Abstract
Soil–water repellency (SWR) is a widely observed phenomenon with severe impacts, but a physicochemical framework to explain the process of SWR development is still a major field of research. Recent studies have shown that microbial biomass residues, in particular cell fragments, contribute significantly to the formation of soil organic matter (SOM) and can decrease wettability. It was also shown that osmotic stress increases the hydrophobicity of bacterial cell surfaces. If microorganisms are an important source of SOM, the attachment of cells and their residues on mineral grains should decrease wettability of minerals, and the effect should be more pronounced in case of osmotic stress. Cultures of Pseudomonas putida, either unstressed or exposed to osmotic stress, and cell fragments were mixed with minerals and the impact on surface wetting properties was investigated by determining the solid-water contact angle (CA). Attachment of bacteria to quartz surfaces resulted in a significant increase in hydrophobicity of the surfaces (CA increase by up to 90°), in particular for stressed cells. Cell fragments and cytosol were also found to decrease wettability significantly (CAs of up to 100°). These findings may explain various phenomena related to SWR, like critical soil–water content, and may be one important explanation for the formation of SWR after irrigation with treated sewage effluents. The results also support the hypothesis of a microbial origin of SWR, in which macromolecular biological structures may have a greater impact than specific classes of organic compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil–water repellency (SWR) is a worldwide phenomenon, which has been reported for a variety of soil types (Dekker et al. 1999; DeBano 2000; Dekker and Ritsema 2000; Doerr et al. 2000). It has important consequences with respect to erosion, water infiltration and surface runoff (Doerr et al. 2000). For example, in Australia two million hectares of sandy soil are affected by severe SWR causing significant economic damage (Blackwell 2000; Franco et al. 2000b). Due to its influence on water availability and water film thickness, SWR affects the biological activity in soils and microbial decomposition (Churaev 2000; Feeney et al. 2006; Goebel et al. 2011). Goebel et al. (2005) found a negative correlation between the soil respiration from grassland and wheat topsoil samples and the soil–water contact angle (CA), a measure for SWR.
In order to improve soil management strategies, the development of a mechanistic framework to explain the formation of SWR is desired. However, exploring the underlying mechanisms of SWR development is still a major field of soil research (Graber et al. 2009).
It is a general consensus that SWR originates from organic compounds with hydrophobic properties interacting with mineral surfaces or interstitial particulate organic material (Bisdom et al. 1993; Doerr et al. 2000; Franco et al. 2000a). The arrangement of these compounds on mineral surfaces ranges from a continuous layer to small patches (Ma’Shum et al. 1988; Bisdom et al. 1993). The adsorption in form of small globules or patches is assumed to be more likely and thus more frequent than the distribution as a uniform monolayer on the mineral surfaces (Ma’Shum et al. 1988; Doerr et al. 2000). According to Doerr et al. (2000), the visualization of hydrophobic coatings is crucial to study SWR formation. However, inconsistent results, ranging from undetectable coatings (Jungerius and de Jong 1989) to clearly visible ones (Tucker et al. 1990), have been published.
The main approach to study the chemical structure of substances responsible for SWR is based on the extraction of water repellent soil material with organic solvents and subsequent application of the extracted compounds to wettable minerals for inducing SWR (Ma’Shum et al. 1988; Doerr et al. 2005). During the last decades, several compound classes like waxes, alkanes, alkanols, fatty acids and humic acids were identified to be capable of inducing SWR (Franco et al. 2000a; Horne and McIntosh 2000; Mainwaring et al. 2004). These compounds were mostly considered to be plant-derived. Studies revealed amphiphilic molecules as highly effective in producing hydrophobic coatings and as important constituents of soil organic matter (SOM; Wershaw 1993; Doerr et al. 2000; Horne and McIntosh 2000; Hurrass and Schaumann 2006; Kleber et al. 2007). As a consequence, Horne and McIntosh (2000) proposed a zonal model, comprised of layers of amphiphilic fragments with either the polar or the non-polar part of the molecule directed towards the pore space, which can explain the formation of SWR after drying. A similar model was later suggested by Kleber et al. (2007) for the surface structure of mineral-associated organic matter. Miltner et al. (2012) proposed that, in addition to plant-derived organic compounds, microbial cell wall envelopes containing various amphiphilic molecules are a significant part of SOM. This may be a further explanation for the occurrence of hydrophobic coatings after drying.
SWR affects the water distribution in soils by decreasing the water infiltration capacity and by the formation of preferential flow paths, which can exclude large areas in soil from water and nutrient flow (Doerr et al. 2000; Goebel et al. 2007, 2011). This may induce water and osmotic stress with severe consequences for the indigenous microbial community and its activity (Feeney et al. 2006; Schimel et al. 2007). Water stress was shown to activate several processes in microorganisms, e.g., production of extracellular polymeric substances (EPS) or exudation of hydrophobic compounds, osmolytes or biosurfactants (Schimel et al. 2007; Morales et al. 2010). Certain bacteria have been shown to degrade SWR-inducing compounds and thus can be used to ameliorate SWR (Roper 2004). Bacteria in general, however, are able to adapt to changing environmental conditions by physicochemical modification of their surface properties, which may result in an increase in cell surface hydrophobicity. This surface modification is a well characterized stress response of bacteria (Lopez et al. 2000; Wick et al. 2003). Changes in the structure of lipopolysaccharides and the release of membrane vesicles were described as underlying mechanisms (Al-Tahhan et al. 2000; Baumgarten et al. 2012). The different wettability of bacterial cells may also be reflected in the properties of their residues and affect soil particle surface properties, when stabilised in soil.
Despite a large body of previous research, the molecular basis of SWR is not yet mechanistically understood (Mainwaring et al. 2004). The role of microbial components like bacteria and their residues and, as a consequence, the potential contribution of stress-related changes in the surface properties of microorganisms to the formation of SWR so far has not been considered.
Therefore, the aim of the present study was to analyze the influence of bacteria and their cell wall envelopes on the wettability of model minerals, like quartz sand and kaolinite (KA). We hypothesize that the bacterial interaction with the minerals leads to a change in particle surface wetting properties and that the salt stress-induced changes in bacterial surface properties are reflected in the wetting properties of organo-mineral associations. In order to evaluate the principal processes, pure cultures of Pseudomonas putida were exposed to osmotic stress, subsequently mixed with several model minerals and the microscopic and macroscopic CA of the organo-mineral associations determined. Scanning electron microscopy (SEM) was used to characterize the surface coverage by structures in the sub-µm size range and environmental SEM (ESEM) was used to determine the micro-scale wettability.
Materials and methods
Culture conditions and chemicals
All chemicals and organisms were obtained from commercial sources. P. putida mt-2, a gram negative bacterium, was obtained from DSMZ (DSM 3931, Braunschweig, Germany) and cultivated in mineral medium [1.55 g K2HPO4, 0.85 g NaH2PO4·2H2O, 2 g (NH4)2SO4, 0.1 g MgCl2·6H2O, 10 mg EDTA, 2 mg ZnSO4·7H2O, 1 mg CaCl2·2H2O, 5 mg FeSO4·7H2O, 0.2 mg Na2MoO4·2H2O, 0.2 mg CuSO4·5H2O, 0.4 mg CoCl2·6H2O, 1 mg MnCl2·2H2O, 1 L demineralized water] according to Hartmans et al. (1989) with 4 g L−1 disodium succinate as sole carbon and energy source. Cells were grown in a baffled flask on a horizontal shaker at 30 °C. For inducing osmotic stress, 1.25 M NaCl (final concentration) was added at the early exponential growth phase. After 2 h of incubation, the cells were harvested by centrifugation (10,000×g for 10 min) and washed three times with 10 mM KNO3 solution. The cell pellet was finally resuspended in 10 mM KNO3 solution and stored for further analysis.
Model minerals
Coarse-grained quartz sand (CQ, Carl Roth GmbH, Karlsruhe, Germany) and a washed and calcined medium-grained quartz (MQ, Merck KGaA, Darmstadt, Germany) were chosen as representative material for two different sand fractions. Fine-grained quartz (FQ, Carl Roth GmbH, Karlsruhe, Germany) and a KA (Fluka, Sigma-Aldrich, St. Louis, USA) were chosen as model minerals for the silt and clay fraction, respectively. All minerals were used as supplied. The specific surface area (SSA) of the minerals was determined by BET measurement with a Nova 4000e (Quantachrome, Odelzhausen, Germany) after drying at 105 °C. The corresponding areas were: CQ: 0.17 m2 g−1, MQ: 0.35 m2 g−1, FQ: 1.06 m2 g−1, KA: 8.92 m2 g−1.
Sample preparation
Unstressed and stressed cells were diluted with 100 µL 10 mM KNO3 solution and added to 500 mg of the mineral phase while gently stirring to prevent damage of the cells and release of cytosolic substances. The concentrations of cells used for the experiment were 108, 109 and 1010 cells g−1 mineral. Cell numbers were previously estimated by optical density measurement (OD560) and cross-checked by using a coulter counter Multisizer 3 (Beckman Coulter, High Wycombe, UK).
Cell fragments and cytosolic compounds from unstressed bacteria were separated by ultrasonic disruption. Briefly, 500 µL samples with cell concentrations as described before were disrupted by applying ultrasound to the suspensions (2 × 30 s, 50 % interval, 50 % power, Sonopuls HD 70, Bandelin electronic, Berlin, Germany). The samples were centrifuged (10,000×g for 20 min), washed two times with 10 mM KNO3 solution and the pellet containing the cell fragments finally stored in 100 µL KNO3 solution. For obtaining the cytosolic compounds, the supernatant of the first centrifugation step was collected (~0.5 mL). Aliquots of 100 µL were used for the experiments. As a protein standard solution, bovine serum albumin (BSA, 200 µg mL−1) was used. Cell fragments, supernatant and BSA solution were finally mixed with 500 mg of the mineral phase. All organo-mineral associations (three replicates for each treatment) were air dried for 2 h at room temperature (~22 °C, ~60 % relative humidity) before analysis.
Water CA measurement
As a measure for cell surface hydrophobicity, the CA of the bacteria was measured with the sessile drop method as described by van Loosdrecht et al. (1987). Briefly, bacterial cells were spread on a 0.45 µm pore size filter (Schleicher and Schuell, Dassel, Germany) by means of filtration. The filters were air dried for 2 h at room temperature and the CA was measured (DSA 100, Kruess GmbH, Hamburg, Germany) with the following settings: water drop volume 3 µL, flow rate 40 µL min−1. The CA, θ (°), of four independently measured drops were evaluated by means of drop shape analysis.
The CA of the organo-mineral associations were determined using the sessile drop method as described by Bachmann et al. (2000). The dried samples were fixed with double-sided tape on glass slides by gentle pressing. Excessive material was removed by tapping to obtain a fixed monolayer. The CA was measured as described before. Samples with CA ≥0–90° show reduced wettability, while values greater than 90° indicate extreme SWR (Goebel et al. 2011).
Scanning electron microscopy
Aliquots of the air-dried organo-mineral associations (i.e., cells and cell fragments) were fixed with a sufficient amount of 2.5 % (w/w) glutaraldehyde in phosphate-buffered saline (8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4, 1 L demineralized water) solution. After 6 h fixation, the fixative was replaced with fresh glutaraldehyde solution and subsequently fixed overnight at 4 °C. Afterwards the samples were washed three times with phosphate-buffered saline and dehydrated by a graded acetone series. The solution was exchanged every 30 min by a solution with a higher acetone concentration (25, 25, 30, 50, 60, 70, 80, 90, 100, 100, 100 %, v/v; Schurig et al. 2013). The samples were subsequently dried by critical point drying (Leica EM CPD300, Wetzlar, Germany) and then coated with a thin layer of gold (Sputtercoater SCD 50, Bal-Tec, Liechtenstein). Afterwards, the sample surfaces were analyzed by means of SEM with an Ultra 55 (Carl Zeiss, Oberkochen, Germany) and the following settings: gun voltage 1 kV, system vacuum 10−6 hPa, detector secondary electron 2, noise reduction frame average/line average.
Environmental SEM
Condensation experiments in an ESEM (Quanta 200-ESEM, FEI Company, Eindhoven, Netherlands) were performed to characterize the micro-scale wettability of the organo-mineral associations (CQ and MQ as mineral compounds). For this, the sample was placed on a Peltier stage in a gaseous environment consisting of water vapour. The initial chamber pressure was 250 Pa and the gun voltage 20 kV. For the wettability experiment according to Goebel et al. (2007), the chamber pressure was increased until condensation of water on mineral surfaces was observable. The CA of the water droplets was analyzed by means of drop shape analysis with the software SCA20 (DataPhysics, Filderstadt, Germany). The number of evaluated drops ranged from two to five for each sample.
Surface coverage determination and data analysis
Mineral surface coverage was determined by multiplying the number of attached bacteria with their cross-section area which was derived from scanning electron micrographs. The determined area was then related to the SSA of the minerals. The cell concentrations were tested for significance using one way repeated-measures ANOVA followed by Holm–Sidak post hoc test for pairwise differences. Some data sets were \(\sqrt {x + 0.5}\) transformed to obtain equal variance. The significance of differences between unstressed and stressed cells without minerals was tested with t test, while the effect of osmotic stress on the organo-mineral associations was tested using one way ANOVA followed by Dunnett’s T3 post hoc test. For all tests, the significance level was set at 0.05. All curve fitting procedures and statistics were performed using SigmaPlot 12.5 (Systat Software, San Jose, USA) and IBM SPSS Statistics 21 (IBM, Armonk, USA).
Results
SEM of organo-mineral associations
Figure 1 shows representative SEM micrographs of the organo-mineral associations with MQ as mineral component. The morphological differences between the unstressed and osmotically stressed cells were clearly visible (compare Fig. 1a–d). While unstressed cells showed a smooth surface and the rod shape of vital cells, some of the stressed cells were flat and partly broken cell envelopes were visible. The cells were heterogeneously distributed over the quartz surface in the form of patchy microcolony-like structures. The same observations were made with CQ as mineral component (see Online Resource 1). The cell fragments, however, showed a spherical and patchy shape (Fig. 1e, f) and were, in contrast to the cells, more homogeneously distributed on the mineral surface. Generally, the surface coverage was higher with increasing cell concentration.
Scanning electron micrographs of unstressed Pseudomonas putida cells (a, b), osmotically stressed cells (c, d) and cell fragments (e, f) attached to medium-grained quartz surfaces (MQ). In micrographs a, c, e, the concentration is 108 cells g−1 quartz and in b, d, f, the concentration is 109 cells g−1 quartz. The smaller micrographs were taken at a higher magnification
Wetting properties of organo-mineral associations
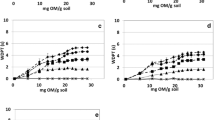
The wetting properties of both quartz sands (MQ and CQ) were significantly influenced by attached bacteria (Fig. 2). Both types of quartz sand showed a negligible CA between 0° and 11° without and with 108 attached cells g−1 mineral. With increasing number of cells, the wettability decreased until the surface was nearly hydrophobic with a CA of about 88° (1010 stressed cells g−1 MQ). In general, the CA of MQ was slightly larger than that of CQ. The CA of unstressed P. putida cells was about 42°, while the stress significantly increased the CA (θ = 65°). This difference was reflected in the CA of the cell–mineral associations for 109 attached bacteria g−1 MQ (θunstressed = 61° and θstressed = 86°) and CQ (θunstressed = 53° and θstressed = 79°). For the higher cell concentrations, the stress effect was less pronounced. From regression analysis, an exponential rise up to maximum could be identified for MQ and CQ for CA as a function of the surface covered by unstressed cells (Fig. 3a, c). For the stressed cell–mineral associations, a hyperbolic function could be fitted for MQ (Fig. 3b) and a sigmoidal function for CQ (Fig. 3d), respectively. The regressions with the best fit were chosen.
Contact angle of Pseudomonas putida (Bac) and medium-grained quartz (MQ) and coarse-grained quartz (CQ)/P. putida [unstressed cells (black bars) and osmotically stressed cells (grey bars)]-associations after 2 h of drying. Different letters indicate significant differences between cell concentrations (lower case letters apply to unstressed cells, upper case letters to stressed cells) and asterisks between unstressed and stressed cells of the same cell concentration (α = 0.05). The values are arithmetic means of four independent measurements with the error bars indicating the standard deviation
Contact angle of Pseudomonas putida/mineral associations in relation to surface coverage with bacteria and corresponding measured data (symbols) and model fits (lines). a Medium-grained quartz MQ, unstressed cells [y = 81.2050 × (1 – e (−0.2136x)), R 2 = 0.96], b medium-grained quartz MQ, osmotically stressed cells (\(y = - 1,379.9223 + \frac{1,466.2952x}{0.0350 + x},\) R 2 = 0.94), c coarse-grained quartz CQ, unstressed cells [y = 72.3755 × (1 – e (−0.0792x)), R 2 = 0.91], and d coarse-grained quartz CQ, osmotically stressed cells (\(y = \frac{75.4528}{{1 + e^{{ - \frac{(x - 4.7012)}{1.1127}}} }},\) R 2 = 0.97)
The CA determined on the cell–mineral associations with small-sized mineral particles (FQ and KA) are shown in Fig. 4. The CA of pure KA (θ = 44°) was higher than that of FQ (θ = 15°). The attachment of cells decreased the wettability of FQ up to a CA of 62° for 1010 unstressed cells g−1 mineral. For KA, no distinct effect could be observed. The difference between the CA of the bacterial cells was not reflected in the CA of these cell–mineral associations. Surprisingly, the attachment of 1010 unstressed cells g−1 FQ led to an approximately two times higher, but not significant, CA than the same amount of stressed cells (θunstressed = 62° and θstressed = 35°).
Contact angle of Pseudomonas putida (Bac) and fine-grained quartz (FQ) and kaolinite (KA)/P. putida [unstressed cells (black bars) and osmotically stressed cells (grey bars)]-associations after 2 h of drying. Different letters indicate significant differences between cell concentrations (lower case letters apply to unstressed cells, upper case letters to stressed cells) and asterisks between unstressed and stressed cells of the same cell concentration (α = 0.05). The values are arithmetic means of four independent measurements with the error bars indicating the standard deviation
Due to the marginal differences between both quartz sands and the generally large CA of the fine-grained minerals, MQ was selected as the mineral matrix for analysing the effect of cell fragments, cytosolic compounds and BSA solution on wettability. Like for the intact cells, attachment of cell wall fragments had no effect on the wettability at low concentrations (108 cells g−1 mineral, Fig. 5). In contrast, the cytosol increased the CA even at low concentrations (θ = 39°). At elevated cytosol concentrations, CA increased up to 101° (109 cells g−1 mineral), while higher concentrations (1010 cells g−1 mineral) caused a slight increase for cell fragments and slightly smaller CA for cytosol (Fig. 5). The mixture of cytosol and cell fragments induced a wettability pattern which was similar to that of cytosol with a maximum CA of 96° at a concentration of 109 cells g−1 mineral (Fig. 5). The addition of BSA solution increased the CA of MQ to 76°, which revealed the proteins in the cytosol to be important factors in determining wettability.
Contact angles of Pseudomonas putida cell fragments (CF), cytosol (CY), mixture of cell fragments and cytosol (CF/CY) and bovine serum albumin solution (BSA; hatched bar)/medium-grained quartz (MQ)-associations after 2 h of drying. Different bacteria concentrations were used (108: black bars, 109: light grey bars, 1010 cells g−1 mineral: dark grey bars). Different letters indicate significant differences between lowest cell concentration of each treatment and control (MQ) and asterisk between cell concentrations of the same treatment (α = 0.05). The values are arithmetic means of four independent measurements with the error bars indicating the standard deviation
Micro-scale wettability analysis by ESEM
ESEM micrographs with MQ as mineral component are shown in Fig. 6. All 108 cells g−1 mineral samples, i.e., unstressed, stressed, cell fragments and cytosol, were completely wettable, i.e., the water condensed homogeneously on the surfaces (Fig. 6a, c, e, g), while the 109 cells g−1 mineral samples showed distinct formation of droplets during condensation (Fig. 6b, d, f, h). The attachment of stressed cells led to the formation of droplets with a higher CA (θ = 85°) compared to the unstressed cells (θ = 66°; Fig. 6b, d). For CQ, the same trends in micro-scale wettability were found (see Online Resource 2). Interaction with the cell fragments (Fig. 6f) and the cytosol (Fig. 6h) led to the formation of droplets for 109 cells g−1 mineral samples with CA of 69° and 76°, respectively.
Micrographs from condensation experiments in an environmental scanning electron microscope with unstressed Pseudomonas putida cells (a, b), osmotically stressed cells (c, d), cell fragments (e, f) and cytosol (g, h) as organic coatings. The mineral phase is medium-grained quartz (MQ). In micrographs a, c, e, g, the concentration is 108 cells g−1 quartz and in b, d, f, h, the concentration is 109 cells g−1 quartz, showing the formation of droplets
Discussion
In this study, we showed that the wettability of mineral surfaces is modified by the coverage with bacteria and biomolecules, in particular cell wall fragments and proteins. The surfaces of two quartz sands (small SSA), different in texture (coarse and medium) and pre-treatment (no pre-treatment, washed and calcined) decreased in wettability due to the interaction with biomass of P. putida mt-2 (i.e., intact cells, cell fragments and cytosol). Exposure of the bacteria to osmotic stress increased their CA similar to what was observed for P. putida DOT-T1E under osmotic stress induced by 2 M NaCl (Baumgarten et al. 2012). This difference in CA between unstressed and stressed cells was reflected in cell–mineral associations with 109 cells g−1 mineral while at higher concentration (i.e., 1010 bacteria g−1 mineral), the CA was high for both stressed and unstressed cells. The exponential increase of the CA up to a maximum indicates that a coverage between 1 and 11 % had the most significant effect on wettability, while a further increase of coverage only had minor additional effects. The stressed cells caused a distinctly greater increase compared to the unstressed cells (Fig. 3a–d), emphasizing a clear effect of osmotic stress on SWR.
The exponential relationship between surface coverage and CA can explain the observed low impact of bacterial attachment on the wettability of fine-grained minerals. Due to their high SSA (FQ: 1.06 m2 g−1, KA: 8.92 m2 g−1), the cell concentrations used were obviously too low for inducing an effect for KA. FQ was only affected at high bacteria concentrations. In this context it should be noted that the larger initial CA of KA and FQ compared to MQ and CQ presumably masked the microbially induced changes.
Nevertheless, in our experiments an effect on wettability was only detected for concentrations of 109 cells g−1 mineral or higher which corresponds to a surface coverage of approximately 10 %. This coverage seems not realistic for natural soils, where coverage of soil particles with living microorganisms is on average less than 1 % (Chenu and Stotzky 2002). However, if microbial necromass is generally contributing to SOM, cell residues equivalent to 109 cells g−1 mineral would be quite common. The SEM micrographs of Miltner et al. (2012) and Schurig et al. (2013) showed a high density of microbial residues on mineral surfaces and therefore the amounts of cell fragments used in this study even seem to be underestimated compared to natural soils. In addition, soils are highly heterogeneous with respect to the spatial distribution of microorganisms. There are hotspots and large bacterial patches, which can induce at least subcritical SWR (Nunan et al. 2002). In soil, high densities of microorganisms are often arranged in biofilms, whose effects on wettability were shown by Schaumann et al. (2007). The matrix of the biofilms consists of EPS, into which microorganisms are embedded (Wingender et al. 1999). Biofilms were often found in water saturated systems and in soil systems when complex substrates were provided in high amounts supporting exponential growth of bacteria. The EPS of such biofilms are typically forming spider-web like structures in SEM micrographs (Miltner et al. 2012). However, this kind of biofilms was not observed in the present study. Hence, the effect of EPS was not tested in our experiments but their formation during the 2 h desiccation period cannot be completely excluded. The formation of EPS during desiccation was shown by Roberson and Firestone (1992) for Pseudomonas strains in soil systems, but the incubation time still was considerably longer than in the present study.
Cell fragments and cytosol were able to strongly change the wettability of quartz. According to Miltner et al. (2012), cell wall fragments contribute to a large extent to SOM formation. A study by Schurig et al. (2013) in the forefield of the Damma glacier (Switzerland) revealed that the coating of minerals with fragments of cell envelopes turned the particle surfaces more hydrophobic. The ability of fatty acids, which are important constituents of the cell fragments, to induce water repellency of acid washed sand has been described in various studies (e.g., Graber et al. 2009; Mainwaring et al. 2013). The stability of cell fragments of dying Streptomyces strains was recently shown by Schütze et al. (2013). For that reason, the accumulation of fragments and the release of cytosol may influence the wettability of natural soils. We found BSA solution to induce a similar effect as cytosol, which suggests that proteins influence the wettability in general as was also described by Bialopiotrowicz and Janczuk (2001). Other types of proteins, like glycoproteins were also found to be positively correlated with the formation of SWR (Young et al. 2012). By using fragments and cytosol in a mixture, the cytosolic compounds could be identified as most important for determining the wetting properties. This may be due to a more homogeneous distribution of the cytosol and a coating of cell fragments by cytosolic substances.
The results of this study are in accordance with findings by Deo and Natarajan (1997, 1998), where the interaction of Bacillus polymyxa and Paenibacillus polymyxa with quartz decreased the wettability of the surfaces. A positive correlation between CA and surface coverage was also found for different size fractions of quartz sand treated with stearic acid (González-Peñaloza et al. 2013). However, previous results did not show the relevance of the physiological adaptation processes, i.e., decreased surface wettability of bacteria, for inducing water repellency.
Decreased wettability due to attachment of cells and cell fragments was also detected on the micro-scale by ESEM condensation experiments. This is in accordance with the observations of Polson et al. (2010), where bacterial and fungal attachment on quartz changed the micro-scale wettability from hydrophilic to hydrophobic. The ESEM micrographs of the hydrophobic material show a distinct drop formation with no direct connection between the drops. This leads to zones on the mineral surface where water is excluded and thus may have an impact on the micro-scale water distribution (Goebel et al. 2007). Furthermore, the micro-scale water repellency and associated variability may also have an influence on water transport (Hallett et al. 2004; Goebel et al. 2011). For that reason, attachment of bacteria and their residues may also have important consequences in case of the small-size fraction minerals used in our study, although the changes in the macroscopic CA were negligible. Our findings support the evidence for a microbial origin of SWR (Hallett and Young 1999; Or et al. 2007). However, so far, no mechanistic understanding of the role of microorganisms, their residues and stress response for the development of SWR is given in the literature. In our study, it could be clearly shown that bacteria, their residues and stress related response can directly affect the wettability of minerals. The accumulation of microbial cell wall fragments and their contribution to SOM formation as shown by Miltner et al. (2012) and Schurig et al. (2013) therefore will have an important effect on the wettability of soil aggregates and soils in general. We have shown that not only specific substances like long chain fatty acids (Horne and McIntosh 2000), but also the microorganisms themselves as well as their cell residues (which always will be present during growth and decay) are able to induce or increase SWR. This would also explain the observations of Hallett and Young (1999), who found a close relationship between increased microbial activity after nutrient additions and changes in SWR. The contribution of cell residues to SWR formation may also, in part, explain the observations by Hallett et al. (2001b), where the use of a bacterial biocide induced high levels of SWR. The induced death and decay of bacteria is accompanied by the release of cytosolic compounds and formation of cell envelope fragments, which may be responsible for the observed increase in SWR.
Furthermore, our findings can contribute to explain various other effects with respect to SWR. During desiccation, soil wettability changes from wettable to water repellent at the so called critical soil–water content (Dekker and Ritsema 1994; Doerr et al. 2000; Dekker et al. 2001). Due to the amphiphilic structure of many cytosolic components and the cell envelope fragments, the hydrophobic domains i.e., membrane lipids and fatty acids can be oriented away from the surface during drying and may form a hydrophobic coating on the mineral surface. The higher concentration of ions and the corresponding osmotic stress on microorganisms at lower water contents may also decrease their surface wettability. The drying and the induced osmotic stress may also partly explain the observed formation of fire-induced water repellency, where the high temperatures cause a drying of soil (Letey 2001).
The coating by cytosolic substances, cell wall fragments and bacterial cells can be the reason for subcritical SWR, where infiltration is interrupted due to hydrophobic spots (Tillman et al. 1989; Hallett et al. 2001a). This is supported by the micro-scale hydrophobicity observed in the ESEM condensation experiments. Small water-repellent spots also induced a shift in surface wettability from hydrophilic to hydrophobic in the study by Ustohal et al. (1998). In addition, the development of SWR after irrigation with treated sewage effluent is a widely observed phenomenon (Wallach et al. 2005). Assuming a high input of microorganisms not adapted to soil and various salts with the wastewater, the subsequent adaptation to osmotic stress of the added microorganisms as well as their death and decay may increase SWR. Development of SWR after nutrient addition with following high respiration rates was also observed by Hallett and Young (1999). In addition, the ability of cell wall fragments to induce SWR may also explain their long-term stabilization in SOM (Miltner et al. 2012). During transient drying of a soil, cell wall fragments may become covered by cell membrane lipids and thereby turn into dry micro spots, which cannot be biodegraded due to reduced water activity.
We used only pure minerals as model compounds and only one type of bacterium. Therefore, it will be necessary to study the observed effects with other microorganisms like gram-positive bacteria and to validate the findings for natural soils. Nevertheless, we demonstrated the general effects in non-complex systems and provided the proof of principle. Atomic force microscopy according to Cheng et al. (2009) to characterize the nano-scale hydrophobicity of the organo-mineral associations would be a helpful tool to get further insights.
Conclusions
By determining macro- and microscale CA, we found evidence for changes in macroscopic and microscopic wettability of model minerals induced by bacteria, their cell walls, cytosol and their response to osmotic stress. From these findings, we were able to derive a mechanistic process that may help in understanding the development of SWR induced by microorganisms, their residues and specific responses to stress. Various related phenomena, like subcritical water repellency and the development of SWR after irrigation with sewage effluents may also be explained by the findings of this study. Our results emphasize that macromolecular biogenic structures are more important for SWR than single classes of substances like fatty acids or waxes. They also highlight the importance of amphiphilic structures, like cell wall fragments, which adds to the hypothesized contribution of cell fragments to SOM formation (Miltner et al. 2012). Overall, the results of this study stress the significant impact of microorganisms and their residues on soil wetting properties.
References
Al-Tahhan RA, Sandrin TR, Bodour AA, Maier RM (2000) Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates. Appl Environ Microbiol 66:3262–3268
Bachmann J, Ellies A, Hartge KH (2000) Development and application of a new sessile drop contact angle method to assess soil water repellency. J Hydrol 231–232:66–75
Baumgarten T, Sperling S, Seifert J, von Bergen M, Steiniger F, Wick LY, Heipieper HJ (2012) Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl Environ Microbiol 78:6217–6224
Bialopiotrowicz T, Janczuk B (2001) Wettability and surface free energy of bovine serum albumin films. J Surfactants Deterg 4:287–292
Bisdom EB, Dekker LW, Schoute JF (1993) Water repellency of sieve fractions from sandy soils and relationships with organic material and soil structure. Geoderma 56:105–118
Blackwell PS (2000) Management of water repellency in Australia, and risks associated with preferential flow, pesticide concentration and leaching. J Hydrol 231–232:384–395
Cheng S, Bryant R, Doerr SH, Wright CJ, Williams PR (2009) Investigation of surface properties of soil particles and model materials with contrasting hydrophobicity using atomic force microscopy. Environ Sci Technol 43:6500–6506
Chenu C, Stotzky G (2002) Interactions between microorganisms and soil particles: an overview. In: Huang PM, Bollag JM, Senesi N (eds) Interactions between soil particles and microorganisms. Impact on the terrestrial ecosystem. Wiley, Chichester, pp 3–40
Churaev NV (2000) Liquid and vapor flows in porous bodies: surface phenomena. In: Topics in chemical engineering, vol 13. Gordon and Breach Science Publishers, Amsterdam
DeBano L (2000) Water repellency in soils: a historical overview. J Hydrol 231–232:4–32
Dekker LW, Ritsema CJ (1994) How water moves in a water repellent sandy soil: 1. Potential and actual water repellency. Water Resour Res 30:2507–2517
Dekker LW, Ritsema CJ (2000) Wetting patterns and moisture variability in water repellent Dutch soils. J Hydrol 231–232:148–164
Dekker LW, Ritsema CJ, Wendroth O, Jarvis N, Oostindie K, Pohl W, Larsson M, Gaudet J (1999) Moisture distributions and wetting rates of soils at experimental fields in the Netherlands, France, Sweden and Germany. J Hydrol 215:4–22
Dekker LW, Doerr SH, Oostindie K, Ziogas AK, Ritsema CJ (2001) Water repellency and critical soil water content in a dune sand. Soil Sci Soc Am J 65:1667–1674
Deo N, Natarajan KA (1997) Interaction of Bacillus polymyxa with some oxide minerals with reference to mineral beneficiation and environmental control. Miner Eng 10:1339–1354
Deo N, Natarajan KA (1998) Studies on interaction of Paenibacillus polymyxa with iron ore minerals in relation to beneficiation. Int J Miner Process 55:41–60
Doerr SH, Shakesby RA, Walsh RP (2000) Soil water repellency: its causes, characteristics and hydro-geomorphological significance. Earth Sci Rev 51:33–65
Doerr SH, Llewellyn CT, Douglas P, Morley CP, Mainwaring KA, Haskins C, Johnsey L, Ritsema CJ, Stagnitti F, Allinson G, others (2005) Extraction of compounds associated with water repellency in sandy soils of different origin. Soil Res 43:225–237
Feeney DS, Crawford JW, Daniell T, Hallett PD, Nunan N, Ritz K, Rivers M, Young IM (2006) Three-dimensional microorganization of the soil–root–microbe system. Microb Ecol 52:151–158
Franco CM, Clarke PJ, Tate ME, Oades JM (2000a) Hydrophobic properties and chemical characterisation of natural water repellent materials in Australian sands. J Hydrol 231–232:47–58
Franco CM, Michelsen PP, Oades JM (2000b) Amelioration of water repellency: application of slow-release fertilisers to stimulate microbial breakdown of waxes. J Hydrol 231–232:342–351
Goebel MO, Bachmann J, Woche SK, Fischer WR (2005) Soil wettability, aggregate stability, and the decomposition of soil organic matter. Geoderma 128:80–93
Goebel MO, Woche SK, Bachmann J, Lamparter A, Fischer WR (2007) Significance of wettability-induced changes in microscopic water distribution for soil organic matter decomposition. Soil Sci Soc Am J 71:1593–1599
Goebel MO, Bachmann J, Reichstein M, Janssens IA, Guggenberger G (2011) Soil water repellency and its implications for organic matter decomposition—is there a link to extreme climatic events? Glob Change Biol 17:2640–2656
González-Peñaloza FA, Zavala LM, Jordán A, Bellinfante N, Bárcenas-Moreno G, Mataix-Solera J, Granged AJ, Granja-Martins FM, Neto-Paixão HM (2013) Water repellency as conditioned by particle size and drying in hydrophobized sand. Geoderma 209–210:31–40
Graber ER, Tagger S, Wallach R (2009) Role of divalent fatty acid salts in soil water repellency. Soil Sci Soc Am J 73:541–549
Hallett PD, Young IM (1999) Changes to water repellence of soil aggregates caused by substrate-induced microbial activity. Eur J Soil Sci 50:35–40
Hallett PD, Baumgartl T, Young IM (2001a) Subcritical water repellency of aggregates from a range of soil management practices. Soil Sci Soc Am J 65:184–190
Hallett PD, Ritz K, Wheatley RE (2001b) Microbial derived water repellency in golf course soils. Int Turfgrass Soc Res J 9:518–524
Hallett PD, Nunan N, Douglas JT, Young IM (2004) Millimeter-scale spatial variability in soil water sorptivity. Soil Sci Soc Am J 68:352–358
Hartmans S, Smits JP, Van der Werf MJ, Volkering F, de Bont JA (1989) Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl Environ Microbiol 55:2850–2855
Horne DJ, McIntosh JC (2000) Hydrophobic compounds in sands in New Zealand—extraction, characterisation and proposed mechanisms for repellency expression. J Hydrol 231–232:35–46
Hurrass J, Schaumann GE (2006) Properties of soil organic matter and aqueous extracts of actually water repellent and wettable soil samples. Geoderma 132:222–239
Jungerius PD, de Jong JH (1989) Variability of water repellence in the dunes along the Dutch coast. Catena 16:491–497
Kleber M, Sollins P, Sutton R (2007) A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 85:9–24
Letey J (2001) Causes and consequences of fire-induced soil water repellency. Hydrol Process 15:2867–2875
Lopez CS, Heras H, Garda H, Ruzal S, Sanchez-Rivas C, Rivas E (2000) Biochemical and biophysical studies of Bacillus subtilis envelopes under hyperosmotic stress. Int J Food Microbiol 55:137–142
Ma’Shum M, Tate ME, Jones GP, Oades JM (1988) Extraction and characterization of water-repellent materials from Australian soils. J Soil Sci 39:99–110
Mainwaring KA, Morley CP, Doerr SH, Douglas P, Llewellyn CT, Llewellyn G, Matthews I, Stein BK (2004) Role of heavy polar organic compounds for water repellency of sandy soils. Environ Chem Lett 2:35–39
Mainwaring K, Hallin IL, Douglas P, Doerr SH, Morley CP (2013) The role of naturally occurring organic compounds in causing soil water repellency. Eur J Soil Sci 64:667–680
Miltner A, Bombach P, Schmidt-Brücken B, Kästner M (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry 111:41–55
Morales VL, Parlange J, Steenhuis TS (2010) Are preferential flow paths perpetuated by microbial activity in the soil matrix? A review. J Hydrol 393:29–36
Nunan N, Wu K, Young IM, Crawford JW, Ritz K (2002) In situ spatial patterns of soil bacterial populations, mapped at multiple scales, in an arable soil. Microb Ecol 44:296–305
Or D, Smets BF, Wraith JM, Dechesne A, Friedman SP (2007) Physical constraints affecting bacterial habitats and activity in unsaturated porous media—a review. Adv Water Resour 30:1505–1527
Polson EJ, Buckman JO, Bowen D, Todd AC, Gow MM, Cuthbert SJ (2010) An environmental-scanning-electron-microscope investigation into the effect of biofilm on the wettability of quartz. SPE J 15:223–227
Roberson EB, Firestone MK (1992) Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol 58:1284–1291
Roper MM (2004) The isolation and characterisation of bacteria with the potential to degrade waxes that cause water repellency in sandy soils. Aust J Soil Res 42:427–434
Schaumann GE, Braun B, Kirchner D, Rotard W, Szewzyk U, Grohmann E (2007) Influence of biofilms on the water repellency of urban soil samples. Hydrol Process 21:2276–2284
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394
Schurig C, Smittenberg RH, Berger J, Kraft F, Woche SK, Goebel MO, Heipieper HJ, Miltner A, Kaestner M (2013) Microbial cell-envelope fragments and the formation of soil organic matter: a case study from a glacier forefield. Biogeochemistry 113:595–612
Schütze E, Miltner A, Nietzsche S, Achtenhagen J, Klose M, Merten D, Greyer M, Roth M, Kästner M, Kothe E (2013) Live and death of Streptomyces in soil—what happens to the biomass? J Plant Nutr Soil Sci 176:665–673
Tillman RW, Scotter DR, Wallis MG, Clothier BE (1989) Water repellency and its measurement by using intrinsic sorptivity. Aust J Soil Res 27:637–644
Tucker KA, Karnok KJ, Radcliffe DE, Landry G, Roncadori RW, Tan KH (1990) Localized dry spots as caused by hydrophobic sands on bentgrass greens. Agron J 82:549–555
Ustohal P, Stauffer F, Dracos T (1998) Measurement and modeling of hydraulic characteristics of unsaturated porous media with mixed wettability. J Contam Hydrol 33:5–37
van Loosdrecht MC, Lyklema J, Norde W, Schraa G, Zehnder AJ (1987) The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol 53:1893–1897
Wallach R, Ben-Arie O, Graber ER (2005) Soil water repellency induced by long-term irrigation with treated sewage effluent. J Environ Qual 34:1910–1920
Wershaw R (1993) Model for humus in soils and sediments. Environ Sci Technol 27:814–816
Wick LY, Pasche N, Bernasconi SM, Pelz O, Harms H (2003) Characterization of multiple-substrate utilization by anthracene-degrading Mycobacterium frederiksbergense LB501T. Appl Environ Microbiol 69:6133–6142
Wingender J, Neu TR, Flemming HC (1999) What are bacterial extracellular polymeric substances? In: Wingender J, Neu TR, Flemming HC (eds) Microbial extracellular polymeric substances. Characterization, structure and function. Springer, Berlin, pp 1–19
Young IM, Feeney DS, O’Donnell AG, Goulding KW (2012) Fungi in century old managed soils could hold key to the development of soil water repellency. Soil Biol Biochem 45:125–127
Acknowledgments
We would like to thank the German Research Foundation (Priority Programme 1315 “Biogeochemical Interfaces in Soil”, MI 598/2-2 and BA 1359/9-2) for funding. We also thank A. Prager (Institute of Surface Modification, Leipzig, Germany) for her kind help with the SEM and J. Reichenbach and L. Y. Wick (both UFZ, Leipzig, Germany) for their help with the CA measurements. We thank three anonymous reviewers for their comments, which improved the manuscript significantly.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Rakesh Dev.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Achtenhagen, J., Goebel, MO., Miltner, A. et al. Bacterial impact on the wetting properties of soil minerals. Biogeochemistry 122, 269–280 (2015). https://doi.org/10.1007/s10533-014-0040-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-0040-9