coronas around ZnO NPs modifying their stability and degree of aggregation. Consequently we also investigated the effects of the HAs on seedling growth in the presence of NPs and any impacts on Zn accumulations in shoot tissue and Zn solubility. The modification of the ZnO NPs by the HAs was studied by imaging with atomic force microscopy and the size of particles determined by dynamic light scattering.
Materials and methods
Sources of materials and characterization
The ZnO NPs were obtained from Sigma-Aldrich Chemical Company, and were described by the manufacturer as having an average diameter of less than 100 nm. Physical and chemical properties of these particles are published in Dimkpa et al. (2011, 2012, 2013a) and Fang et al. (2013). These studies demonstrate the purity of the product by metal analysis using acid digests processed by inductively coupled plasma spectrometry (ICP-MS), chemical and physical attributes by XRD and SEM with EDAX analysis; aggregation and surface charge of particles in water and medium as well as release of soluble Zn under different conditions.
The calcareous agricultural soil was obtained from the top 10 cm of a field in northern Utah at 41°47′6.10″ N 111°49′2.94″ W. The soil had been used to raise potatoes. The acidic soil was from the Okefenokee swamp flat woods area of SE Georgia at 30°59′22.4″N 82°37′45.4″W from fields under commercial blueberry production.
Soils were air dried, sieved to <2-mm and stored in closed containers at 4 °C until used. Soil properties were determined by the Utah State University Analytical Laboratories using standard soil procedures (Gavlack et al. 2003). Soil pH was determined in saturation paste extracts, and bioavailable trace elements were assayed from extractions using ICP emission spectroscopy. Bioavailable K and P were extracted with sodium bicarbonate with analysis by atomic absorption spectroscopy for K and colorimetrically for P. Nitrate-nitrogen was determined after a KCl extraction with colorimetric analysis. Organic carbon was assessed by the Walkley–Black procedure. Particle size distribution was by the pipette method.
When used as a control solid growth matrix, white silica sand was washed extensively with distilled water and dried in an oven at 120 °C before use. The elemental composition of the sand described in Dimkpa et al. (2012) showed Zn was present at less than 0.01 mg/kg.
Plant growth
Triticum aestivum seeds (Handy Pantry Organic Sprouting Seed Hard Red Wheat, Utah) were surface sterilized in 10 % hydrogen peroxide for 10 min with shaking. The seeds were washed with sterile double-distilled (dd) water five times before planting into the native agricultural soil in 20 cm-high Magenta boxes (Sigma Aldrich) or into sterilized sand. The microcosms created in each box used 200 g of growth matrix with 35 mL sterile distilled water for the soils and 50 mL sterile distilled water when sand was used. These levels were below water saturation. Water was present to similar extents (30 ml/200 g of matrix) at the end of the 7 days growth period.
Three seeds were placed at equidistant points in each Magenta box at depths of approximately 0.5 cm. Boxes were incubated at 26 °C under fluorescent lamps with daily rotations in a random manner to minimize light-gradient effects. Each treatment was run with at least three replicates and each study was repeated at least three times. After 7 days, wheat seedlings were harvested by gentle removal from the growth mix and the roots rinsed by immersion into sterile water. Microscopic analysis of the roots revealed little breakage during their removal and examination of the growth matrices found no broken root segments. The seedlings were washed gently in sterile water and measured for growth characteristics: shoot height and root length.
Soil or sand was amended with ZnO NPs at 125, 250 and 500 mg Zn/kg; previous studies had shown that 500 mg/kg dose was phytotoxic in sand, with reduced root elongation being a visible symptom (Dimkpa et al. 2012). The ZnO NPs were dispersed in dry sand or soil by thorough shaking before adding sterile water at volumes noted above. The similarity of the values of soluble Zn between samples taken from one box was evidence of homogeneity of loading the NPs in the growth matrix.
The soluble aromatic material in the alkaline soil (selected because the organic content was higher than the acidic soil) was estimated by determining the absorbance at 280 nm characteristic of phenolics of water extracts of a soil–water saturation paste. Sterile water was added 1 mL at a time to 200 g of the agricultural soil to reach complete saturation but with no ponded water: 35 mL were added and 8.5 mL was recovered as filtrate passing through a GFA filter by application of a vacuum. Aliquots (1 mL) of filtrate were centrifuged at 8,000 g for 15 min and absorbance (280 nm) of the dilutions measured. A standard curve of absorbance at 280 nm for the commercial HA (0.1 mg/mL) was generated to deduce the concentration that equaled that of the soluble soil fraction. Consequently sand in the microcosms was amended with an aqueous solution of HA (1, 4 and 16 mg/box) to represent this concentration (4 mg/box) as well as higher (16 mg/box) and a lower (similar to the acid soil) levels.
Determination of soluble metal levels
Levels of soluble zinc in the experimental microcosms were determined after plant harvest by removing three 20 g samples of sand or soil from each box. Each sample was suspended in 50 mL sterile distilled water with shaking at 120 rpm for 30 min. The suspensions were allowed to settle for 30 min and approximately 30 mL the solution was centrifuged at 10,000×g for 20 min to generate a supernatant. After two centrifugations of the supernatants at 15,000×g to pellet NPs, soluble metals were determined using ICP-MS with an Agilent 7500C.
Determination of shoot accumulated Zn
Shoots from the harvested plants were excised by cutting at the root-shoot interface. The residual wheat kernels were removed but coleoptiles were retained. The tissues were air dried, cut into small pieces, and weighed before digestion in nitric acid according to Jones and Case (1990). The samples were diluted as needed with dd water and analyzed for metals using ICP-MS.
Effects of HA on particle size of ZnO NPs
HA (4 mg/L) was incubated without shaking at 26 °C under room lighting for 7 days with or without 500 mg/L ZnO NPs. These conditions were to model potential interactions between HA and the ZnO NPs that could occur in the aqueous phase of the microcosms. Samples were examined for particle size by atomic force microscopy (AFM) and by dynamic light scattering (DLS) using a DynaPro NanoStar (Wyatt Technology Corporation, Santa Barbara, CA) as described previously (Dimkpa et al. 2012). Data shown are from two replicates with four samples taken from each replicate.
Results
Characterization of the field soils
The calcareous alkaline field soil, a Millville series soil, was a loam with particle size distribution of 13 % sand, 59 % silt and 28 % clay. The acid soil, a Mascotte series soil, was a loamy sand (85 % sand 7 % silt and 8 % clay). Carbonates were present in the Millville soil (16 % calcium carbonate equivalent) but none were detected in the acid Mascotte soil. Compositions of the soils with respect to plant nutrition are provided in Table 1. Both soils had an array of culturable microbes at planting. Culturable microbes also were obtained from wheat seedling root surfaces at similar numbers (6 ± 1 × 108/g root) after harvest with and without amendments with ZnO NPs (data not shown).
Effect of NPs on wheat growth in the acidic and alkaline soils
Wheat seedlings grew in both field soils; shoots were not chlorotic (Fig. 1). The addition of ZnO NPs over the dose range of 125–500 mg/kg had no effect on shoot or root length in the alkaline soil (Fig. 1a). Although the number of roots did not change and varied between four and five, the addition of ZnO NPs in the alkaline soil increased lateral root production (e.g. control soil (11 ± 2) alkaline soil with 500 mg/L ZnO NPs 18 ± 5). However, addition of the ZnO NPs to the acid soil resulted in dramatic decreases in root growth (Fig. 1b, c), although shoot growth was little affected (Fig. 1b). Weights were not recorded because we currently are unable to reproducibly strip the root surfaces of associated NPs and soil particles without using chemicals that cause plant cell damage (data not shown). The extent of surface particle contamination varied between roots and the particles would influence weight.
Effects of ZnO NPs (500 mg Zn/kg soil) on wheat growth in acid (a) and alkaline (b) soils. Plants were grown in soil for 7 days prior to harvest and measurement of shoot height, root length, and numbers of lateral roots developing from those roots. Images of roots typical of those grown in the acid soil at the different ZnO NPs doses are shown. Different letters for the bars indicate statistically significant results based on 2-way ANOVA with P = 0.05. Each treatment involved at least three growth boxes each with three plants. Data are typical of two separate studies
Effects of soluble metals in aqueous fractions from the growth matrices
Soluble Zn in the aqueous fraction extracted at the time of plant harvest increased when the NPs were present in soils, confirming findings in sand (Table 2). There was a dose dependent increase in the levels of soluble Zn. However the extent of release varied with soil type; release in the acid soil was at a level similar to that in sand (about 3 mg/L extracting solution from the 500 mg/L dose) compared with the 100-fold lower solubility (less than 0.03 mg/L with the 500 mg/kg dose) observed in the alkaline soil (Table 2). The pH of the extracts (Table 2) from the acid soil remained acidic but those of the alkaline soil were buffered near pH 8 by the high carbonate content (Table 1) of the soil. Analysis of the soil extracts also showed, as anticipated (Alloway 2009), that the levels of other soluble metals (Ca, Mg and K) were higher in the alkaline soil than for the preparations from the acid soil and sand (Table 2).
Uptake of Zn into shoot material
Only shoot material was assayed for metal uptake because of the visible presence of clumps of ZnO NPs/soil on the root surface when examined microscopically; these attached materials were not removed by washing in water. The shoot tissue included the coleoptile sheath from which the true leaves erupt as the seedlings were grown for 7 days. The data summarized in Fig. 2a) show that shoots had increased Zn when grown with the ZnO NPs even in the alkaline soils, although there was little effect of dose. Tissues showed about a twofold increase in Zn loading from growth with the NPs over the levels in the control plant to achieve levels about 100 mg Zn/kg.
Accumulations of Zn in shoot tissues after growth in the acid (a) or alkaline soils (b) Data are means with the standard errors of three replicates each with nine plants from one of two studies with similar results. The different letters indicate significant differences in load based on ANOVA at P = 0.05
In the acid soils (Fig. 2b), Zn loading into the shoot tissue was about tenfold higher than with the alkaline soils and there was no notable effect of the challenge dose of NPs. Levels of about 1,000 mg/kg were recorded in the total shoot tissue.
Interactions between HA and ZnO NPs
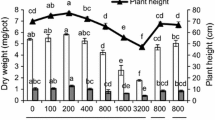
The acid and the alkaline soils differed in organic content and to explore the role of humic like materials sand was amended with a commercial source of HA to determine whether phytotoxicity of the NPs would be altered. Soluble aromatic compounds were present in the filtrate of the alkaline soil saturation paste at an equivalent of 4 mg commercial HA/box. The HA solution at the concentration of 4 mg/50 mL used in the microcosm had a pH 8.04 and thus was similar to the pH of the pore water from the alkaline soil, pH 8.00. Plants grown in sand amended with HA at this level, as well as above (16 mg/box) and below this level (1 mg/box) showed no consistent differences on shoot height. Root length was little altered by the amendment of sand with HA (Fig. 3a). However, seedling roots did not elongate to the lengths observed in the alkaline soil-grown plants (Fig. 1a). Although there was no change in the number of primary roots in each of three studies, there was increased formation of lateral roots (P = 0.10) compared with control plants when HA alone was present.
a Effects of HA addition to sand, with and without ZnO NPs (500 mg Zn/kg sand), on wheat growth (shoot height, root length and lateral branching). The HA was added to the sand at 1, 4 and 16 mg/box. The letters indicate statistical differences based on 2-way ANOVA at P = 0.05. Data are from one of two studies with similar results. Light bars are growth in sand and with HAs at 0, 1, 4 and 10 mg/box from left to right, and darker bars are sand with HAs and with ZnO NPs at 0, 1, 4 and 10 mg/box from left to right. b Effect of HA on solubility of Zn from ZnO NPs after growth of wheat seedlings in sand. The HA was present at concentrations of 0, 1,4 and 16 mg/box. ZnO NPs were added at 500 mg Zn/kg. Data are means from five replicates of each treatment each with three plants from one of two studies with similar results. Letters show statistical differences based on 2-way ANOVA with post hoc testing using Tukey’s test. c Effect of HA on accumulation of Zn in shoots of wheat seedlings grown in sand with and without amendments of ZnO NPs. The HA was present at concentrations of 0, 1, 4 and 16 mg/box. ZnO NPs were added at 500 mg Zn/kg. Data are means and standard deviations from 25 plants for each treatment
The presence of HA with NPs in the sand did not change the soluble Zn (Fig. 3b) from levels measured in samples of sand with NPs after plant harvest. Neither was there a statistical change caused by HA in the accumulation of zinc into the shoots of the 7 day-old seedlings (Fig. 3c). Lateral root numbers were increased in the treatments of HA with ZnO NPs (Fig. 3a).
Physical interactions between HA and ZnO NPs
DLS and AFM analyses of the HA solution incubated for 7 days at 26 °C revealed discrete particles as illustrated in Fig. 4. Particles with two size distributions were observed for the HA preparation: the smaller particles in the four replicates showed were below 100 nm and the larger particles over 300 nm (Fig. 4a). For ZnO NPs suspended in water, single peaks showing aggregation were found, with diameter about 400 nm (Fig. 4a). In contrast the mix of HA with 500 mg/L ZnO NPs again showed particles in two size ranges with means of about 400 nm and much larger aggregates of greater than 2,500 nm (Fig. 4a). AFM analysis (Fig. 4b) showed particles with nano-scale height for HA, and with greater height for the ZnO NPs. Very large aggregates were imaged for the suspension of HA and ZnO NPs.
Effect of HA on particle size of ZnO NPs. The ZnO NPs were suspended in water or HA (4 mg/L) for 7 days prior to analysis by DLS (a) or AFM imaging (b) The data shown are of one representative image of four replicates prepared as discussed in “Materials and methods” section
Discussion
Phytotoxicity of ZnO NPs, exhibited as the inhibition of root elongation of wheat, occurred when seedlings were raised in a native, acid soil. Inhibition of root elongation also occurred in sand (Dimkpa et al. 2013a). In contrast in an alkaline calcareous loam phytotoxicity was mitigated. These plants grew as well with NPs in the alkaline soil as without. However shoot accumulations of Zn increased about twofold, to about 100 mg/kg dry tissue, from growth with the NPs. Welch (1995) and Welch and Graham (2002) indicated Zn levels between 20 and 50 mg/kg dry tissue were typical for normal growth, with toxicity to wheat growth arising with Zn levels above 500 mg/kg. Leaf toxicity, visible as chlorosis, was apparent in seedlings grown for 14 days in sand with 500 mg Zn/kg from ZnO NPs, when levels of Zn at 2,076 ± 670 mg/kg dry shoot tissue were recorded (Dimkpa et al. 2012). These symptoms were typical of the phytoxic symptoms observed after high Zn ion treatments (Potters et al. 2007).
This increased level of Zn in the shoots of plants grown with ZnO NPs in alkaline soil occurred even although soluble Zn in the aqueous extracts from the growth matrix after plant growth was very low. Low Zn solubility was anticipated because of the alkaline pH and the sorption of Zn onto soil components including clays, ferric oxides and carbonates (McBride 1994). For example Spark et al. (1995) found that Zn was adsorbed by kaolinite. We speculate that the increased shoot accumulation was the result of superior delivery of ions released from the NPs at the root surface and/or from NPs gaining entry into the root cells. The speciation of Zn primarily as Zn phosphate within sand-grown wheat shoots (Dimkpa et al. 2012) agreed with dissolution of ZnO NPs within the plant or growth matrix.
Alloway (2009) included ion concentration and organic matter in addition to pH as factors affecting metal bioavailability in soils. For example, Zn ion rhizotoxicity in hydroponically-grown wheat was reduced by micromolar concentrations of Mg and K ions (Pedler et al. 2004), and Zn uptake was reduced by the presence of Ca, starting at 0.2 mM (8 mg Ca/L) (Hart et al. 1998). The ICP-MS analysis of the aqueous extract from the alkaline soil in the growth box at the end of the growth period showed Ca to be present well above the 8 mg/L level in the growth matrix after factoring the dilution of the soil solution during preparations of extracts. Consequently, although Ca, K, and Mg were present in this soil at levels that could be restricting Zn uptake as a free ion, Zn remained bioavailable from the ZnO NPs.
Our studies did not support mitigation of toxicity of ZnO NPs by the commercial HA product we used as an organic matter amendment to sand. Although DLS and AFM analyses demonstrated the HA to promote aggregation of particles when mixed with ZnO NPs, supporting observations with HA by Omar et al. (2013). Surprisingly these changes had no effect on bioavailability under the test conditions where soluble Zn levels in the growth matrix and shoot accumulations were not altered from controls where plants were raised with ZnO NPs alone. The effect of Zn may be related to the observation in Arabidopsis that lateral root formation has been observed to require Zn (Richard et al. 2011). The aggregation of ZnO NPs with the HAs could be advantageous in retention of bioavailable Zn due to reduced transport of the ZnO aggregates through soil pores (Uyusur et al. 2010). Also we did observe stimulation of lateral root formation with the HA amendments as well as with exposure to ZnO NPs. Thus, these amendments altered root morphology to increase root density through lateral root formation, a factor that could increase nutrient uptake for the plants (Russel and Sanderson 1967; Drew and Saker 1978; Ma et al. 2001). Increases in lateral root formation with HA in maize was correlated with activation of proton pumps in the plasmalemma (Canellas et al. 2002) and tonoplast (Zandonadi et al. 2007).
The phytotoxicity observed as severely stunted plant roots in the acid soil was dependent on dose which correlated with at least 100-fold higher soluble Zn in the growth matrix than for the alkaline soil. The levels of shoot accumulation in the seedlings grown in acid soil also were about tenfold higher than in the alkaline soil, and being above 500 mg/L (Welch 1995 Welch and Graham 2002), were consistent with phytotoxicity. The lower concentrations of soluble Ca, Mg and K present in the acid soil extracts than in alkaline soil solution would offer lesser competition for Zn uptake. However the amount of soluble zinc detected in the extracts was not at the anticipated level from complete solubility. In other studies we observed complete solubility of 100 mg Zn/L dose of ZnO NPs in 2 days when suspended at an initial pH of pH 5 with a chelator, 300 mg/L citrate (data not shown). Citrate and other organic acids, such as malate for wheat, are secreted from plant roots (Jones 1998) and increased quantities have been correlated with tolerance of the plant to a metal challenge, such as Al (Pellet et al. 1995). Also whereas solubility of Zn was dose-dependent, the uptake into shoot tissues was little affected by dose. These observations suggest that shoot uptake of Zn from the NPs was not totally driven by the soluble levels of Zn in the growth matrix.
In summary, the phytotoxicity of ZnO NPs to young wheat seedlings was dependent on the soil properties: phytotoxicity was observed in acid but not alkaline soils. However, although the extent of solubility of Zn from the NPs was a 100-fold less in the alkaline than the acid soil, an increased uptake of Zn into the shoots from the NPs occurred in the calcareous alkaline soil. These findings indicate that use of NPs such as ZnO NPs as a fertilizer or a pesticide would have to be tuned to the soil being treated to avoid phytotoxic effects yet retain beneficial Zn uptake.
References
Adani F, Genevi P, Zaccheo P, Zocchi G (1998) The effect of commercial humic acid on tomato plant growth and mineral nutrition. J Plant Nutr 21:561–575
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31:537–548
Beyer WN, Green CE, Beyer M, Chaney RL (2013) Phytotoxicity of zinc and manganese to seedlings grown in soil contaminated by zinc smelting. Environ Pollut 179:167–176
Bian SW, Mudunkowotuwa IA, Tupasjngjhe T, Grassian VH (2011) Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size and adsorption of humic acid. Langmuir 27:6059–6068
Borill P, Connorton JM, Balk J, Miller AJ, Sanders D, Uauy C (2014) Biofortification of wheat grain with iron and zinc: integrating novel genomic resources and knowledge from model crops. Front Plant Sci 5:53. doi: 10.3389/fpls.2014.00053, 21 Feb 2014
Boussen S, Soubrand M, Bril H, Ouerfelli K, Abdeljaouad S (2013) Transfer of lead, zinc and cadmium from mine tailings to wheat (Tricum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 192:227–236
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification. Plant Soil 302:1–17
Canellas LP, Olivares FL, Okorokova-Façanha AL, Façanha AR (2002) Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol 130(4):1951–1957
Dimkpa CO, Calder A, Britt DW, McLean JE, Anderson AJ (2011) Responses of a soil bacterium, Pseudomonas chlororaphis O6 to commercial metal oxide nanoparticles compared with responses to metal ions. Environ Pollut 159:1749–1756
Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, Boyanov MI, Anderson AJ (2012) CuO and ZnO nanoparticles: phytotoxicity, metal speciation and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14(9):1125–1129
Dimkpa CO, Latta DE, McLean JE, Britt DW, Boyanov MI, Anderson AJ (2013a) Fate of Cuo and ZnO nano- and microparticles in the plant environment. Environ Sci Technol 47:4734–4742
Dimkpa CO, McLean JE, Britt DW, Anderson AJ (2013b) Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals 26:913–924. doi:10.1007/s10534-013-9667-6
Drew MC, Saker LR (1978) Nutrient supply and the growth of the seminal root system in barley. J Exp Bot 29(2):435–451
Fang T, Watson JL, Goodman J, Dimkpa CO, Martineau N, Das S, McLean JE, Britt DW, Anderson AJ (2013) Does doping with aluminum alter the effects of ZnO nanoparticles on the metabolism of soil pseudomonads? Microbiol Res 168(2):91–98
Fernandez D, Garcia-Gomez C, Babin M (2013) In vitro evaluation of cellular responses induced by ZnO nanoparticles, zinc ions and bulk ZnO in fish cells. Sci Total Environ 452–453:262–274
Gavlack RG, Horneck DA, Miller RO, Kotuby-Amacher J (2003) Plant, soil, and water reference methods for the Western region. Western Regional Extension Publications 125, University of California, Davis
Hart JJ, Norvell WA, Welch RM, Sullivan LA, Kochian LV (1998) Characterization of zinc uptake, binding and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol 118:219–226
He L, Liu Y, Mustapha A, Lin M (2011) Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res 166(3):207–215
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Jones JB Jr, Case VW (1990) Sampling, handling, and analyzing plant tissue samples. In: Westerman RL (ed) Soil testing and plant analysis, 3rd edn. Soil Sci Soc Am., Inc, Madison
Karakurt Y, Unlu H, Unlu H, Padem H (2009) The influence of foliar and soil fertilization humic acid on yield and quality of pepper. Acta Agric Scand Sect B – Soil Plant Sci 59:233–237
Kairyte K, Kadys A, Luksiene Z (2013) Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J Photochem Photobiol B 128:78–84
Kopec DM. Humorous humics: soil amendments worth applying. Univ AZ (2000) Cooperative Extension Turf Tips October 7(10). http://turf.arizona.edu/tipsoct00.htm. Accessed Jan 24, 2014
Li L, Schuster M (2014) Influence of phosphate and solution pH on the mobility of ZnO nanoparticles in saturated sand. Sci Total Environ 472:971–978
Li BY, Zhou DM, Cang L, Zhang HL, Fan ZH, Qin SW (2007) Soil micronutrient availability to crops as affected by long-term inorganic and organic fertilizer applications. Soil Tillage Res 96:166–173
Li M, Pokhrel S, Jin X, Mädler L, Damoiseaux R, Hoek EMV (2011) Stability, bioavailability, and bacterial toxicity of ZnO and iron-doped ZnO nanoparticles in aquatic media. Environ Sci Technol 45(2):755–761
Lin DH, Xing BS (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585
López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2010) Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol 44:7315–7320
Ma JF, Goto S, Tamai K, Ichii M (2001) Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiol 127:1773–1778
Ma X, Gieser-Lee J, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408:3053–3061
Ma H, Williams PL, Diamon SA (2013) Ecotoxicity of manufactured ZnO nanoparticles: a review. Environ Pollut 172:76–85
Malik KA, Azam F (1985) Effect of humic acid on wheat (Triticum aestivum L.) seedling growth. Environ Exp Bot 25:245–252
Martínez C, Yáñez C, Yoon S, Bruns MA (2007) Biogeochemistry of metalliferous peats: sulfur speciation and depth distributions of dsrAB genes and Cd, Fe, Mn, S, and Zn in soil cores. Environ Sci Technol 41:5323–5329
McBeath TM, McLaughlin MJ (2014) Efficacy of zinc oxides as fertilisers. Plant Soil 374:843–855
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, New York, p 339
Milani N, McLaughlin MJ, Hettiaratchchi GM, Beak DG, Kirby JK, Stacey S (2010) Fate of nanoparticulate zinc oxide fertilisers in soil: diffusion and solid phase speciation. In: Soil solutions for a changing world: 19th world congress of soil science, Brisbane, QLD, Australia
Myers SS, Zanobetti A, Kloog I, Huybers P, Leakey AD, Bloom AJ, Carlisle E, Dietterich LH, Fitzgerald G, Hasegawa T, Holbrook NM, Nelson RL, Ottman MJ, Raboy V, Sakai H, Sartor KA, Schwartz J, Seneweera S, Tausz M, Usui Y (2014) Increasing CO2 threatens human nutrition. Nature 510:139–142. doi:10.1038/nature13179
Omar FM, Aziz HA, Stoll S (2013) Aggregation and disaggregation of ZnO nanoparticles: influence of pH and adsorption of Suwannee river humic acid. Sci Total Environ 468–469:195–201
Pedler JF, Kinraide TB, Parker DR (2004) Zinc rhizotoxicity in wheat and radish is alleviated by micromolar levels of magnesium and potassium in solution culture. Plant Soil 259:191–199
Pellet DM, Grunes DL, Kochian LV (1995) Organic acid exudation as an aluminium-tolerance mechanism in maize (Zea mayes L.). Planta 196:788–795
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105
Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Gelb J, Walker SL, Nisbet RM, An YJ, Schimel JP, Palmer RG, Hernandez-Viezcas JA, Zhao L, Gardea-Torresdey JL, Holden PA (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc Natl Acad Sci USA 109:E2451–E2456
Richard O, Pineau C, Loubet S, Chalies C, Vile D, Marques L, Berthomieu P (2011) Diversity analysis of the response to Zn within the Arabidopsis thaliana species revealed low contribution of Zn translocation to Zn tolerance and a new role for Zn in lateral root development. Plant Cell Environ 34:1065–1078
Roy RN, Finck A, Blair GJ, Tandon HLS (2006) Plant nutrition for food security: a guide for integrated nutrient management. FAO Fertil Plant Nutr Bull.ftp://ftp.fao.org/docrep/fao/009/a0443e/a0443e.pdf . Accessed 17 July 2013
Russel SR, Sanderson J (1967) Nutrient uptake by different parts of the intact roots of plants. J Exp Bot 18(3):491–508
Spark KM, Wells JD, Johnson BB (1995) Characterizing trace metal adsorption on kaolinite. Eur J Soil Sci 46:633–640
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. John Wiley & Sons, New York
Uyusur B, Darnault CJG, Snee PT, Kokën, Jacobson AR, Wells RR (2010) Coupled effects of solution chemistry and hydrodynamics on the mobility and transport of quantum dots nanomaterials in the vadose zone. J Contam Hydrol 118(3–4):184–198. doi:10.1016/j.jconhyd.2010.09.013
Welch RM (1995) Micronutrient nutrition of plants. Crit Rev Plant Sci 14:49–82
Welch RM, Graham RD (2002) Breeding crops for enhanced micronutrient content. Plant Soil 245:205–214
Yang K, Lin D, Xing B (2009) Interactions of humic acid with nanosized inorganic oxides. Langmuir 25:3571–3576
Zandonadi DB, Canellas LP, Façanha AR (2007) Indolacetic and humic acids induce lateral root development through concerted plasmalemma and tonoplast H+ pumps. Planta 225:1583–1595
Acknowledgments
This work was supported by a Grant from the USDA, (USDA-CSREES 2011-03581), the Utah Water Research Laboratory and the Utah Agricultural Experiment Station. Utah Agricultural Experiment Station paper number 8741. We thank undergraduates Melanie Wright, Kjersti Matherson, and Elliot Morrell for their help with the growth studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watson, JL., Fang, T., Dimkpa, C.O. et al. The phytotoxicity of ZnO nanoparticles on wheat varies with soil properties. Biometals 28, 101–112 (2015). https://doi.org/10.1007/s10534-014-9806-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-014-9806-8