Abstract
Given the environmental challenge caused by the wide use of polyacrylamide (PAM), an environmental-friendly treatment method is required. This study demonstrates the role of Acidovorax sp. strain PSJ13 isolated from dewatered sludge in efficiently degrading PAM. To be specific, the strain PSJ13 can degrade 51.67% of PAM in 96 h (2.39 mg/(L h)) at 35 °C, pH 7.5 and 5% inoculation amount. Besides, scanning electron microscope, X-ray photoelectron spectroscopy, liquid chromatography–mass spectrometry and high-performance liquid chromatography were employed to analyze samples, and the nitrogen present in the degradation products was investigated. The results showed that the degradation of PAM by PSJ13 started from the side chain and then mainly the –C–C– main chain, which produced no acrylamide monomers. As the first study to report the role of Acidovorax in efficiently degrading PAM, this work may provide a solution for industries that require PAM management.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyacrylamide (PAM), a synthetic linear polymer with a high molecular weight, is extensively utilized as a viscosity enhancer, flocculant, or soil conditioning agent (Xiong et al. 2018) in various industries, including sewage treatment (Guezennec et al. 2015), oil production (Abidin et al. 2012), and papermaking (Wong et al. 2006). However, its widespread application is associated with several challenges. In the sewage treatment process, for instance, PAM accumulates in dewatered sludge during the sludge dewatering process. Consequently, the water content of the dewatered sludge containing PAM remains at 70–80%, leading to agglomeration and difficulties in dispersion, as well as increased drying costs (Yu et al. 2015).
In oil recovery, PAM is employed to enhance the viscosity of injected water, subsequently improving water mobility and oil recovery (Al-Kindi et al. 2022). This process generates significant amounts of PAM-containing water, which necessitates treatment and disposal. Although PAM is considered nontoxic, its natural degradation produces acrylamide monomer, a substance that poses risks to both ecological environments and human health (Kolya and Tripathy 2014). Acrylamide monomer is a skin and respiratory irritant that can be absorbed through the skin and mouth. In higher animals, acrylamide absorption can damage the central nervous system, resulting in peripheral axonal disease (Erkekoglu and Baydar 2014; Pundir et al. 2019). Furthermore, acrylamide is classified as a Class 2A carcinogen by international research bodies, such as the European Food Safety Authority (Song et al. 2018). Consequently, the development of safe degradation methods for PAM is of utmost importance.The biodegradation of PAM has garnered significant attention due to its environmentally friendly nature and absence of secondary pollution (Joshi and Abed 2017; Nyyssola and Ahlgren 2019). Research on PAM biodegradation commenced in the 1990s, with subsequent studies exploring its degradation efficacy. Kay-Shoemake et al. (1998) investigated the biotransformation potential of PAM and demonstrated its use as an organic nitrogen source for soil microorganisms. Matsuoka et al. (2002) isolated Acinetobacter from soil samples, which could degrade 16–19% PAM under aerobic conditions at 37 °C. Ma et al. (2008) reported Clostridium bifermentans H1’s ability to degrade PAM under anaerobic conditions, achieving a degradation efficiency of 30.8%. Yu et al. (2015) isolated Pseudomonas putida HI47 from dewatered sludge, with the strain metabolizing PAM as its sole nutrient source and achieving a degradation efficiency of 31.1% within 7 days. Bao et al. (2010) isolated Bacillus cereus and Bacillus sp. from polymer flooding-produced water, which degraded 36.3% PAM under aerobic conditions at 40 °C for 7 days. Despite the demonstrated ability of various bacterial strains to biodegrade PAM, its large molecular volume and highly stable C–C and C–H covalent bonds within the main chain render it biologically resistant, resulting in low biodegradation efficiency (Li et al. 2015; Nyyssola and Ahlgren 2019; Sun et al. 2016).
To improve the biodegradability of PAM, researchers have shifted their focus to using biological combination strategies to degrade polymers after pretreatment, which demonstrates huge potential (Chen et al. 2021; Zhang et al. 2019). For example, Pi et al. (2015) constructed a Fenton/combined biological treatment ABR process for PAM-containing wastewater, reaching a removal rate of PAM up to 71.81% under the conditions of H2O2 5.3 mmol/L, Fe2+ 1.44 mmol/L, and pH 3.0 at 30 °C. Song et al. (2021) constructed an OUA multi-stage treatment system combining ozone and biological treatment, with the degradation efficiency of 500 mg/L PAM increasing from 33.29 to 48.40% when ozone changed from 15 to 30 g O3/g TOC. Such biological combination methods, which improve the degradation effect, brings about high energy consumption and considerable secondary pollution. In addition, the physical/chemical combined biological treatments usually produce acrylamide monomers (Xiong et al. 2018). Accordingly, it remains worthwhile to investigate and isolate efficient PAM-degradation strains and whether they will produce acrylamide monomer after biodegradation.
This study isolates PSJ13, an efficient PAM-degrading strain, from dewatered sludge, which can decompose polymers without producing acrylamide monomer. The degradation efficiency reaches 50% within 96 h under the optimized conditions. Strain PSJ13 is identified as Acidovorax sp. To our knowledge, this is the first report on the degradation of PAM by Acidovorax sp. The effects of factors, including temperature and pH, on bacteria degradation performance are investigated. Furthermore, this paper also studies the degradation characteristics of PAM by strain PSJ13.
Methods and materials
The sample of sludge and PAM
Sludge samples were collected from sewage treatment plants in Beibei District, Chongqing. The debris was removed with tweezers and other tools, and excess activated sludge was stored in a refrigerator at 4 °C. The PAM sample was obtained from Musheng Biotechnology Co. Ltd (Chongqing, China). The relative molecular weight of PAM was about 5 × 106.
Media
This study adopted two kinds of media. One was a Polymer Medium (PM) with PAM as the sole carbon and nitrogen source, and the other was Luria–Bertani (LB) as the enrichment medium. The composition of PM medium was as follows: NaCl 0.5 g/L, MgSO4·7H2O 0.51 g/L, CaCl2·2H2O 0.1 g/L, NaH2PO4·2H2O 0.65 g/L, K2HPO4·3H2O 1.4 g/L, FeSO4·7H2O 0.018 g/L. The composition of LB medium is as follows: NaCl 10 g/L, Yeast extract 5 g/L, Tryptone 10 g/L. The pH of LB was adjusted to 7.0, and the pH of PM was adjusted to 7.2–7.4 by adding NaOH or HCl, and sterilized in an autoclave sterilizer at 121 °C for 20 min before use.
Isolation of PAM-degrading strain
PM liquid medium containing 500 mg/L, 1000 mg/L, 1500 mg/L, and 2000 mg/L PAM was prepared. The culture flask containing 100 mL LB liquid medium and 10 g sludge sample was cultured on a shaker at 150 r/min and 25 °C for 2 days. Subsequently, 5 mL of the initial culture was added to 100 mL 500 mg/L PM and incubated at 150 r/min and 25 °C for 7 days. At the end of this stage, 5 mL of the medium was transferred to a fresh 1000 mg/L PM and incubated for 7 days. The steps were repeated until the PAM concentration in the PM medium reached 2000 mg/L. 1 mL of the final culture solution was obtained and put into 9 mL sterile water to prepare 6 gradient diluents of 10–1–10–6. 200 μ of each gradient dilution was transferred to PM solid medium, spread with a triangular coating rod, and cultured at 25 °C in an incubator.
Colonies with different morphological characteristics on PM medium were isolated and purified by continuous streaking. The purified strain was inoculated in 150 mL PM liquid medium. The culture was subjected to PAM degradation tests at 150 r/min and 25 °C aerobic conditions, during which the cell concentration and PAM degradation ability were evaluated. In addition, three bottles of uninoculated PAM medium were used as blank controls. The strain PSJ13 could grow with PAM as the sole nutrient source and showed a strong degradation ability to PAM, so the strain was selected for further study.
Identification of PAM-degrading strain PSJ13
Bacterial isolate identification was carried out via the amplification and sequencing of approximately 1400 bp of the bacterial 16S ribosomal DNA (rDNA). The universal primers employed for bacterial amplification were 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-AAGGAGGTGATCCAGCCGCA-3′). DNA extraction from the isolates was performed using a column-based method. The PCR amplification procedure involved a reaction system containing 21 μL PCR mix, 1 μL Primer F, 1 μL Primer R, and 2 μL DNA templates. This process was carried out over 35 cycles in a GeneAmp PCR 9700 thermal cycler from Applied Biosystems, with each cycle consisting of 96 °C for 20 s, 62 °C for 30 s, and 72 °C for 10 s. Initial denaturation was performed at 96 °C for 5 min, and final extension was carried out at 72 °C for 7 min.
The sequencing was completed by Wuhan Huada Gene Technology Co. Ltd. Sequence data were subsequently analyzed using BLAST (NCBI) to identify the bacteria. The phylogenetic tree was constructed using MEGA-X software, and the phylogenetic tree was drawn using the adjacency algorithm (bootstrap number = 1000).
The physiological and biochemical properties of strain PSJ13 were tested according to Bergey’s decisive bacteriology manual. The characteristics of Gram reaction, ammonia production, nitrate reduction, catalase, oxidase and amylase activity were studied. In addition, the morphology of strain PSJ13 was observed by SEM (Song et al. 2019a).
Inocula preparation for degradation studies
PSJ13 was cultured in LB medium at 25 °C, 150 r/min for 12 h. The bacteria were collected after centrifugation at 8000 r/min for 5 min, and then washed three times with phosphate buffer to prepare inoculum (OD600 = 1.0).
Biodegradation of PAM by strain PSJ13
Effects of culture conditions on degradation of PAM by PSJ13
In order to investigate the effect of culture conditions on strain PSJ13, the prepared inoculum was inoculated into the PM medium containing 500 mg/L PAM. The effects of culture temperature (20 °C, 30 °C, 35 °C, 45 °C, 55 °C), initial pH (4.5, 5.5, 6.5, 7.5, 8.5) and initial inoculum (1%, 2%, 3%, 4%, 5%) on the degradation of PAM by strain PSJ13 were studied. When designing the experiment on the effect of culture temperature, pH and initial inoculum on the degradation of PAM by strain PSJ13, five treatment groups and a control group were designed for each condition to determine the relevant indicators. The PAM concentration was determined after centrifugation of the degradation solution on the 2nd and 4th days, while cell concentration was determined on the 4th day.
Effects of exogenous additives on PAM degradation by strain PSJ13
Glucose, sucrose, tryptophan, yeast extract, NaNO3, NH4NO3, NH4Cl and (NH4)2SO4 were added to the PM as exogenous additives, and the concentration of exogenous additives was 100 mg/L. Similarly, the PAM concentration was determined after centrifugation of the degradation solution on the 2nd and 4th days, while cell concentration was determined on the 4th day.
Degradation characteristics of PAM by strain PSJ13
To study the degradation characteristics of strain PSJ13 on PAM, solid and liquid samples of original PAM and treated samples were prepared. The solid sample was prepared as follows: the culture solution before and after degradation was centrifuged at 8000 r/min for 15 min to remove the bacteria. And the supernatant was mixed with anhydrous ethanol (1:2, volume ratio) to make a mixture. After standing for 10 min, the mixture was centrifuged at 3000 r/min for 15 min to collect precipitates, which was repeated thrice. The precipitates were collected, washed with chloroform (3 times), and then dried in a freeze dryer for subsequent study. As for the liquid sample, the culture solution before and after degradation was centrifuged at 8000 r/min for 5 min to remove the bacteria, and the supernatant was filtered through a 0.45 μm filter membrane.
The morphological changes of PAM before and after degradation were observed by SEM (Hitachi S-4800, Japan). Changes in functional groups before and after PAM degradation were analyzed by FT-IR. FT-IR (Thermo Scientific Nicolet 6700, America) detection wavenumber is 400–4000/cm, detection mode is transmittance. Further, changes in functional groups before and after PAM degradation were analyzed by XPS, which was performed on an ESCALAB 250 spectrometer (Thermo Fisher Escalab 250Xi10300, America) equipped with a monochromatic Mg Kα X-ray source (1253.6 eV). The C1s electron binding energy corresponding to graphitic carbon was set at 284.6 eV and used as a reference to position the other peaks on the XPS spectra. High-performance liquid chromatography (HPLC) was used to detect the existence of acrylamide monomer. HPLC (Toshimazu LC-20AD, Japan) equipped with Ultimate XB-C18 (4.6 × 250 mm) chromatographic column. The mobile phase consisted of 90% water and 10% methanol. The flow rate was 1.0 mL/min. Detection wavelength 210 nm. The gradation of PAM molecules was preliminarily explored by LC–MS. LC–MS (Agilent 1290UPLC-Agilent QTOF6550) equipped with Waters BEH C18 (2.1 × 100 mm) chromatographic column. ESI + mode: voltage 4000 V.Total nitrogen, ammonium nitrogen (NH4+–N), nitrate nitrogen (NO3−–N), nitrite nitrogen (NO2−–N) and hydroxylamine (NH2OH) were determined by ultraviolet spectrophotometer to explore the changes of nitrogen forms during the degradation of PAM by strain PSJ13. In addition, solid samples were adopted for SEM, FI-IR, LC–MS, and XPS analysis, while liquid samples for HPLC, LC–MS and nitrogen forms analysis.
Analysis method
PAM concentration was determined by the starch-cadmium iodide method (Bao et al. 2010). The removal efficiency of PAM was calculated as follows:
where C0 and C1 represent the concentration of PAM before and after degradation, respectively. The concentration of TN, NH4+–N, NO3−–N, NO2−–N and NH2OH during PAM degradation were determined according to the water and wastewater test standard method (Gui et al. 2021).
Triplicate measurements were performed for each sample, and the results were expressed as mean ± standard error. In addition, Excel 2016, SPSS Statistics 19, and Avantage 5.5 were adopted for statistical analysis, and Origin Pro 9 and MEGA-X for plotting.
Result and discussion
Bacteria isolation and identification
23 strains that can grow on polymer medium were isolated from sludge during preliminary screening. Among them, strain PSJ13 exhibited high removal efficiency of PAM, with a degradation efficiency of 25% after culturing for 48 h, and 42% after 96 h, which outperformed microorganisms reported previously (Bao et al. 2010; Ma et al. 2008; Yu et al. 2015). In addition, the pH of the culture medium increased during the degradation, which was contrary to previous reports that microbial degradation of PAM produces carboxylic acids resulting in a decrease in pH (Sang et al. 2015). It’s speculated that such difference is caused by degradation pathway.
The 16S rDNA of the strain was amplified and sequenced. BLAST search results showed that the isolated strain had the closest relationship with the Acidovorax strain B78, with the similarity being 99%. The phylogenetic tree of strain PSJ13 was constructed by the neighbor-joining method (Fig. 1a). The nucleotide sequence determined in this study was stored in the GenBank database with accession number ON740684. Further, the authors explored the morphological, physiological and biochemical properties of Acidovorax PSJ13, and the morphological characteristics are shown in Fig. 1b and c, which reveals strain PSJ13 to be rod-shaped with a smooth surface and a length of 0.46 × 0.84 μm. Besides, the physiological and biochemical properties of strain PSJ13 were studied, and the biochemical results are listed in Table 1. The strain PSJ13 is Gram-negative, and its ammonia production and nitrate reduction performance was positive. The strain’s properties are similar to those of Acidovorax facilis, a rhizosphere microbe reported by Kim and Cho (2006) for the remediation of oil-contaminated soil.
Acidovorax sp., belonging to Proteobacteria β subclass, plays an important role in the degradation of refractory organic pollutants (Liao et al. 2021; Shehu and Alias 2019). There have been reports on its role in degrading benzene, chlorobenzene, polychlorinated biphenyl (Hirose et al. 2019), high concentrations of phenanthrene (Liao et al. 2021; Singleton et al. 2018), nitroaromatic compounds (Li et al. 2021; Pantke et al. 2012), cyclohexane (Salamanca et al. 2021) and other organic substances. Instead, there exists no report on its role in PAM degradation, which makes this paper the first one.
Effect of culture conditions on PAM degradation by Acidovorax PSJ13
The effects of temperature, pH and inoculum amount on the degradation efficiency of the polymer were also investigated. As shown in Fig. 2, the biodegradation of the polymer became weakened with increasing temperature. To be specific, the degradation percentage was only 12.02% when the strain was cultured at 55 °C for 96 h. The degradation rate of PAM by microorganisms was 26.21% after incubation at 30 °C (the optimum temperature for the growth of strain PSJ13) for 48 h, and the value reached 52.17% after 96 h (2.45 mg/(L h)). Moreover, low pH negatively impacts PAM degradation by strain PSJ13. Notably, when the pH ranges from 4.5 to 5.5, the degradation efficiency of PAM by microorganisms remains low, with the efficiency reaching only 20.69% after 96 h of incubation at pH 5.5. However, the degradation efficiency reaches its peak at a pH of 7.5. The degradation efficiency of the polymer was 29.37% at 48 h and 53.68% at 96 h (2.79 mg/(L h)). In terms of the inoculation amount of the culture medium, it was in direct proportion to the degradation efficiency of strain PSJ13, that is, the degradation efficiency of bacteria increased with higher inoculation amount. When the inoculation amount was 5%, the degradation rate of PAM reached 51.67% after 96 h culture (2.39 mg/(L h)). Therefore, the degradation efficiency of Acidivorous PSJ13 was far higher than that of Bacillus sp. (0.60 mg/(L h)) reported by (Bao et al. 2010) and Pseudomonas putida HI47 (0.93 mg/(L h)) reported by (Yu et al. 2015).
Previous studies have shown that the degradation efficiency of polymers by microorganisms varies greatly with different types of nutrients added, and the degradation pathways of polymers by strains also differ (Wen et al. 2010). This study adopted glucose, sucrose, peptone, yeast extract, NaNO3, NH4NO3, NH4Cl and (NH4)2SO4 as exogenous nutrients to explore the degradation effect of PSJ13 on polymer, and the concentration of each nutrient was 0.1 g/L. According to the results, the degradation efficiency of PAM after 48 h was low when glucose, sucrose, peptone and yeast extract were added, recording only 4%, 2%, 9% and 11%, respectively; while that of PAM was 18%, 30%, 26% and 22%, respectively, when NaNO3, NH4NO3, NH4Cl and (NH4)2SO4 were added. This indicated that when PAM was used as the sole nitrogen source, the addition of the carbon source was not conducive to the degradation of PAM. In contrast, when PAM was used as the sole carbon source, the addition of the nitrogen source could notably increase the bacteria degradation efficiency. Despite the lower degradation efficiency of PAM when adding an exogenous carbon source than that of adding a nitrogen source, the biomass concentration was higher, which supported that PSJ13 mainly used PAM as the carbon source. Accordingly, PSJ13 mainly employed the main chain –C–C– bond for the efficient biodegradation of PAM. Sun et al. (2016) also found that the degradation efficiency of PAM depends on the –C–C– backbone structure rather than the NH2-group.
The excess sludge normally features a temperature of 20–35 °C and pH 6.5–8.0, which puts strain PSJ13 in a favorable position to degrade PAM. In addition, the larger inoculation amount of the culture medium benefits the degradation efficiency. The addition of an external nitrogen source can effectively improve the degradation efficiency of the polymer with NH4NO3 being the most effective nitrogen source. Therefore, to improve the degradation effect of PSJ13 in actual practice, the inoculation amount of bacteria can be appropriately increased, and some nitrogen source substances such as NH4NO3 can be added.
Degradation characteristics of PAM by Acidovorax PSJ13
Morphological changes of PAM after degradation
Samples before and after the biodegradation of PAM were observed at ×10,000 and ×30,000 magnification by SEM to study the morphological changes of PAM degraded by strain PSJ13, as shown in Fig. 3. The samples before biodegradation had many cavities and complete structures, which varied greatly with those after biological treatment. Besides, the degradation products of strain PSJ13 exhibited cavity collapse, quantity reduction, loose and irregular structure, indicating the obvious treatment effect of strain PSJ13 on PAM.
Structure analysis of PAM biodegradation products
To explore the changes of functional groups in the process of PAM biodegradation, FT-IR, XPS and LC–MS were used to analyze the PAM without biodegradation and with biodegradation. According to Fig. 4a that illustrates the FT-IR results, the bands of 2800–3000/cm can be assigned to –C–C– extension (Song et al. 2017), and the energy band intensity of the degradation products at 2800–3000/cm decreased, which indicated the breaking of the main chain of –C–C– of PAM after biological treatment. Besides, the bands at 3400/cm and 1600/cm are –NH2 and –COOH vibration peaks of –CONH2, respectively (Song et al. 2019b), which weakened, indicating that the amide group has been hydrolyzed and the side chain group was decreasing. The reason may be that the breakage of the carbon main chain caused the shedding of the side chain group. In addition, the structure of PAM and degradation products was analyzed by XPS as shown in Fig. 4b. In the XPS spectrum, 284.8 eV, 287.88 eV and 288.90 eV correspond to the characteristic peaks of C- (C, H), CONH2, COOH of PAM (Sun et al. 2016). The peak area of C- (C, H) and COOH decreased from 59.46% and 7.16% to 23.18% and 4.34%, respectively, while –CONH2 disappeared during the degradation process, which is consistent with Sun’s findings (Sun et al. 2016). This implies that microorganisms play a role in degrading PAM, leading to alterations in its molecular structure. Figure 4c shows the LC–MS chromatography analysis of the polymer degradation products, according to which, the retention time and number of peaks of PAM degradation products increased, indicating that PAM was degraded into small molecular fragments with different degrees of polymerization (Yu et al. 2015). Moreover, we detected a –CN peak in the breakdown products. This, along with the observation that adding a nitrogen source boosts the efficiency of microbial degradation, suggests that the breakdown of PAM by Acidovorax PSJ13 mostly takes place in the main carbon chain structure, not the side chain group. Furthermore, these broken-down fragments have the ability to rejoin and form –CN. The chain breakage of PAM under aerobic condition was accompanied by the production of carbon dioxide. However, a peak also appeared at 286.23 eV, and the relative peak area after degradation was reduced, which has not been reported. The peak, which is assigned to bond –C–O–C/–C–OH according to the XPS spectrum library, may be caused by the introduction of related structural substances during the synthesis of PAM.
Changes of nitrogen forms during PAM biodegradation
Furthermore, the concentration of nitrogenous substances (TN, NH4+–N, NH2OH, NO2−–N and NO3−–N) during PAM degradation was determined as shown in Fig. 5. Specifically, the content of TN and NH4+ changed dramatically during PAM biodegradation. The concentration of TN decreased from 81.11 to 64.27 mg/L after 120 h, which indicates its conversion into other forms of nitrogen. The concentration of NH4+–N climbed rapidly with time going by, from 0.64 to 20.52 mg/L within 24 h and finally to 34.52 mg/L at 120 h, which demonstrates that the strain PSJ13 could degrade PAM to produce NH4+–N, and the strain’s good ammoniation ability. The rapid increase of NH4+–N concentration in 24 h indicated that PAM underwent deamination reaction during biodegradation, which further triggered the breakdown of the PAM main chain. To sum up, the large reduction of TN concentration and the formation of NH4+-N corresponded to the phenomenon that the peak of –CONH2 in the XPS spectrum disappeared during the degradation process. The concentration of NH2OH increased from 4.98 to 8.14 mg/L, showing an upward trend. The NO2−–N and NO3—N, whose amount was small with concentration of 0.1–0.2 mg/L, exhibited negligible changes. NO2− showed an upward trend, while NO3− showed a downward trend. The results demonstrated the good ammonification ability but weak nitrification ability of strain PSJ13. Although NH4+–N was oxidized to the intermediate product NH2OH, the further oxidation products NO2−–N and NO3−–N exhibited small concentration. Eventually, the accumulation of NH4+–N and the decrease of –COOH led to higher pH in the culture medium. The biodegradation of PAM is usually initiated by deamination (Nyyssola and Ahlgren 2019). Combined with the SEM results that showed the structural changes of the degradation products, the degradation of polymer by Acidovorax PSJ13 started with deamination, and then the main chain part, followed by the breaking of side chain.
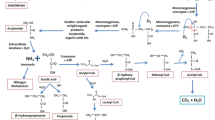
PAM biodegradation pathway hypothesis
The biodegradation of PAM is complex, with the degradation pathways of different bacteria varying. Bao et al. (2010). proposed that the main degraded part of PAM was –NH2, and the oxidized part was first the main chain part α-C and then –COOH, resulting in lower pH (Bao et al. 2010). Nyyssola et al. concluded that in addition to the oxidation of α-C, the oxidation of β-C also occurred in the main chain of PAM, that is, such oxidation led to the formation of a –C=C– between α-C and β-C, followed by the hydration of the –C=C–, and oxidation of the resulting β-OH group to –C=O (Nyyssola and Ahlgren 2019). Instead, this paper showed that the degradation of PAM by PSJ13 had an α-C oxidation pathway (–COOH change detected), and the existence of β-C oxidation could not be proved (–C=O change not detected).
The authors proposed a hypothetical PAM degradation pathway by Acidovorax PSJ13. The degradation process begins with deamination (①), as microorganisms require growth substrates for subsequent life activities (Nyyssola and Ahlgren 2019). The polymer –C–C– is then broken down into smaller molecules with varying degrees of polymerization and carbon dioxide (②④). This step involves weak oxidation of α-C, and the produced –COOH is utilized by microorganisms through metabolic pathways similar to the tricarboxylic acid cycle (Yin et al. 2015), resulting in a decrease in –COOH (⑤). Subsequently, –NH2 is removed to form NH4+–N; a small portion of NH4+–N is oxidized to NH2OH, NO2–N, and NO3–N, accompanied by gaseous nitrogen production due to the decrease in TN concentration (①). Moreover, the emergence of the –CN structure suggests that small molecular polymer fragments generated by the rupture of the polymer backbone may recombine in an unknown manner, and the –C=O of CONH2 may be broken (③). This process is illustrated in Fig. 6. The primary mode of degradation after physical and chemical treatment is typically the PAM main chain rupture. The main chain undergoes free radical chain reactions resulting from chain degradation, which can enhance PAM degradation efficiency (Song et al. 2018).
The backbone’s degradation may explain the role of strain PSJ13 in degrading polymers, and the fracture mode is similar to the way of polymer degradation by plasma reported by Song et al. (2019b). Such degradation mode seems to be beneficial to PAM degradation.
Detection of acrylamide monomer after PAM biodegradation
The PAM samples before and after degradation were analyzed to determine the existence of acrylamide monomer after degradation, which revealed a small amount of acrylamide monomer before and after the degradation of the polymer by HPLC. Instead of attributing such phenomenon to the degradation of PAM by strain PSJ13, the authors attribute it to PMthe trace amount of acrylamide monomer produced by PM, which results from the weak decomposition of the polymer caused by sterilization treatment (Table 2). Previous studies have also reported that PAM treated with physical methods, such as temperature and light, produces acrylamide monomers (Caulfield et al. 2002; Joshi and Abed 2017). The concentration of acrylamide monomer before and after biodegradation was 0.609 ± 0.058 μg/mL and 0.544 ± 0.093 μg/mL, respectively, which was not statistically significant (P < 0.05). Therefore, the degradation of PAM by strain PSJ13 do not produce acrylamide monomers.
Conclusion
This paper is the first to demonstrate the role of Acidovorax sp. in efficiently degrading PAM, and Acidovorax sp. Strain PSJ13 could degrade more than 50% PAM under optimal culture conditions after 96 h, with the degradation rate reaching 2.39 mg/(L h), significantly higher than previous reports. The degradation of PAM by PSJ13 started from the side chain and α-C oxidation, then mainly –C–C– main chain. The experiment also proved the absence of toxic acrylamide monomers during the biological breakdown of PAM.
References
Abidin AZ, Puspasari T, Nugroho WA (2012) Polymers for enhanced oil recovery technology. Procedia Chem 4:11–16. https://doi.org/10.1016/j.proche.2012.06.002
Al-Kindi S, Al-Bahry S, Al-Wahaibi Y, Taura U, Joshi S (2022) Partially hydrolyzed polyacrylamide: enhanced oil recovery applications, oil-field produced water pollution, and possible solutions. Environ Monit Assess 194(12):875. https://doi.org/10.1007/s10661-022-10569-9
Bao MT, Chen QG, Li YM, Jiang GC (2010) Biodegradation of partially hydrolyzed polyacrylamide by bacteria isolated from production water after polymer flooding in an oil field. J Hazard Mater 184(1–3):105–110. https://doi.org/10.1016/j.jhazmat.2010.08.011
Caulfield MJ, Qiao GG, Solomon DH (2002) Some aspects of the properties and degradation of polyacrylamides. Chem Rev 102(9):3067–3083. https://doi.org/10.1021/cr010439p
Chen H, Chen Z, Nasikai M, Luo G, Zhang S (2021) Hydrothermal pretreatment of sewage sludge enhanced the anaerobic degradation of cationic polyacrylamide (cPAM). Water Res 190:116074. https://doi.org/10.1016/j.watres.2020.116704
Erkekoglu P, Baydar T (2014) Acrylamide neurotoxicity. Nutr Neurosci 17(2):49–57. https://doi.org/10.1179/1476830513Y.0000000065
Guezennec AG, Michel C, Bru K, Touze S, Desroche N, Mnif I, Motelica-Heino M (2015) Transfer and degradation of polyacrylamide-based flocculants in hydrosystems: a review. Environ Sci Pollut Res 22(9):6390–6406. https://doi.org/10.1007/s11356-014-3556-6
Gui X, Li Z, Wang Z (2021) Kitchen waste hydrolysate enhances sewage treatment efficiency with different biological process compared with glucose. Bioresour Technol 341:12590. https://doi.org/10.1016/j.biortech.2021.125904
Hirose J, Fujihara H, Watanabe T, Kimura N, Suenaga H, Futagami T, Goto M, Suyama A, Furukawa K (2019) Biphenyl/PCB degrading bph genes of ten bacterial strains isolated from biphenyl-contaminated soil in Kitakyushu, Japan: comparative and dynamic features as Integrative Conjugative Elements (ICEs). Genes 10(5):404. https://doi.org/10.3390/genes10050404
Joshi SJ, Abed RMM (2017) Biodegradation of polyacrylamide and its derivatives. Environ Process 4(2):463–476. https://doi.org/10.1007/s40710-017-0224-0
Kay-Shoemake JL, Watwood ME, Lentz RD, Sojka RE (1998) Polyacrylamide as an organic nitrogen source for soil microorganisms with potential effects on inorganic soil nitrogen in agricultural soil. Soil Biol Biochem 30(8–9):1045–1052. https://doi.org/10.1016/S0038-0717(97)00250-2
Kim JY, Cho KS (2006) Bioremediation of oil-contaminated soil using rhizobacteria and plants. Kor J Microbiol Biotechnol 34(3):185–195
Kolya H, Tripathy T (2014) Biodegradable flocculants based on polyacrylamide and poly N, N-dimethylacrylamide grafted amylopectin. Int J Biol Macromol 70(8):26–36. https://doi.org/10.1016/j.ijbiomac.2014.06.028
Li YM, Sang GL, Pi YG, Lu JR, Bao MT (2015) Biodegradation for hydrolyzed polyacrylamide in the anaerobic baffled reactor combined aeration tank. Ecol Eng 84:121–127. https://doi.org/10.1016/j.ecoleng.2015.07.028
Li T, Gao YZ, Xu J, Zhang ST, Guo Y, Spain JC, Zhou NY (2021) A recently assembled degradation pathway for 2,3-dichloronitrobenzene in Diaphorobacter sp. strain J53051. Mbio 12(4):e02231-e2321. https://doi.org/10.1128/mBio.02231-21
Liao X, Luo J, Cassidy DP, Li Y, Tao H, Zhao Y (2021) Biodegradation of phenanthrene at high concentrations by Acidovorax sp JG5 and its functional genomic analysis. J Chem Technol Biotechnol 96(11):3142–3151. https://doi.org/10.1002/jctb.6867
Ma F, Wei L, Wang L, Chang CC (2008) Isolation and identification of the sulphate-reducing bacteria strain H1 and its function for hydrolysed polyacrylamide degradation. Int J Biotechnol 10(1):55–63. https://doi.org/10.1504/IJBT.2008.017979
Matsuoka H, Ishimura F, Takeda T, Hikuma M (2002) Isolation of polyacrylamide-degrading microorganisms from soil. Bioprocess Eng 7(5):327–330. https://doi.org/10.1007/bf02932844.pdf
Nyyssola A, Ahlgren J (2019) Microbial degradation of polyacrylamide and the deamination product polyacrylate. Int Biodeterior Biodegrad 139:24–33. https://doi.org/10.1016/j.ibiod.2019.02.005
Pantke C, Obst M, Benzerara K, Morin G, Ona-Nguema G, Dippon U, Kappler A (2012) Green rust formation during Fe(II) oxidation by the nitrate-reducing Acidovorax sp strain BoFeN1. Environ Sci Technol 46(3):1439–1446. https://doi.org/10.1021/es2016457
Pi Y, Zheng Z, Bao M, Li Y, Zhou Y, Sang G (2015) Treatment of partially hydrolyzed polyacrylamide wastewater by combined Fenton oxidation and anaerobic biological processes. Chem Eng J 273:1–6. https://doi.org/10.1016/j.cej.2015.01.034
Pundir CS, Yadav N, Chhillar AK (2019) Occurrence, synthesis, toxicity and detection methods for acrylamide determination in processed foods with special reference to biosensors: a review. Trends Food Sci Technol 85:211–225. https://doi.org/10.1016/j.tifs.2019.01.003
Salamanca D, Buehler K, Engesser KH, Schmid A, Karande R (2021) Whole-cell biocatalysis using the Acidovorax sp CHX100 Delta 6HX for the production of omega-hydroxycarboxylic acids from cycloalkanes. New Biotechnol 60:200–206. https://doi.org/10.1016/j.nbt.2020.10.009
Sang G, Pi Y, Bao M, Li Y, Lu J (2015) Biodegradation for hydrolyzed polyacrylamide in the anaerobic baffled reactor combined aeration tank. Ecol Eng 84:121–127. https://doi.org/10.1016/j.ecoleng.2015.07.028
Shehu D, Alias Z (2019) Dechlorination of polychlorobiphenyl degradation metabolites by a recombinant glutathione S-transferase from Acidovorax sp KKS102. FEBS Open Bio 9(3):408–419. https://doi.org/10.1002/2211-5463.12405
Singleton DR, Lee J, Dickey AN, Stroud A, Scholl EH, Wright FA, Aitken MD (2018) Polyphasic characterization of four soil-derived phenanthrene-degrading Acidovorax strains and proposal of Acidovorax carolinensis sp. nov. Syst Appl Microbiol 41(5):460-472. https://doi.org/10.1016/j.syapm.2018.06.001
Song W, Zhang Y, Gao Y, Chen D, Yang M (2017) Cleavage of the main carbon chain backbone of high molecular weight polyacrylamide by aerobic and anaerobic biological treatment. Chemosphere 189:277–283. https://doi.org/10.1016/j.chemosphere.2017.09.079
Song T, Li S, Ding W, Li H, Bao M, Li Y (2018) Biodegradation of hydrolyzed polyacrylamide by the combined expanded granular sludge bed reactor-aerobic biofilm reactor biosystem and key microorganisms involved in this bioprocess. Bioresour Technol 263:153–162. https://doi.org/10.1016/j.biortech.2018.04.121
Song T, Li S, Lu Y, Yan D, Sun P, Bao M, Li Y (2019a) Biodegradation of hydrolyzed polyacrylamide by a Bacillus megaterium strain SZK-5: functional enzymes and antioxidant defense mechanism. Chemosphere 231:184–193. https://doi.org/10.1016/j.chemosphere.2019.05.143
Song W, Zhang Y, Yu J, Gao Y, Naitoc T, Oinumac G, Inanagac Y, Yang M (2019b) Rapid removal of polyacrylamide from wastewater by plasma in the gas–liquid interface. J Environ Sci 083(009):1–7. https://doi.org/10.1016/j.jes.2019.03.015
Song T, Li S, Yin Z, Bao M, Lu J, Li Y (2021) Hydrolyzed polyacrylamide-containing wastewater treatment using ozone reactor-up flow anaerobic sludge blanket reactor-aerobic biofilm reactor multistage treatment system. Environ Pollut 269:116111. https://doi.org/10.1016/j.envpol.2020.116111
Sun M, Tong ZH, Cui YZ, Wang J (2016) Microbial metabolism induced chain shortening of polyacrylamide with assistance of bioelectricity generation. Sci Pollut Res 23(12):12140–12149. https://doi.org/10.1007/s11356-016-6409-7
Wen Q, Chen Z, Zhao Y, Zhang H, Feng Y (2010) Biodegradation of polyacrylamide by bacteria isolated from activated sludge and oil-contaminated soil. J Hazard Mater 175(1–3):955–959. https://doi.org/10.1016/j.jhazmat.2009.10.102
Wong SS, Teng TT, Ahmad AL, Zuhairi A, Najafpour G (2006) Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J Hazard Mater 135(1–3):378–388. https://doi.org/10.1016/j.jhazmat.2005.11.076
Xiong B, Loss RD, Shields D, Pawlik T, Hochreiter R, Zydney AL, Kumar M (2018) Polyacrylamide degradation and its implications in environmental systems. Npj Clean Water 1:17. https://doi.org/10.1038/s41545-018-0016-8
Yin X, Li J, Shin HD, Du G, Liu L, Chen J (2015) Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: advances and prospects. Biotechnol Adv 33(6):830–841. https://doi.org/10.1016/j.biotechadv.2015.04.006
Yu F, Fu RM, Xie Y, Chen WL (2015) Isolation and characterization of polyacrylamide-degrading bacteria from dewatered sludge. Int J Environ Res Public Health 12(4):4214–4230. https://doi.org/10.3390/ijerph120404214
Zhang L, Su F, Wang N, Liu S, Yang M, Wang YZ, Huo D, Zhao T (2019) Biodegradability enhancement of hydrolyzed polyacrylamide wastewater by a combined Fenton-SBR treatment process. Bioresour Technol 278:99–107. https://doi.org/10.1016/j.biortech.2019.01.074
Acknowledgements
This work was financially supported by the National Natural Science Fund of China (No. 42077217).
Author information
Authors and Affiliations
Contributions
Study design and organization were performed by ZW, XG, Experiment operation and sample analysis, writing-review and editing were performed by ZW, ZL, KL Conceptualization, formal analysis, writing-review and editing were performed by ZW, KL All authors improved the manuscript through comments and text suggestions and all authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Li, K., Gui, X. et al. Acidovorax PSJ13, a novel, efficient polyacrylamide-degrading bacterium by cleaving the main carbon chain skeleton without the production of acrylamide. Biodegradation 34, 581–595 (2023). https://doi.org/10.1007/s10532-023-10036-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-023-10036-3