Abstract
Conversion of landscapes to large-scale agriculture has substantially increased the loading of bioavailable nitrogen (N) to stream networks through extensive artificial drainage and fertilizer application. Floodplain restoration may enhance N cycling in agricultural stream systems by increasing residence time of floodwaters in contact with bioreactive surfaces that retain or remove excess N. Microbially-mediated denitrification potentially plays a significant role in constructed floodplains by permanently removing excess N through conversion of bioavailable nitrate (NO3-N) to dinitrogen gas, either as N2O or N2. Restoring channelized streams via the construction of inset floodplains can increase the total denitrification capacity of agricultural watersheds, but little is known about the abiotic factors that control the proportion of NO3-N that is converted to the potent greenhouse gas N2O versus N2 (i.e., N2O yield). We used an in-situ static core design to assess the importance of constructed floodplain age, inundation time, and carbon (C) availability on total denitrification rates and N2O yield. Novel use of membrane-inlet mass spectrometry (MIMS) allowed us to simultaneously measure N2O and N2 to capture total denitrification and the proportion of bioavailable N converted to each end-product. Floodplain age did not influence total denitrification rates, but rather denitrification increased with the duration of floodplain inundation until C limitation occurred at approximately 24 h. In addition, we found that N2O yields from floodplain soils were higher than those reported for other aquatic systems. Finally, while floodplain restoration in agricultural streams generally increases N retention at the watershed scale, regardless of the restoration age, the impact of added floodplains on N2O emissions should be considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the agricultural Midwestern US, extensive fertilizer application and artificial drainage have resulted in high nitrogen (N) loading rates to adjacent waterways (Vitousek et al. 1997), which can contribute to decreased water quality in downstream receiving waters (Carpenter et al. 1998). Stream channelization and subsurface tile drainage allow for the efficient removal of excess water from low-gradient agricultural lands (Landwehr and Rhoads 2003), but these practices reduce streamwater contact time with stream sediments and floodplain soils that may promote biological N removal (Randall et al. 1997; Bukavekas 2007). Moreover, in many agricultural streams, nitrate (NO3-N) uptake is typically saturated due to very high loading from the surrounding landscape (Bernot et al. 2006). Therefore, optimizing N removal is a priority for restoration strategies in agricultural streams (Groffman and Crawford 2003; Sheibley et al. 2006; Kaushal et al. 2008; Roley et al. 2012a, b). A shared goal among farmers and resource managers is to maintain agricultural productivity while minimizing negative environmental impacts. We investigate how one approach (i.e., floodplain restoration) contributes to that goal.
A common goal for agricultural conservation practices is to optimize biological N removal while still maintaining the drainage needs of the adjacent agricultural land (Roley et al. 2012a). While many streams in the agricultural Midwest have been channelized to improve drainage from the landscape, the resulting channel often suffers from bank slumping, undercutting and sediment deposition (Landwehr and Rhoads 2003). Therefore, to increase N retention and channel stability while accommodating the needs of surrounding farmland, land managers have implemented an alternative strategy whereby inset floodplains are constructed alongside the stream channel, called two-stage floodplains (Powell et al. 2007), which allow tile drain water to flow directly onto floodplains during baseflow conditions, and during storms, slows down floodwaters which inundate adjacent floodplains (Roley et al. 2012a). Previous studies have shown that the increased residence time on these constructed floodplains, whose design mimics that of natural floodplains, enhances denitrification (Roley et al. 2012a, b; Mahl et al. 2015; Hanrahan et al. 2018), which is a microbially-mediated process by which NO3-N is converted to dinitrogen gases under anoxic conditions and in the presence of organic carbon (C; Knowles 1982). While studies have compared denitrification in two-stage versus channelized ditches (Powell and Bouchard 2010; Roley et al. 2012a) as well as unmaintained, “naturalized” ditches (Hanrahan et al. 2018), few studies have compared denitrification in constructed floodplains of different ages.
Although denitrification results in a permanent removal of dissolved N from the system as the inert gaseous compound of N2 (Galloway et al. 2003), it can also produce N2O in its incomplete form. The production of N2O as an intermediate form occurs when nitrous oxide reductase activity (nos), the enzyme that reduces N2O to N2, is suppressed and denitrifying bacteria cannot complete the full reduction pathway (Firestone et al. 1979). The suppression of this enzyme has been found by previous studies to occur under high soil NO3-N concentrations (Jalota et al. 2018; Weier et al. 1991), among other abiotic factors including temperature, pH, and soil moisture (Smith et al. 2003). The fraction of denitrified N that escapes the system as N2O versus N2 (i.e., N2O yield) is an important metric, but our understanding of factors that control this ratio in stream floodplains is limited (Beaulieu et al. 2011).
Nitrous oxide is a potent greenhouse gas, with ~ 300 times the warming potential than that of carbon dioxide (IPCC 2007), and anthropogenic N loading to river networks may be an important source of N2O to the atmosphere (Beaulieu et al. 2011). Moreover, N2O yield is an important metric that has supported global regulatory efforts (Nevison 2001), and as such, previous research has sought to understand what drives the relative proportion of denitrification end-products (Firestone et al. 1979; Letey et al. 1980; Vinther 1984; Weier et al. 1993; Beaulieu et al. 2011; Phillips et al. 2015). For example, N2O yield (calculated as N2O/N2) decreased with incubation time and was lowest under conditions of high soil water content and C availability, while high NO3-N increased N2O yields (Weier et al. 1993). Constraining estimates of N2O yield for aquatic systems receiving excess N from agriculture (i.e., “indirect emissions”; Nevison 2001; Klein et al. 2006) motivated early studies (Ryden et al. 1979), but these estimates have proven to be variable and analytically challenging.
Field measurements of N2 and N2O produced in floodplain soils are challenging (Phillips et al. 2015), mostly because estimating N2 is complicated by high atmospheric concentrations of the gas. Most denitrification studies have been performed using techniques that block the reduction of N2O to N2, allowing for the accumulation of N2O, which is easily measured via gas chromatography. Others have quantified denitrification using flow-through cores that allow for the accumulation and analysis of N2 and N2O simultaneously (Burgin et al. 2010; Burgin and Groffman 2012). The development of membrane-inlet mass spectrometry (MIMS) has allowed for rapid, high-precision measurements of dissolved N2, O2, and Ar in water (Kana et al. 1994), and does not require headspace equilibrations (as for gas chromatography) or large sample volumes, which facilitates in-situ field experiments. In recent studies, standard MIMS methodologies have been modified to measure the presence of other dissolved gasses, including CO2 (Gueguen and Tortell 2008), methane (CH4; Schlüter and Gentz, 2008), and nitric oxide (NO; Conrath et al. 2004; Goodwin et al. 2018). However, we are aware of only a single study that has explored the capacity of MIMS to accurately measure N2O and N2 simultaneously (Genthner et al. 2013), who used an ultralow freezer as a cryotrap for the measurement of N2O.
In this study, we compared total denitrification rates (measuring both N2 + N2O accumulation) in constructed floodplain soils (via the two-stage ditch) constructed in 2007 and 2017, and examined the environmental factors that potentially control N2O yields in floodplain soils. Specifically, we tested the role of inundation and C availability using in situ cores containing overlying “floodwater” of ambient and elevated dissolved C concentrations. We hypothesized that denitrification would be higher on older floodplains, regardless of C amendment, because the combination of stream sediment deposition during flooding combined with the seasonal growth and decomposition cycles of floodplain grasses would promote C accrual. We also predicted that the end-product of denitrification would shift from N2O to N2 as a function of increased inundation time and C availability.
Methods
Study site
We conducted this study in the Shatto Ditch, a 9.4 km tributary of the Tippecanoe R. located in north-central Indiana, USA (Fig. 1a, c). The Shatto Ditch drains a watershed of primarily tile-drained, row crop agriculture in maize-soybean rotation, and is a system that has historically been maintained, through periodic dredging, as a drainage ditch, resulting in incised banks with steep slopes (Fig. 1c). As a result, the system has the flashy discharge regime typical of first-order Midwestern agricultural streams (Roley et al. 2012b, 2014); its baseflow discharge ranges from 12 to 174 L/s, but flows can be up to 1000 L/s during storms (Roley et al. 2012a). Large amounts of fine benthic organic matter (FBOM) and sand are transported during high-discharge events, as the streambed is composed mainly of a homogenous mix of sand, FBOM, and small gravel typical of the outwash plain in which Shatto Ditch is located. Given the adjacent agricultural land use, > 11 years of data collection shows elevated inorganic nutrient concentrations with NO3−-N generally > 5 mg L−1 on average (Griffiths et al. 2013; Roley et al. 2014; Hanrahan et al. 2018).
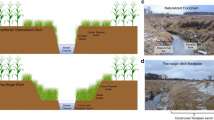
Diagrams of a established (EST) and b newly constructed (NEW) two-stage ditch within the c Shatto Ditch watershed. Each study site was equipped with a sampling station (d) containing a carboy for each amendment flowing passively into a contained area with the in-situ cores (e). Cores are installed at 5–10 cm depth (D) and remain in-situ for the duration of the experiment. f Cross-sectional diagram of in-situ experimental setup
In November 2007, lateral inset floodplains were constructed along a 600 m reach of Shatto Ditch via the construction of a two-stage ditch (Roley et al. 2012a, b, 2014; Fig. 1a). Channel slopes on each side of the stream were excavated to create ~ 3 m wide inset floodplains that were allowed to vegetate naturally (Reed canary grass; Phalaris arundinacea), and floodplains inundate regularly (~ 12 times per year) during high flow events. In August 2017, an additional 3700 m of two-stage ditch was constructed directly upstream of the first two-stage segment (Fig. 1b) to make a total of 4.3 km of continuous two-stage ditch, the longest such construction in the world. We utilized the two different constructed floodplain reaches to compare the influence of inundation time and C amendment on denitrification rates between constructed floodplains of different ages.
Denitrification incubations using in situ, intact cores: experimental field-based design
In June 2018, we conducted in situ denitrification incubations during baseflow conditions when floodplain soils were recently wetted during a storm, but not saturated or flooded. We randomly selected a plot approximately 1 m away from the stream, on the floodplain bench, within each of the two study reaches (EST, NEW) to perform the chamber incubations. After manually clearing away the overlying vegetation to expose the floodplain soils, we deployed 0.25 m long clear acrylic cores at 5–10 cm depth to capture the zone shown previously to have the highest denitrification activity (Roley et al. 2012a; Fig. 1e).
To inundate the chambers, we carefully collected 10L of site water from the center of the stream, with minimal artificial aeration, in two identical carboys (Fig. 1d). The first carboy provided unamended, ambient (AMB) site water and the second carboy was amended with dextrose to elevate carbon levels (+ C) in site water by 10–15 mg L−1 above ambient conditions. Tables provided an elevated surface to fill cores from carboys passively using a network of air-tight tubing fitted with stopcocks to regulate flow (Fig. 1d). We deployed 3 soil cores per treatment (AMB, + C) protected from the elements by a bottomless 5-gallon bucket at each site (EST, NEW), and included a single control core filled with treatment water only in each bucket (n = 4; Fig. 1e). We sealed the cores with rubber stoppers (size 13) fitted with an inlet tube regulated with a stopcock and an outlet tube fitted with a stopcock used for sampling (Fig. 1f). The inlet tubes for each core within each treatment (n = 4) were directly connected to a T-junction at the spigot of the carboy and therefore allowed for direct, gravity-fed regulation of water flow to the cores at the time of sampling. These deployed cores remained intact and stationary for the entire duration of the experiment, approximately 56 h.
To characterize stream conditions at the time of sampling, we collected and filtered three replicate water samples (60 mL) adjacent to each core deployment site and kept them frozen until analysis for NO3−-N using the cadmium reduction method (APHA 2012). We took 3 replicate floodplain soil cores with a push corer (5-cm depth X 2.2. cm diameter; Model HA, Oakfield Apparatus, Inc., WI, USA) to characterize soil extractable nutrients. We stored samples in a sealed container at 4C, until extraction (within 3 days of collection) by adding 40 mL of 2 mol L−1 potassium chloride (KCl) to 4 g of soil at field moisture. We agitated samples at 100 rpm for 1 h, filtered, and the supernatant was frozen until later analysis. Extractable nutrients were expressed per unit dry mass. We analyzed surface water and soil extractions on a Lachat QC8500 Flow Injection Autoanalyzer (Lachat Instruments, Loveland, Colorado, USA).
In-situ sampling over incubation time
After we deployed the floodplain cores, and set up the gravity-feed system, we filled all cores simultaneously with water from their respective treatment carboy, and sealed them by pressing the rubber stopper securely into the top of the acrylic core, pushing any excess water and air through the outlet tube until no bubbles present within the core. Once both the inlet and outlet tubes were closed, we began the incubation period. We allowed the cores to incubate for 1 h before taking the first dissolved gas sample to allow the system to come to equilibrium (Groffman et al. 1999). At 1 h post-sealing, we opened the spigot to the carboy and opened the stopcock to the respective core, to allow water from the carboy to passively be delivered at the water–sediment surface, displacing water at the top of the core, pushing it through the outlet tube, and filling three replicate 12 mL Exetainer vials (Labco Ltd. Lampeter, UK). We overfilled each vial three times from the bottom to minimize the sample interaction with the atmosphere and collected the overflow volume in a container to later account for dilution within the core. We added 0.2 mL of 50% w/v ZnCl2 solution to each vial for preservation, and 0.2 mL of saturated sodium hydroxide (NaOH) solution to convert all CO2 within the sample to bicarbonate, as the presence of CO2 within the sample interferes with the quantification of N2O by MIMS (Genthner et al. 2013). We sealed these vials with double-wadded Teflon Exetainer caps and stored submerged upside-down until analysis for dissolved gas concentration. We recognize the potential of disproportionate accumulation of gases in the water column above the sediments, which previous studies have controlled for by actively mixing the water column within the cores (Gihring et al. 2010) or designing a fully flow-through system (Kana et al. 1998). However, given the constraints of a fully in situ field design with high replication, we chose to proceed without physical mixing. We believe that by filling cores at each time period from the sediment–water interface allowed for periodic mixing of the water column.
We repeated this process for each treatment (AMB, + C) at each site (EST, NEW) at four sampling times, approximately 2 h apart, to total ~ 8 h incubation period (+ 1 h equilibration) for each inundation time. In addition to samples taken from the cores, we took replicate dissolved gas samples via siphon from the treatment carboys to account for dilution within the cores at each time point. Prior to leaving the site at the end of the incubation, we flushed each core with fresh water from the treatment carboys and loosely covered them with their respective stoppers. We repeated this process at 24 h post-deployment, and again at 48 h without disturbing the cores to examine how variation in the duration of floodplain inundation influences denitrification (3 inundation times: 0HR, 24HR, 48HR). After the incubation was complete for the 48HR inundation time, we removed the intact cores, transported them back to the lab, and stored them at 4C until further soil analysis.
Dissolved gas analysis by MIMS
We simultaneously analyzed dissolved di-nitrogen gas (N2) and nitrous oxide (N2O) using membrane inlet mass spectrometry (MIMS; Bay Instruments, Easton, MD, USA) (Kana et al. 1994, 1998). Briefly, samples are drawn from the Exetainer vial by peristaltic pump and dissolved gases are diffused over a membrane under a vacuum before being analyzed by mass spectrometry, which directly quantifies the concentration of 28N2, 32O2 and 28N2:Ar, 32O2:Ar in the water sample. Similar to Genthner et al. (2013), we modified a typical MIMS protocol to simultaneously measure the concentration of N2 and N2O in our samples by utilizing a cryotrap (typically used to extract water vapor and other compounds prior to dissolved gases reaching the mass spec) held between − 85 and − 90 °C (Genthner et al. 2013). This range is below the freezing point of CO2 (− 78 °C), but above the freezing point for N2O (− 91 °C), which allowed the mass spec to interpret any gas with the molecular mass of 44 as N2O instead of the equivalent CO2, which gave us the ability to simultaneously measure the concentration of 44N2O and 28N2 in each sample. We created this cryotrap using a cost-effective mixture of dry ice and isopropanol in the absence of a portable ultralow freezer. This cryotrap contained approximately a 50:50 mixture of dry ice and isopropanol by volume in a cryogenic storage dewar, monitored constantly by an ultralow thermometer probe (VWR International, Radnor, PA, USA) inserted into the mixture. Every five samples, we ran an air-equilibrated standard consisting of a dilute NaOH solution (6.25 mL saturated NaOH solution in 1L of nanopure water at 18 M Ω resistance) held at the temperature of sample collection (19 − 23°C) using a water bath (VWR International, Radnor, PA, USA) and stirring at constant speed (Lab Egg RW11 Basic, IKA Works Inc., Wilmington, NC, USA). These standards were used to correct for instrument drift throughout the course of a daily run. If core temperature differed significantly (> 2 °C) over the course of an incubation, we ran samples in separate batches with air-equilibrated standards reflecting the given temperature.
We calculated the concentration of dissolved N2 and N2O in each sample by multiplying the N2 and N2O to Ar ratios from the MIMS by the equilibrium concentration of Ar using equations based on temperature of the water and barometric pressure of the laboratory at the time of analysis (Hamme and Emerson 2004) and corrected for field conditions. We used Ar ratios to calculate concentrations, as the MIMS is more precise with ratios than absolute concentrations. Once we calculated initial sample N2 and N2O concentrations in μmol L−1, we multiplied each value by its respective molar mass (N2 = 28 g mol−1, N2O = 44 g mol−1) to acquire concentrations in μg L−1. Once compiled by inundation time, site, and amendment, each set of 3 replicate dissolved gas samples were corrected by the potential dilution by the carboy water addition at the previous time point.
We used simple linear regression to determine whether there was a significant change in N2 and N2O concentrations (in mg L−1) over incubation time for all replicates at each time point, assuming a positive linear relationship in N2 indicates detectable complete denitrification (CDN; Groffman et al. 1999) and an increase in N2O indicates detectable incomplete denitrification (IDN). The slope of the line equals the rate of the process (as μg N2 (or N2O) L−1 h−1). We multiplied these rates by the volume of overlying water present within each respective core, measured in the field (distance in cm from water–sediment surface to bottom of the rubber stopper x horizontal surface area of core), expressed in μg h−1. We assume that these measurements are representative of the entire water column within the core due to the mixing that occurred as a result of adding water near the sediment–water interface. If regressions were statistically insignificant, we considered denitrification to be below our detection limits. To place CDN and IDN in the same units, we expressed rates of N2 and N2O production as N2-N and N2O-N, respectively, as well as adding them together to express total denitrification (TDN; in μg N h−1), reflecting the sum of the rates of N2-N and N2O-N denitrification. In the cases when denitrification was significant for only one of the N gas species, we calculated TDN as simply the rate of the significant species. To compare with previous studies, we expressed rates of TDN in three different ways: (1) as areal fluxes by dividing by the core surface area (0.0032 m2; μg N m−2 h−1), (2) per g of sediment ash-free dry mass (AFDM; μg N [g AFDM]−1 h−1, denoted as DNAFDM), and (3) per g of sediment dry mass (DM; μg N [g DM]−1 h−1, denoted as DNDM).
Laboratory analysis of floodplain soil characteristics
Once all inundation times were complete, we analyzed the stored soil cores for a suite of biophysical characteristics to examine controls on denitrification between the two sites (EST, NEW). We transferred the soil from each core into separate, pre-weighed aluminum tins. We dried the entirety of the core at 60 °C until the weight was consistent (~ 90 h) to calculate DM. We crushed and homogenized any large pieces of soil to ensure even and thorough ashing in the muffle furnace at 550 °C for 4 h to calculate AFDM. We estimated percent organic matter (OM) for each soil core using the ratio of AFDM to DM (Arango et al. 2007). Prior to ashing the cores, we also took three replicate subsamples from each core to determine total carbon (C) and nitrogen (N) content. We weighed ~ 20 mg of ground soil into 5 × 9 mm tin capsules (Costech, Valencia, CA) and measured total C, total N, and C:N ratio of each core on an elemental analyzer (Costech ECS 4010, Valencia, CA).
Upscaling denitrification along the stream reach
After measuring denitrification rates in cores, we scaled our results to the constructed floodplain area of Shatto Ditch, to determine total N removal via denitrification, multiplying the area of floodplain (in m2) by the areal denitrification rate (μg N m−2 h−1) for a given inundation. We scaled denitrification for two different periods of floodplain restoration: (1) post-2007 and (2) post-2017. Post-2007 refers to the period of 2007–2017 during which the initial 600 m reach was constructed (1800 m2). Post-2017 refers to the restoration of a 3700 m (11,104 m2) reach performed in August 2017 and period in which the remaining available stream reach was constructed in 2018 (8208 m2). In order to estimate reach-scale N-removal potential of Shatto Ditch, we applied a recently-published denitrification rate for naturalized floodplains (726 μg N m−2 year−1; Hanrahan et al. 2018) to all unconstructed reaches of within the watershed. We performed this analysis under the theoretical conditions of ambient C floodwaters after 24 h of inundation.
Statistical analyses
To meet the assumptions of parametric statistics, we tested all data for normality with the Shapiro–Wilk test (P > 0.05). We determined significant DN using linear regression of N2 or N2O concentration versus incubation time (p < 0.1) following methods of Hanrahan et al. (2018). Each core within the same site and amendment were treated as a field replicate within a given inundation time (n = 3) if DN was significant by linear regression. We compared DN rates and floodplain characteristics using a three-way analysis of variance (ANOVA, p < 0.05), including [Inundation time × Site × Amend] as an interaction term to determine if the C amendment influenced denitrification at each experimental site over the different inundation times. We conducted all statistics in R (3.4.2).
Results
There were significant differences in floodplain soil characteristics between sites. Soil N (Fig. 2a) and C content (g kg dry soil−1) (Fig. 2b) were significantly lower in the NEW floodplain compared to EST floodplains (t-test, p < 0.001 for both;), resulting in a 40% higher C:N ratio for soils at NEW (t-test, p < 0.001; Fig. 2c). Soil N content was positively correlated with soil organic matter (Pearson’s r, r = 0.85, P < 0.001; Fig. 2d) as well as with soil C (Pearson’s r, r = 0.95, p < 0.001; Fig. 2e). The correlations both grouped by site, whereby EST soils had higher organic matter (t-test, p < 0.01) compared to NEW. Soil C:N ratio was negatively correlated with organic matter (Pearson’s r, r = -0.78, p = 0.003; Fig. 2f).
Boxplots of floodplain soil characteristics, including a N content, b C content, and c C:N ratio by site. Means were compared by a two-sample t-test and coefficients are shown within each plot. Scatterplots of soil d N content, e C content, and f C:N ratio versus soil organic matter (%) are evaluated by Pearson’s correlation coefficient and the results are shown within each plot. Colors of points indicate site. (Color figure online)
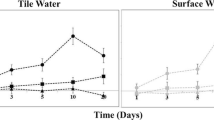
We found a linear increase in N2O concentrations over incubation time (Fig. 3), indicating measurable incomplete denitrification (IDN), in 11 of 12 cores during the 0H inundation (p < 0.1, r2 = 0.44–0.79), 3 of 12 cores during the 24H inundation (p < 0.02, r2 = 0.60–0.68), and 2 of 12 cores during the 48H inundation (p < 0.002, r2 = 0.40–0.73). Hourly rates of IDN ranged from 0.26 to 0.95 μg N2O-N h−1. Similarly, we found a linear increase in N2 concentrations over the 8 h incubation time (Fig. 3), indicating measurable complete denitrification (CDN), in 10 of 12 cores during the 24H inundation (p < 0.06, r2 = 0.38–0.90) and 7 of 12 cores during the 48H inundation (p < 0.09, r2 = 0.25–0.71). None of the cores showed a significant N2 increase during the 0HR inundation. Hourly rates of CDN ranged from 12.8 to 98.0 μg N2-N h−1. Considering both metrics of denitrification, only 4 of 36 cores showed both measurable CDN and ICN.
N2O (left side of figure) and N2 (right side of figure) concentrations (in mg L-1) versus incubation time in hours. Plots are faceted vertically by trial and horizontally by site and amendment, respectively. Data for all three field cores shown (depicted by shade of point color), but only significant linear regressions (p < 0.1) are presented as lines on the plot. Coefficients for each significant linear regression are given within the facet of the relevant plot
Using the rates calculated from each core, we can examine the effect of floodplain age, inundation time, and C amendments (+ C) using denitrification expressed in three ways: μg N m−2 h−1, μg N [g AFDM]−1 h−1, and μg N [g DM]−1 h−1. We visualized data among all factors (site, inundation time, and C amendment) to understand primary drivers of denitrification rates in these constructed sites (Fig. 4, left panels). First, DNDM ranged from 0.0013 to 0.7357 μg N [g DM]−1 h−1 from all cores throughout all inundation times in each floodplain site. Expressed per unit organic matter, DNAFDM ranged from 0.03 to 18.5 μg N [g AFDM]−1 h−1 in all cores, while areal DN ranged from 81.4 to 3,00,934.8 μg N m−2 h−1. Generally, DNDM was ~ 60% lower in NEW cores than in EST (3-way ANOVA; Site: F1,18 = 33.9, p < 0.0001), but this variation differed with inundation time (3-way ANOVA; Inundation time x Site: F2,18 = 9.2, p = 0.002). However, when we analyzed floodplain site in the context of the other two factors, floodplain site was not a significant driver of areal denitrification or DNAFDM (Fig. 4, left panels).
Boxplots of denitrification rates expressed as µg N gDM−1 h−1, µg N gAFDM−1 h−1, and µgN m−2 h−1. Plots on the left-side of figure are faceted by inundation time, amendment, and site. Box colors indicate the amendment and results of a 3-way ANOVA are shown within each plot. Given the lack of singificant difference between site, plots on right side of panel are faceted by inundation time and amendment. Lower case letters indicate significant differences (Tukey’s HSD, P < 0.05)
Given that floodplain site was not a major driver of denitrification in our study, we pared our analyses to include only inundation time and C amendment for each expression of denitrification (Fig. 4, right panels). A two-way ANOVA indicated that both inundation time and carbon amendment were significant drivers of denitrification (2-way ANOVA; inundation time: F2,24 = 22.5, p < 0.0001; amend: F1,24 = 12.4, p < 0.002). However, we found a significant interaction between inundation time and C amendment for all expressions of denitrification (2-way ANOVA; Inundation time x amend: F2,24 = 9.14, p < 0.001). Our results show that under both AMB and + C conditions, total denitrification significantly increases from 0 to 24 h of inundation time. However, total denitrification remains constant or decreases under ambient conditions between 24 and 48 h of inundation. Conversely under the + C amendment, total denitrification increases between 24 and 48 h of inundation. Therefore, our data shows that both inundation time and C availability are significant drivers of total denitrification. Specifically, total denitrification increases with inundation time in until approximately 24 h of floodplain inundation, after which an additional source of C increases total denitrification after 48 h of inundation time. Given our novel analytical approach, supported by the MIMS instrumentation, we were also able to calculate the often-used N2O yield (as (N2O/(N2O + N2))*100) (Ciarlo et al. 2008; Beaulieu et al. 2011). In instances when no significant rate of production was detected for either gas, we assumed a rate of 0. We found that inundation time plays a significant role in the end-product of denitrification (Fig. 3), so we calculated a mean N2O yield for each inundation time (0, 24, 48HR) by taking the average of all cores within a given site and C amendment (n = 4 per inundation time treatment). We found that N2O yield dropped dramatically after initial inundation, with mean N2O yields of 100% for 0HR, 0.78% for 24HR and 1.1% for 48HR (Fig. 7).
Finally, when we scaled up the areal denitrification rates for actual floodplain area of Shatto Ditch during two stages of restoration (Fig. 5), we found that the combination of constructed and “naturalized” floodplains could remove a significant amount of bioavailable N within the watershed. Upon the initial 600 m construction of floodplain (2007 period), denitrification could potentially remove 0.6 kg N d−1 after 24 h of inundation and assuming an 8 h residence time. Upon restoration of all available stream length in the Shatto Ditch after 2017, denitrification could remove 4.9 kg N d−1 after 24 h of inundation. Therefore, constructed floodplains throughout Shatto Ditch in 2017 could potentially reduce N loads in stream water at a rate 8 times greater than in 2007.
Potential N removal via denitrification in the Shatto Ditch watershed during two stages of floodplain restoration: 2007 and 2017. Areas that have not been restored are considered “naturalized” and a constant denitrification rate of 726 µg N m−2 year−1 is applied. Inundation time is assumed to be 24 h
Discussion
Our study employed a novel in-situ, intact static core design to identify factors that control denitrification end-products in constructed floodplains of different ages. Results from this study underline the importance of floodplain restoration as mechanism to increase residence time of floodwaters on bioreactive surfaces, allowing for increased removal of N via denitrification. We hypothesized that three factors would influence floodplain denitrification rates and subsequent N2O yield: (1) floodplain age, (2) C limitation, and (3) inundation time. Briefly, we found that age did not influence denitrification, and rates did increase with duration of inundation, until C limitation became a driver after 24 h. Additionally, N2O yields from floodplains were higher than assumed previously (IPCC 2001), and while floodplain restoration can increase N retention in waterway, the impact of added floodplains on N2O emissions should be considered.
Streamwater nitrate outweighs the impact of soil C and N heterogeneity on denitrification
We found that constructed floodplain soils were heterogeneous in composition and available soil C and N varied predictably with floodplain age. Available soil C and N increased with percent organic matter and were significantly higher in older floodplains (Fig. 2), which we hypothesized based on similar findings within the same watershed (Hanrahan et al. 2018) and other similar studies (McMillan and Noe 2017). Contrary to our hypothesis, we did not observe higher denitrification in the oldest floodplains. Previous research found that floodplain age increased channel stability (McMillan et al. 2014) and soil organic matter (Weier et al. 1993; McMillan and Noe 2017). Yet in this system, even newly constructed floodplains had the potential to remove N due to adequate soil C and NO3-N delivery from adjacent stream water, which aligns with previous studies examining drivers of denitrification in floodplains (Groffman and Crawford 2003; Orr et al. 2007; Gift et al. 2010; Mahl et al. 2015). However, we must also address the potential impact of in-situ vegetation on FP site differences. While floodplain vegetation was dense on EST benches and could therefore not feasibly be excluded from our in situ cores, vegetation was sparse on NEW benches. The potential exists that oxygen produced by vegetation in EST cores may have influenced the ability of underlying soils to reach anoxia, thereby reducing denitrification in a FP site that we hypothesized would support greater denitrification.
To place our results in the context of other denitrification rates measured on constructed floodplains, we plotted areal DN (in μg N m−2 h−1) versus water column NO3−-N (in mg L−1; Fig. 6), using data from previously published studies in Shatto Ditch (Roley et al. 2012a, b; Mahl et al. 2015; Hanrahan et al. 2018). While all measurements were made in this same system, we note that both Roley et al. (2012a, b) and Mahl et al. (2015) estimated denitrification via the chloramphenicol-amended acetylene block technique on sediment slurries incubated in the laboratory, which optimized redox conditions (Smith and Tiedje 1979; Royer et al. 2004; Arango et al. 2007). Hanrahan et al. 2018 also used slurries, but used the MIMS for analyses of dissolved N2 only, implementing a sacrificial assay method, which places their results somewhere between potential and actual denitrification. We note that while these studies are comparable, slurries have lower water to sediment ratios than our cores, which assume that the entirety of enclosed floodplain soils may contribute to denitrification. Therefore, estimates of denitrification based on measurements using sediment slurries conducted under redox-optimized conditions may overestimate the in-situ rates presented in our study.
Scatterplot of denitrification (ug N m−2 h−1) versus nitrate concentration (mg NO3-N L−1) measured after 0 (light green), 24 (medium green), and 48 (dark green) hours of floodplain inundation in the context of previous studies conducted on two-stage floodplains (Roley et al. 2012a, b; Mahl et al. 2015; Hanrahan et al. 2018). Roley et al. and Mahl et al. used the chloramphenicol-amended acetylene inhibition technique (Smith and Tiedje 1979; Royer et al. 2004) and Hanrahan et al. used sacrificial microcosm incubations with MIMS to estimate in-situ denitrification rates in laboratory assays
While our study period sits at the upper threshold of the baseflow NO3−-N concentration range of the Shatto Ditch Watershed, denitrification measured with our in-situ static core method after 24 and 48 h of inundation indicate that denitrification within these floodplains soils are similar to those in other two-stage ditches, regardless of method and season (Roley et al. 2012a; Mahl et al. 2015; Hanrahan et al. 2018; Fig. 6). While floodplain denitrification responds linearly to instream nitrate concentrations under 1–2 mg NO3−-N L−1 (Hanrahan et al. 2018), we found that great variability exists in those floodplains with floodwaters exceeding that threshold. Under the conditions of our study, ambient floodwaters nitrate concentration (6.4—7.7 mg NO3−-N L−1) exceeded the point at which denitrification is saturated with respect to this particular reactant (Garcia-Ruiz et al. 1998; Hanrahan et al. 2018). Therefore we suggest that while constructed floodplains in this particular watershed are heterogeneous in form and function under saturated NO3−-N conditions, these systems still possess the potential for significant NO3−-N removal via denitrification.
Influence of C limitation on denitrification rates increases with inundation time
We documented denitrification in the floodplain soils in the Shatto Ditch within the first 8 h of inundation regardless of restoration age; however, the limitation of denitrification rate by C availability occurred between 24 and 48 h after inundation. Since restoration age was not a significant driver of denitrification rate (Fig. 3, left panel), we reduced our analysis to the factors of inundation time and C amendment (Fig. 3, right panel). When expressed in μg N [g DM]−1 h−1, denitrification increased between 0 and 24 h of inundation in both ambient and + C amended cores (Fig. 3, right panel). However, after 48 h of inundation under ambient conditions, total denitrification in floodplain soils appears to be limited by C availability; denitrification rate declines from 24 to 48 h in ambient cores, while rates increase under C addition. Previous studies in Shatto Ditch floodplains found that denitrification in constructed floodplain soils were not limited by C availability (Roley et. al 2012a, b; Mahl et al. 2015), however, they used the chloramphenicol-amended acetylene block technique (Smith and Tiedje 1979; Royer et al. 2004; Arango et al. 2007) on soil slurries, which did not directly test the role of inundation. Additionally, chloramphenicol-amended acetylene block technique of Roley et al. (2012a, b) and Mahl et al. (2015) inundates soils for only 4 h after extracting soils from the field. Similarly, the sacrificial slurry of Hanrahan et al. (2018) determines denitrification rate after soils are inundated for a total of only 8 h. While Roley et al. (2012a, b) found no C limitation of denitrification in the same floodplain soils of our study using a method analogous to our 0HR inundation treatment, we suggest that this may be a result of limited inundation time. Therefore, we suggest that chloramphenicol-amended acetylene block or sacrificial slurry denitrification studies addressing C limitation in floodplain soils may find different results if soils are inundated prior to incubation.
Shifts from incomplete to complete denitrification occur with inundation time
We found that the contributions of N2O to total denitrification in constructed floodplains were greatest immediately upon flooding, and that the end-product shifts from N2O to N2 over the course of 48 h of inundation. Within the first 8 h of flooding, regardless of whether they were recently constructed or well-established, floodplains exclusively produced N2O (Fig. 3). After 24 h of continuous inundation, however, the majority of cores began producing the terminal end-product of denitrification (N2). After 48 h, N2O production nearly ceases and N2 production begins to taper as well. Previous studies have found that this transition from incomplete (terminus at N2O) to complete (terminus at N2) denitrification occurs as a result of NO3-N limitation in soils; at higher soil NO3-N concentrations, incomplete denitrification occurs as a result of the suppression of nos activity, which is the enzyme responsible for microbial conversion of N2O to N2 (Jalota et al. 2018; Weier et al. 1991; Firestone et al. 1979). Therefore, while our study replenished cores with a fresh supply of high NO3-N water prior to each successive incubation, the transition from incomplete to complete denitrification may have occurred as underlying floodplain soils were depleted of NO3-N within 0 to 24 h of inundation time.
While soil NO3-N availability may primarily explain the transition from N2O to N2 production, inundation time and bioavailable C could influence denitrification end products. Previous studies have shown that with increasing anoxia, induced by water-filled pore space, the percentage of N2 as the denitrification end-product increases (Rolston et al. 1978; Weier et al. 1993). Weier et al. (1993) simultaneously tested the combined effect of soil water content, C and NO3-N availability on denitrification end-products, where N2 was produced at the highest rate relative to N2O under high C conditions. While we did not directly test the impact of inundation time on soil porewater anoxia, we suggest that a hierarchical framework exists by which chemical factors that influence microbial activity control the completion of denitrification as inundation time increases. That is, the successive depletion of NO3-N and C from soils while soil anoxia increases may allow for idealized conditions for the microbial community to perform complete denitrification. Further exploration is required to identify the hierarchical control regarding NO3-N, C availability, and inundation time on denitrification end-products, particularly for in-situ field-based experiments.
Floodplain N2O yields differ strongly with inundation time
Even with incomplete denitrification, the concentration of N2O produced is small relative to that of N2 (Fig. 3) regardless of inundation time or C availability. Groffman et al. (2000) found that the N2O yield may vary from 1:500 to 99:1 in riparian zones, indicating that this ratio is highly variable. Here we place our N2O yield estimates in the context of a previous meta-analysis (Beaulieu et al. 2011) for multiple ecosystem types including agricultural soils, marine, lake, stream, and other floodplain types (Fig. 7). We show that N2O yields from our study differed strongly with inundation time, as cores produced N2O yields at 100% immediately after inundation (0HR) and were significantly lower with longer inundation times (0.78% for 24HR and 1.1% for 48HR). Thus, 0HR cores may be comparable to high N2O yields found in agricultural soils, but cores inundated for longer periods (24, 48HR) fall well within the lower range of for N2O yields measured in other freshwater and marine systems. Additionally, the Intergovernmental Panel on Climate Change (IPCC) has used a N2O yield of 0.5% for nitrification and denitrification in systems impacted by agricultural activity, termed “indirect emissions” (Nevison 2000; IPCC 2001), to calculate emission factors (EF) that inform global N2O budgets. In a study by Tian et al. 2019 that informed the recent refinement of IPCC’s indirect N2O EFs (IPCC 2019), the authors recognize the formative use of N2O yields in developing EFs. We note that the mean N2O yields generated from floodplain soils in our study exceed this static value of 0.5% (Fig. 7), particularly at shorter inundation times (0, 24 h). While we recognize the dynamic nature of estimating EFs and the iterative approach taken by the IPCC to do so effectively, we posit that this comparison reiterates how continued research aimed at constraining these estimates may have implications for global predictions and model parameterization.

modified from Beaulieu et al. (2011). Note the difference in scale between the two panels
Boxplots of nitrous oxide (N2O) yield (percentage of denitrified N released as N2O) measured in this study (separated by inundation time) in comparison with other ecosystems. The number of data points included in each boxplot is indicated at the top of the plot. Meta-analysis data
Future research may use this field and MIMS-based methodology to isolate additional drivers of denitrification end products. In particular, evidence from numerous studies suggest that lower soil pH increases the likelihood of incomplete denitrification (Šimek and Cooper 2002) and understanding the role of pH management in the context of agricultural soils is critical (Adams and Adams 1983; Waring and Gilliam 1983). Previous studies in agricultural soils have cited several variables that impact yields from agricultural soils, including carbon availability, O2 partial pressure, soil moisture content, pH and temperature (Nevison 2001). A comprehensive review by Saggar et al. (2013) concluded that the heterogeneity of soils and environmental conditions in these complex systems makes it difficult to accurately predict N2O. For example, Hénault et al. (2012) describes considerable spatial and temporal variation for N2O yield estimation found in the literature, emphasizing that the most variability is found at the smallest spatial scales (< 1 m2) and over the shortest time periods, which has direct implications for the study results described here. Moreover, this context emphasizes the ongoing challenges in understanding a complex transformation that occurs across a combination of heterogeneous soils/sediments and under very dynamic environmental conditions (Hénault et al. 2012). In addition, while constructed floodplains may present a temporary source of N2O to the atmosphere on very short timescales (i.e., < 24 h), direct emissions from upland agricultural soils in the surrounding watershed far outweigh emissions from “indirect” aquatic sources that may occur as a result of agricultural runoff of fertilizer N. Many studies have suggested alternative ways to prevent agricultural land use from enhancing N2O emissions, but Saggar et al. (2013) suggest that the difficulty of constraining N2O yields “may explain why to date no clear picture has emerged as to the efficacy of soil management practices in reducing N2O emissions”. However, Weier et al. (1993) suggested that decreasing application of N-based fertilizer may reduce NO3-N accumulation in soils and subsequently increase the conversion rate of N2O to N2, while the best way to reduce N2O emissions from agricultural watersheds may be to increase N use efficiency on farmlands (Hénault et al. 2012).
Floodplain restoration jumpstarts N removal capacity of agricultural waterways
Floodplain restoration along the length of Shatto Ditch significantly accelerated N watershed scale N removal, regardless of the age of floodplain restoration. Constructed floodplains throughout the Shatto Ditch Watershed and currently remove 8 × more N than if those same areas were allowed to naturalize (Fig. 5, right map), representing ~ 4.9 kg N removed per day. The floodplains constructed by 2017 (Fig. 5, shaded) flood regularly, with inundation times ranging from 50–150 days depending on the water year (Mahl et al. 2015), and they currently cover > 46% of the total stream length and represents the total area within the watershed available for restoration. In contrast, if restoration had stopped with 0.6 km in 2007, the short two-stage ditch and the remaining “naturalized” stream length would have removed only 0.6 kg N per day (Hanrahan et al. 2018; Fig. 5, left). Our study demonstrates that constructed floodplains increased permanent N removal from overlying water immediately upon inundation, and also triggered complete denitrification to N2 after only 24 h of flooding. Given the role of inundation time in driving incomplete vs. complete denitrification, the role of storms will be a key driver of N2O yields. Predicted increases in both the variability and intensity of precipitation are predicted for the Midwestern U.S., which are expected to increase water quality issues (Sinha et al. 2017), as such, floodplain restoration in agricultural headwaters could add resilience by increasing complete denitrification in order to mitigate downstream export of N.
References
Adams TM, Adams SN (1983) The effects of liming and soil pH on carbon and nitrogen contained in the soil biomass. J Agric Sci 101:553–558
APHA (2012) Standard method for the examination of water and wastewater. American Public Health Association, Washington
Arango CP, Tank JL, Schaller JL, Royer TV, Bernot MJ, David MB (2007) Benthic organic carbon influences denitrification in streams with high nitrate concentration. Freshw Biol 52:1210–1222. https://doi.org/10.1111/j.1365-2427.2007.01758.x
Beaulieu JJ, Tank JL, Hamilton SK, Wollheim WM, Hall RO, Mulholland PJ et al (2011) Nitrous oxide emission from denitrification in stream and river networks. PNAS 108:214–219. https://doi.org/10.1073/pnas.1011464108
Bernot MJ, Tank JL, Royer TV, David MB (2006) Nutrient uptake in streams draining agricultural catchments of the midwestern United States. Freshw Biol 51:499–509. https://doi.org/10.1111/j.1365-2427.2006.01508.x
Bukaveckas PA (2007) Effects of channel restoration on water velocity, transient storage, and nutrient uptake in a channelized stream. Environ Sci Technol 41:1570–1576. https://doi.org/10.1021/es061618x
Burgin AJ, Groffman PM (2012) Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J Geophys Res Biogeosci. https://doi.org/10.1029/2011JG001799
Burgin AJ, Groffman PM, Lewis DN (2010) Factors regulating denitrification in a riparian wetland. Soil Sci Soc Am J 74:1826–1833. https://doi.org/10.2136/sssaj2009.0463
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568. https://doi.org/10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2
Ciarlo E, Conti M, Bartoloni N et al (2008) Soil N2O emissions and N2O/(N2O+N2) ratio as affected by different fertilization practices and soil moisture. Biol Fertil Soils 44:991–995. https://doi.org/10.1007/s00374-008-0302-6
Conrath U, Amoroso G, Kohle H, Sultemeyer DF (2004) Non-invasive online detection of nitric oxide from plants and some other organisms by mass spectrometry. Plant J Cell Mol Biol 38:1015–1022. https://doi.org/10.1111/j.1365-313X.2004.02096.x
Firestone MK, Smith MS, Firestone RB, Tiedje JM (1979) The influence of nitrate, nitrite, and oxygen on the composition of the gaseous products of denitrification in soil. Soil Sci Soc Am J 43:1140–1144. https://doi.org/10.2136/sssaj1979.03615995004300060016x
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356. https://doi.org/10.1641/0006-3568(2003)053[0341:TNC]2.0.CO
Garcia-Ruiz R, Pattinson SN, Whitton BA (1998) Denitrification in river sediments: relationship between process rate and properties of water and sediment. Freshw Biol 39:467–476
Genthner FJ, Marcovich DT, Lehrter JC (2013) Estimating rates of denitrification enzyme activity in wetland soils with direct simultaneous quantification of nitrogen and nitrous oxide by membrane inlet mass spectrometry. J Microb Biochem Technol 5:95–101. https://doi.org/10.4172/1948-5948.1000108
Gift DM, Groffman PM, Kaushal SS, Mayer PM (2010) Denitrification potential, root biomass, and organic matter in degraded and constructed urban riparian zones. Restor Ecol 18:113–120
Gihring TM, Canion A, Riggs A, Huettel M, Kostk JE (2010) Denitrification in shallow, sublittoral Gulf of Mexico permeable sediments. Limnol Oceanogr 55(1):43–54. https://doi.org/10.4319/lo.2010.55.1.0043
Goodwin JM, Chrestensen CA, Moomaw EW (2018) Detection of nitric oxide by membrane inlet mass spectrometry. Methods Mol Bio 1747:35–47. https://doi.org/10.1007/978-1-4939-7695-9_4
Griffiths NA, Tank JL, Royer TV, Roley SS, Rosi-Marshall EJ, Whiles MR et al (2013) Agricultural land use alters the seasonality and magnitude of stream metabolism. Limnol Oceanog 58:1513–1529. https://doi.org/10.4319/lo.2013.58.4.1513
Groffman PM, Holland EA, Myrold DD, Robertson GP, Zou X (1999) Denitrification. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standard Soil Methods for Long-Term Ecological Research. Oxford University Press, New York
Groffman PM, Gold AJ, Addy K (2000) Nitrous oxide production in riparian zones and its importance to national emission inventories. Chemosphere Global Change Sci 2(3):291–299. https://doi.org/10.1016/S1465-9972(00)00018-0
Groffman PM, Crawford MK (2003) Denitrification potential in urban riparian zones. J Environ Qual 32:1144–1149
Hamme RC, Emerson SR (2004) The solubility of neon, nitrogen and argon in distilled water and seawater. Deep Sea Res 51:1517–1528. https://doi.org/10.1016/j.dsr.2004.06.009
Hanrahan BR, Tank JL, Dee MM, Trentman MT, Berg EM, McMillan SK (2018) Constructed floodplains enhance denitrification compared to naturalized floodplains in agricultural streams. Biogeochem 141:419–437. https://doi.org/10.1007/s10533-018-0431-4
Hénault C, Grossel A, Mary B, Roussel M, Léonard J (2012) Nitrous oxide emission by agricultural soils: a review of spatial and temporal variability for mitigation. Pedosphere 22(4):426–433. https://doi.org/10.1016/S1002-0160(12)60029-0
IPCC (2001) Climate change 2001: the scientific basis. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA (eds) Contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
IPCC (2007) Climate change 2007. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. In: Pachauri RK, Reisinger A (eds) Synthesis Report. IPCC, Geneva, p 104
IPCC (2019) 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Volume 4: Agriculture, Forestry and Other Land Use. Chapter 11: N2O emissions from managed soils, and CO2 emissions from lime and urea application. IPCC, Geneva
Jalota SK, Vashisht BB, Sharma S, Kaur S (2018) Emission of greenhouse gases and their warming effect. In: Jalota SK, Vashisht BB, Sharma S, et al. (eds) Understanding climate change impacts on crop productivity and water balance. Academic Press, Cambridge, pp 1–53
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal Chem 66:4166–4170. https://doi.org/10.1021/ac00095a009
Kana TM, Sullivan MB, Cornwell JC, Groxzkowski KM (1998) Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol Oceanog 43:334–339. https://doi.org/10.4319/lo.1998.43.2.0334
Kaushal SS, Groffman PM, Mayer PM, Striz E, Gold AJ (2008) Effects of stream restoration on denitrification in an urbanizing watershed. Ecol Appl 18:789–804
Klein CD, Novoa RSA, Ogle S, Smith KA, Rocette P, Wirth TC (2006) N2O emissions from managed soils, and CO2 emissions from lime and urea application. In: 2006 IPCC guidelines for national greenhouse gas inventories. Intergovernmental Panel on Climate Change. Available at https://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Landwehr K, Rhoads BL (2003) Depositional response of a headwater stream to channelization, East Central Illinois, USA. River Res Appl 19:77–100. https://doi.org/10.1002/rra.699
Letey J, Valoras N, Hadas A, Focht DD (1980) Effect of air-filled porosity, nitrate concentration, and time on the ratio of N2O/N2 evolution during denitrification. J Env Qual 9:227–231. https://doi.org/10.2134/jeq1980.00472425000900020013x
Mahl UH, Tank JL, Roley SS, Davis RT (2015) Two-stage ditch floodplains enhance N-removal capacity and reduce turbidity and dissolved P in agricultural streams. J Am Water Resour Assoc 51:923–940. https://doi.org/10.1111/1752-1688.12340
McMillan SK, Tuttle AK, Jennings GD, Gardner A (2014) Influence of restoration age and riparian vegetation on reach-scale nutrient retention in constructed urban streams. J Am Water Resour Assoc 50:626–638. https://doi.org/10.1111/jawr.12205
McMillan SK, Noe G (2017) Increasing floodplain connectivity through urban stream restoration increases nutrient and sediment retention. Ecol Eng 108:284–295. https://doi.org/10.1016/j.ecoleng.2017.08.006
Nevison C (2000) Review of the IPCC methodology for estimating nitrous oxide emissions associated with agricultural leaching and runoff. Chemosphere Global Change Sci 2(3–4):493–500. https://doi.org/10.1016/S1465-9972(00)00013-1
Nevison C (2001) Indirect N2O Emissions from Agriculture. In: Good practice guidance and uncertainty management in national greenhouse gas inventories. Intergovernmental Panel on Climate Change. Available at https://www.ipcc-nggip.iges.or.jp/public/gp/english/
Orr CH, Stanley EH, Wilson KA, Finlay JC (2007) Effects of restoration and reflooding on soil denitrification in a leveed midwestern floodplain. Ecol Appl 17:2365–2376. https://doi.org/10.1890/06-2113.1
Phillips RL, McMillan AMS, Palmada T, Dando J, Giltrap D (2015) Temperature effects on N2O and N2 denitrification end-products for a New Zealand pasture soil. N Z J Agric Res 58:89–95. https://doi.org/10.1080/00288233.2014.969380
Powell GE, Ward AD, Mecklenburg DE, Jayakaran AD (2007) Two-stage channel systems: part 1, a practical approach for sizing agricultural ditches. J Soil Water Conserv 62:277–286
Powell KL, Bouchard V (2010) Is denitrification enhanced by the development of natural fluvial morphology in agricultural headwater ditches? J N Am Benthol Soc 29:761–773. https://doi.org/10.1899/09-028.1
Randall GW, Huggins DR, Russelle MP, Fuchs DJ, Nelson WW, Anderson JL (1997) Nitrate losses through subsurface tile drainage in conservation reserve program, alfalfa, and row crop systems. J Env Qual 26:1240–1247. https://doi.org/10.2134/jeq1997.00472425002600050007x
Roley SS, Tank JL, Stephen ML, Johnson LT, Beaulieu JJ, Witter JD (2012a) Floodplain restoration enhances denitrification and reach-scale nitrogen removal in an agricultural stream. Ecol Appl 22:281–297. https://doi.org/10.1890/11-0381.1
Roley SS, Tank JL, Williams MA (2012b) Hydrologic connectivity increases denitrification in the hyporheic zone and constructed floodplains of an agricultural stream. Geophys Res 117:G00NO4. https://doi.org/10.1029/2012JG001950
Roley SS, Tank JL, Griffiths NA, Hall RO, Davis RT (2014) The influence of floodplain restoration on whole-stream metabolism in an agricultural stream: insights from a 5-year continuous data set. Freshw Sci 33:1043–1059. https://doi.org/10.1086/677767
Rolston DE, Hoffman DL, Toy DW (1978) Field measurement of denitrification: I. flux of N2 and N2O. Soil Sci Soc Am Journal 42:863–869. https://doi.org/10.2136/sssaj1978.03615995004200060004x
Royer TV, Tank JL, David MB (2004) Transport and fate of nitrate in headwater agricultural streams in illinois. J Environ Qual 33:1296–1304. https://doi.org/10.2134/jeq2004.1296
Ryden JC, Lund LJ, Letey J, Focht DD (1979) Direct measurement of denitrification loss from soils: II. Development and application of field methods. Soil Sci Soc Am J 43:110–118. https://doi.org/10.2136/sssaj1979.03615995004300010020x
Saggar S, Jha N, Deslippe J, Bolan NS, Luo J, Giltrap DL, Kim D-G, Zaman M, Tillman RW (2013) Denitrification and N2O:N2 production in temperate grasslands: processes, measurements, modelling and mitigating negative impacts. Sci of The Tot Env 465:173–195. https://doi.org/10.1016/j.scitotenv.2012.11.050
Schlüter M, Gentz T (2008) Application of membrane inlet mass spectrometry for online and in situ analysis of methane in aquatic environments. J Am Soc for Mass Spectrom 19:1395–1402. https://doi.org/10.1016/j.jasms.2008.07.021
Sheibley RW, Ahearn DS, Dahlgren RA (2006) Nitrate loss from a constructed floodplain in the Lower Cosumnes River, California. Hydrobiologia 571:261–272. https://doi.org/10.1007/s10750-006-0249-2
Šimek M, Cooper JE (2002) The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years. Eur J Soil Sci 53:345–354. https://doi.org/10.1046/j.1365-2389.2002.00461.x
Sinha E, Michalak AM, Balaji V (2017) Eutrophication will increase during the 21st century as a result of precipitation changes. Science 357:405–408
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. European J Soil Sci 54:779–791. https://doi.org/10.1046/j.1351-0754.2003.0567.x
Smith MS, Tiedje JM (1979) The effect of roots on soil denitrification. Soil Sci Soc Am J 43:951–955. https://doi.org/10.2136/sssaj1979.03615995004300050027x
Tian L, Cai Y, Akiyama H (2019) A review of indirect N2O emission factors from agricultural nitrogen leaching and runoff to update of the default IPCC values. Environ Pollut 245:300–306. https://doi.org/10.1016/j.envpol.2018.11.016
Vinther FP (1984) Total denitrification and the ratio between N2O and N2 during the growth of spring barley. Plant Soil 76:227–232. https://doi.org/10.1007/BF02205582
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW et al (1997) Technical report: human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750. https://doi.org/10.2307/2269431
Waring SA, Gilliam JW (1983) The effect of acidity on nitrate reduction and denitrification in lower coastal plain soils. Soil Sci Soc Am J 47:246–251
Weier KL, Doran JW, Power JF, Walters DT (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci Soc Am J 57:66–72. https://doi.org/10.2136/sssaj1993.03615995005700010013x
Weier KL, Macrae IC, Myers RJK (1991) Seasonal variation in denitrification in a clay soil under a cultivated crop and a permanent pasture. Soil Biol Biochem 23:629–635. https://doi.org/10.1016/0038-0717(91)90075-U
Acknowledgements
This research was supported by Illinois-Indiana Sea Grant College Program 074483-15949. MMD was supported in part by a University of Notre Dame Center for Environmental Science and Technology (CEST)/Bayer Predoctoral Fellowship. We thank numerous members of the Tank Lab for field and lab assistance, including Ursula Mahl and Abigail White. We are grateful to Annie Bowling of Dr. Todd Royer’s lab for sample analysis. We thank Dr. Sara McMillan for input on design; Dr. Todd Kana for valuable MIMS advisement; Jon Loftus and Suzyanne Guzicki of CEST for analytical instrument troubleshooting and Dr. Jason Taylor for an insightful review of an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: James Sickman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dee, M.M., Tank, J.L. Inundation time mediates denitrification end products and carbon limitation in constructed floodplains of an agricultural stream. Biogeochemistry 149, 141–158 (2020). https://doi.org/10.1007/s10533-020-00670-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00670-x