Abstract
This study shows vanadate (V(V)) reduction in a methane (CH4) based membrane biofilm batch reactor when the concentration of dissolved oxygen (O2) was extremely low. V(IV) was the dominant products formed from V(V) bio-reduction, and majority of produced V(IV) transformed into precipitates with green color. Quantitative polymerase chain reaction and Illumina sequencing analysis showed that archaea methanosarcina were significantly enriched. Metagenomic predictive analysis further showed the enrichment of genes associated with reverse methanogenesis pathway, the key CH4-activating mechanism for anaerobic methane oxidation (AnMO), as well as the enrichment of genes related to acetate synthesis, in archaea. The enrichment of aerobic methanotrophs Methylococcus and Methylomonas implied their role in CH4 activation using trace level of O2, or their participation in V(V) reduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vanadium (V), a transition heavy metal, is derived from a variety of natural and human activities, including V-rich rocks and V associated industries (Bosch et al. 1989; Nriagu 1998; Wright and Belitz 2010; Yoo 1998). Although trace amount of V is beneficial to the organisms, V in water is always a concern due to its mutagenicity and cytotoxicity to human bodies (Altamirano-Lozano 1998). The maximum permitted V concentration in drinking water established by China was 0.05 mg/L (Standardization Administration of People’s Republic of China, 2006). It has been reported that the V concentration in the surface water in Panzhihua region, China, reached up to 0.3 mg/L, far exceeding the permitted limit (Zhang et al. 2019a). V in natural water usually exist as pentavalent (V(V)) and tetravalent (V(IV)) (Crans et al. 2004). V(V) is highly toxic and soluble, while V(IV) has much less toxicity and easily form into precipitates (Patel et al. 1989). Therefore, transforming V(V) to V(IV) is considered as an effective method to remove V from wastewaters (Ortiz-Bernad et al. 2004).
Several physico-chemical technologies have been applied to remove V in wastewaters, such as adsorption by metal (hydr) oxide and nanoparticles, ion exchange and co-precipitation (Naeem et al. 2007; de Godoi et al. 2013; Keränen et al. 2015; Blackmore et al. 1996). Due to the low expense and simplicity, bio-reduction of V(V) for V detoxification draws more attention in recent years (Carpentier et al. 2003; Xu et al. 2015; Zhang et al. 2014, 2018, 2019b). Electron donors, e.g., lactate, acetate, and glucose were needed for the bio-reduction process (Ortiz-Bernad et al. 2004; Carpentier et al. 2003; Zhang et al. 2014). In recent years, CH4 has been deemed to be a more promising electron donor compared with the traditional electron donors, as CH4 is nontoxic to environment, leaves less electron residues (due to its low water solubility), and has lower cost per electron equivalent (Lai et al. 2016a, b, 2018a, b, c; Lv et al., 2019). A variety of electron acceptors, including nitrate (NO3−), nitrite (NO2−), selenate (SeO42−), chromate (CrO42−), and bromate (BrO3−) have been documented to be reduced in CH4 supplying systems (Lai et al. 2016a, b, 2018a, b, c; Haroon et al. 2013; Ettwig et al. 2010; Lu et al. 2016).
Based on stoichiometric and energetic calculation, the occurrence of methane oxidation coupled to V(V) reduction is feasible (Without considering biomass synthesis, Rittmann and McCarty 2012):
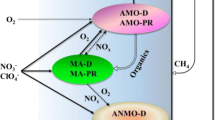
In our previous work, we demonstrated bio-reduction of V(V) and other electron acceptors in CH4 based membrane biofilm reactors (MBfR) (Lai et al. 2016a, b, 2018a, b; Luo et al. 2015). In that work, aerobic methane oxidation (AMO) played the major role, and released intermediate electrons that were utilized by oxyanions-reducing bacteria, thus transferring oxidized oxyanions to reduced form. Due to the involvement of O2, AMO process is energy intensive and has low electron utilizing efficiency. In contrast, anaerobic methane oxidation (AnMO) process does not need O2, saving more energy and expense. However, AnMO coupled to V(V) reduction (AnMO-VR) has never been reported.
AnMO could be carried out by bacteria affiliated to NC10 and anaerobic methanotrophic archaea (ANME) (Haroon et al. 2013; Ettwig et al. 2010). The bacteria affiliated to NC10 activated CH4 by “Intra-Aerobic” pathway. In this process, electron acceptor NO2− was reduced to NO, and further disproportionated to O2 intracellularly, which was used to oxidize CH4. ANME activated CH4 by “Reverse Methanogesis” pathway. Raghoebarsing et al. (2006) hypothesized ANME activate CH4 by reverse methanogenesis to generate electrons, which were then shuttled to denitrifying bacteria for denitrification. Moreover, Haroon et al. (2013) reported ANME-2d alone was able to carry out NO3− dependent AnMO through “Reverse Methanogesis” pathway. Key enzymes, e.g., methylcoenzyme M reductase (Mcr), tetrahydromethanopterin S-methyltransferase (Mtr), and methylenetetrahydromethanopterin cyclohydrolase (Mch) were involved in the “Reverse Methanogesis” pathway (Haroon et al. 2013). Although AnMO coupled to bio-reduction of NO3−, NO2−, SO42−, and Fe3+ have been reported (Haroon et al. 2013; Ettwig et al. 2010; Beal et al. 2009; Ettwig et al. 2016; Milucka et al. 2012), whether AnMO is able to reduce more oxyanions remained unknown.
Compared with AnMO, AMO is usually carried out by two bacterial groups: methanotrophs and oxyanions-reducing bacteria such as denitrifying bacteria. In this process, methanotrophs derive electrons from CH4 by incorporating O2, and methane mono-oxygenase plays a crucial role in activating CH4 (Modin et al. 2007). Electrons were released as intermediates such as acetate, methanol, formic acid and then used by co-existing denitrifying bacteria for bi-reduction of NO3− and NO2− (Modin et al. 2007).
However, a series of researches demonstrated methanotrophs played an important role in CH4 supplied culture when the concentration of surrounding O2 was extremely low or even zero. Bar-Or et al. (2017) reported increasing gene copies of methane monooxygenase pmoA, a key enzyme for AMO, in an iron-dependent AnMO culture. Martinez-Cruz et al. (2017) carried out anaerobic incubation for sub-Arctic lake sediments, finding aerobic methanotrophs, e.g., Methylobacter, assimilated most of carbon from CH4. Moreover, Siniscalchi et al. (2017) found the high abundance of aerobic methanotrophs dominating in a culture which performed AnMO coupled to denitrification process. So the absolute distinction between AMO and AnMO under low oxygen concentration was vague.
Thus, the first aim of this study is to test bio-reduction of V(V) in a CH4 based membrane biofilm batch reactor (MBBR) when the concentration of surrounding O2 was extremely low, and the second objective is to reveal the possible microbial mechanisms in the process. We assessed the microbial community in the biofilm by quantitative polymerase chain reaction (qPCR) and Illumina sequencing targeting methanogens’ and bacterial 16S rRNA genes, and predicted the metabolic genes associated with CH4 oxidation and V(V) reduction through PICRUSt pipline.
Materials and methods
Experimental setup
The MBBR system was comprised of a 1 L glass container, 42 hollow fibers, plastic caps and norprene tubings (Lai et al. 2018c). The fibers were inserted into a glass tube, glued together with AB gel (EC2216, Scotch-Weld, USA) and sealed within Norprene tubing. The MBBR system was similar to that of Lai et al. (2018c) except that a gas collection bag (Delin, Dalian) was connected to the system all the time without clamp by pinchcocks, which may cause O2 permeation at a very low concentration. All fibers were supplied with CH4 gas from both ends at the pressure of 10 psi, while a magnetic stirrer was applied to mix the liquids. O2 in the gas collection bag was determined every 5 days.
Startup and continuous operation
A culture from our previous V(V) reducing MBfR was inoculated into the MBBR (Lai et al. 2018a). The medium contained following ingredients (per L of demineralized water): Na2HPO4·12H2O 0.8 g, MgSO4· 7H2O 5 mg, NH4Cl 20 mg, CaCl2 1 mg, KaH2PO4 0.4 g, trace element solution 1 mL (Luo et al. 2015). The medium was degassed with argon (Ar) for 15 min before use. The MBBR operation was in sequencing batch mode and was divided into three stages, in which the introduced V(V) concentration was 10, 10 and 20 mg V/L in Stage 1, 2 and 3, respectively. V(V) was added again after V(V) was completely consumed for each stage. The MBBR contains 200 mL of headspace and 800 mL of liquid, and the working temperature was kept at 35 ± 1 °C. O2 in the MBBR was determined by GC (model 7890A, Agilent Technologies Inc., U.S.A), and the concentration in headspace was bellow detection limit (200 ppm, equivalent to 8 µg/L dissolved O2 in liquids by Henry’s law).
Chemical analysis
We sampled 0.5 mL liquids from the MBBR and filtered them using a 0.22 µm membrane filter for removing microbial biomass. The liquid samples were centrifuged (15,000×g, 10 min) to remove V(IV) precipitates. The concentrations of V(V) and V(IV) were assayed by spectrophotometric method as previously reported by Ensafi et al. (1999) and Safavi et al. (2000), respectively. pH in the liquids of MBBR system were measured by a pH meter and it was in the range of 7.0–7.5.
Biofilm sampling, DNA extraction and imagining
We conducted biofilm samplings in an anaerobic glove box (AW200SG, Electrotek, England). At the end of each stage, we cut off a section from a biofilm attached fiber (~ 5 cm in length) for DNA extraction, and the remained fiber was sealed. We extracted DNA from the biofilms using the DNeasy Blood and Tissue Kit as described previously (Lai et al. 2016a). At the end of Stage 3, ten fibers were cut off for SEM observation (Lai et al. 2014). After more than 250 days of running time (the V(V) removal data after day 156 was not shown in Fig. 1), precipitates were collected for characterization.
qPCR analysis of mcrA, and archaeal and bacterial 16S rRNA genes
We amplified the MBBR biofilms with primes MLf/MLr targeting mcrA (unit of methyl-coenzyme reductase) (Ceccarelli et al. 2014), ARC787F/ARC1059R targeting archaeal 16S rRNA gene (Yu et al. 2005), and 16SF/16SR targeting bacterial 16S rRNA gene (Maeda et al. 2003). We used plasmids that contain target fragments to build standard curves and serve as positive control for qPCR analysis. We utilized the SYBR Premix to perform qPCR for DNA samples and standard plasmids according to the instruction. Sterile water served as negative template, and three repetitions in qPCR reaction were performed for all samples.
Illumina sequencing
We amplified V9 and V4–V5 regions of 16S rRNA genes for methanogens and bacteria, respectively using Premix Taq 2.0 plus dye (Takara, Japan). The primer sets were 1106F/1378R (targeting methanogens) and 515F/907R (targeting bacteria) (Lai et al. 2016b; Feng et al. 2012). We purified the amplicons and sent them to Novogene Technology company to perform Illumina sequencing. The raw data were trimmed and processed using QIIME (version 1.9.1) (Lai et al. 2014). The metagenomic information associated with archaeal and bacterial 16S rRNA gene data were predicted using PICRUSt piplines (Langille et al. 2013; Xie et al. 2011).
V(IV) precipitates characterization
We recovered the V(IV) precipitates by sequential washing using deionized water, and 100% acetone for three times, and centrifugation (10,000×g, 2 min) was applied to separate the precipitates and supernatant. We applied SEM (Hitachi TM 1000) and EDS analyzer (Hitachi Se3400N VP-SEM–EDS) to determine the constituent of V(IV) precipitates, and conducted XPS (ESCALAB 250, Thermo Electron, USA) to measure the valent state.
Results and discussion
V(V) reduction in the CH4 based MBBR
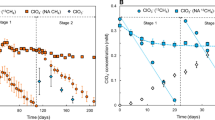
Figure 1a, b illustrated the measured V(V) concentrations throughout the whole experiments. CH4 supply was not limited, since the theoretical CH4 demand was much lower than the supplied CH4 flux shown in Table S1. The V(V) removal rates in Stage 2 (0.66 mg/L day) were higher than that in Stage 1 (0.43 mg/L day) (Table S1), as the microorganisms in the biofilm need adaption in Stage 1. However, the V(V) removal rates decreased slightly in Stage 3 (0.52 mg/L day) compared with Stage 2, might due to the negative impact of higher loading of V(V). Table S2 showed that the V(V) reduction mainly followed zero-order kinetics elucidated by linear regression model.
The color of the bulk liquids in the MBBR turned into green during the running of MBBR, implying the occurrence of V(IV) resulting from V(V) reduction. However, after centrifugation of the liquid samples, precipitates with green color occurred and the liquid phase turned into colourless. Figure 1b shows that less than 1.7 mg/L of V(IV) was detected by spectrophotometry. The concentration of precipitated V was up to 73.3 mg/L that was calculated by subtracting measured V(IV) from total added V(V) after 156 days’ running. These results suggest most of V(IV) formed as precipitates during V(V) reduction, which is further confirmed by SEM–EDS, and XPS characterization (Fig. S1).
We previously achieved bio-reduction of V(V) in a CH4 based MBfR, and the O2 concentration in the influent and effluent was between 0.1 and 0.2 mg/L. However, in this study, we found V(V) could be reduced in the CH4 based MBBR when O2 concentration was extremely low, as O2 in the MBBR system was undetectable by using GC (the lowest detection limit was 8 µg/L O2). Besides, V(IV) precipitates as the major production formed form this bio-process might also bring additional commercial benefits. V has been used in multiple industries, including pharmaceutical and atomic energy industry, petroleum refining, phthalic anhydrides and sulfuric acid production (Bosch et al. 1989; Nriagu 1998; Wright and Belitz 2010; Yoo 1998), and is also an important alloying element which can improve the hardness and durability of many kinds of materials (Gupta and Krishnamurthy 1992). The expanding demand and wide application of V makes it one of the most important metals for modern technology (Nriagu 1998; Ortiz-Bernad et al. 2004).
Microbial community
Figure 2a illustrated the qPCR results normalized to copies/cm2 of biofilm for archaeal 16S rRNA and mcrA, and bacterial 16S rRNA genes. The abundance of these genes increased continuously through whole experiments, implying both of archaea and bacteria were enriched. Figure 2b showed two archaeal methanogens, Methanobacterium (Kim et al. 1995) and Methanosarcina (Rotaru et al. 2014), dominated in the biofilm by Illumina sequencing targeting methanogenic 16S rRNA gene. The proportion of Methanosarcina increased from 13.5 to 34.5%, accompanied with decreased abundance of Methanobacterium from 38.6% to 4.6%, through Stage 1 to Stage 3.
Three clades of archaea, ANME-1, ANME-2, and ANME-3, has been reported to couple AnMO to bio-reduction of electron acceptors such as SO42−, NO3−, and Fe3+ (Haroon et al. 2013; Ettwig et al. 2016; Meyerdierks et al. 2010). Among these three clades, ANME-2 and ANME-3 belong to the Methanosarcinales, while Methanosarcina also belongs to this family (Meyerdierks et al. 2010; Sheehan et al. 2015). Therefore, the genus Methanosarcina has intimate phylogenetically relationship with ANME, implying Methanosarcina might have the similar metabolic capability with ANME. Considering some Methanosarcina species harbor the ability to reduce ferric iron (Fe(III)), this genus probably also played as vanadate reducer (Liu et al. 2011).
Figure 3 shows that methanotrophs, e.g., Methylococcus and Methylomonas, were the predominant bacteria in the biofilm. Although the relative abundance of these two genera decreased from inoculum to Stage 1, it increased again in the latter two stages. Notably, the relative abundance of Methylococcus increased from 5.4 to 23.7% throughout the whole experiments.
Here we propose three possible reasons explaining the persistence of aerobic methanotrophs Methylococcus and Methylomonas. Firstly, the inoculum contained high abundance of these aerobic methanotrophs and they were robust and able to bear hypoxia. Methylomonas denitrificans has been found to carry out CH4 oxidation even the surrounding O2 concentration was lower than 50 nM (Kits et al. 2015), while Methylococcaceae was able to live where O2 was scarce (Danilova et al. 2016). These two genus might oxidize CH4 using trace level of O2 in the system. Secondly, these aerobic methanotrophs might also play a role in AnOM, although their real contribution hasn’t been identified. Bar-Or et al.(2017) incubated anoxic slurry which had iron-dependent AnMO activity and found 13C-labeled CH4 was mainly assimilated by aerobic methanotrophs They proposed AnMO might be carried out by synergy between methanogens and aerobic methanotrophs. Similarly, Martinez-Cruz et al.(2017) applied stable isotope probing technologies targeting sub-Arctic lake sediments and found type I methanotroph Methylobacter assimilated carbon from CH4 in anaerobic condition. Thirdly, these aerobic methanotrophs might act as vanadate-reducers, as aerobic methanotrophs have versatile functions and they contain multiple reductases. Methylococcus capsulatus (Bath) has been peported to detoxify mercury ions (Hg(II)) through dissimilatory Hg(II) reduction (Boden and Murrell 2011), while Methylomonas denitrificans, sp. nov. type strain FJG1 had metabolic modules for NO3− reduction (Kits et al. 2015).
Proposed microbial mechanisms
We applied PICRUSt pipeline to in-depth excavate predicted metagenomic information to reveal the possible microbial mechanisms in the CH4 fed biofilm. As to methanogens, predicted genes encoding key enzymes related to (reverse) methanogenesis pathway, e, g., methyl-coenzyme M reductase (Mcr), tetrahydromethanopterin S-methyltransferase (Mtr), and methylenetetrahydromethanopterin cyclohydrolase (Mch), and formylmethanofuran dehydrogenase (Fmd), were detected (Fig. 4). Besides, majority of these genes were enriched during Stage 1 to Stage 3, suggesting the possible occurrence of AnMO by Methanosarcina-like methanogen through reverse-methanogenesis pathway. The detection of predicted gene encoding acetate kinase (Ack) in methanogens’ community suggested Methanosarcina-like archaea might be able to produce acetate, a versatile intermediate electron donor that can be used by other group for V(V) reduction (Ortiz-Bernad et al. 2004). The obvious increased abundance of predicted genes encoding ATP synthase (Atps) and sodium/proton antiporter (Mrp) suggested the AnMO coupled to V(V) reduction process might produce large amount of energy (ATP) for Methanosarcina’s biomass synthesis and metabolic activities (Stock et al. 1999; Beck and Rosen 1979).
For bacterial community, the predicted genes encoding nitrate reductase (Nar) and cytochrome C (Cytc) were enriched during Stage 1–3 (Fig. 5). Nitrate reductase has multiple functions and has been reported to reduce many types of oxyanions including SeO42−, ClO4− and BrO3− (Lai et al. 2016b, 2018b; Zhao et al. 2011), while Antipov and Khijiniak (2016) found nitrate reductase in Haloalkaliphilic Halomonas was involved in V(V) reduction. Myers et al. (2004) found Shewanella oneidensis MR-1 required Cytochrome C for carrying out V(V) reduction. These evidences supported that nitrate reductase and cytochrome C might also contribute to V(V) reduction in the CH4 fed biofilm. The genes associated with these enzymes might be assigned to aerobic methanotrophs such as Methylococcus and Methylomonas (Kits et al. 2015), or assigned to other bacterial groups performing V(V) reduction.
These results indicate the possible microbial mechanisms in the V(V) reducing CH4 fed biofilm when the concentration of O2 was extremely low: simultaneous AnMO that was carried out by Methanosarcina through reverse-methanogenesis pathway, and AMO that was carried out by Methylococcus and Methylomonas using trace level of O2 occurred, releasing intermediates such as acetate. Methanosarcina, aerobic methanotrophs or other bacterial group then utilized these intermediates as electron donors for V(V) bio-reduction.
Conclusion
In this research, continuous V(V) reduction was achieved in a CH4 fed biofilm with extremely low concentration of O2. Archaea Methanosarcina was enriched in the biofilm, accompanied with the increased of abundance of predictive genes associated with AnOM, implying its possible role in CH4 activation. Aerobic methanotrophs Methylococcus and Methylomonas might also activated CH4 by trace level of O2. More in-depth researches by using advanced molecular technologies including metatranscriptomics and proteomics are needed to confirm the microbial mechanisms.
References
Altamirano-Lozano M (1998) Genotoxic effects of vanadium compounds. Invest Clin 39(Suppl 1):39–47
Antipov AN, Khijiniak TN (2016) Vanadate reduction under alkaline conditions by haloalkaliphilic Halomonas strains. Microbiology 85:658–663. https://doi.org/10.1134/s0026261716060023
Bar-Or I, Elvert M, Eckert W, Kushmaro A, Vigderovich H, Zhu Q, Ben-Dov E, Sivan O (2017) Iron-coupled anaerobic oxidation of methane performed by a mixed bacterial-archaeal community based on poorly reactive minerals. Environ Sci Technol 51:12293–12301. https://doi.org/10.1021/acs.est.7b03126
Beal EJ, House CH, Orphan VJ (2009) Manganese- and iron-dependent marine methane oxidation. Science 325:184–187. https://doi.org/10.1126/science.1169984
Beck JC, Rosen BP (1979) Cation/proton antiport systems in Escherichia coli: properties of the sodium/proton antiporter. Arch Biochem Biophys 194:208–214. https://doi.org/10.1016/0003-9861(79)90611-8
Blackmore DPT, Ellis J, Riley PJ (1996) Treatment of a vanadium-containing effluent by adsorption/coprecipitation with iron oxyhydroxide. Water Res 30:2512–2516. https://doi.org/10.1016/0043-1354(96)00080-2
Boden R, Murrell JC (2011) Response to mercury (II) ions in Methylococcus capsulatus (Bath). FEMS Microbiol Lett 324:106–110. https://doi.org/10.1111/j.1574-6968.2011.02395.x
Bosch H, Bongers A, Enoch G, Snel R, Ross JRH (1989) Lithium-vanadium bronzes as model catalysts for the selective reduction of nitric oxide. Catal Today 4:139–154. https://doi.org/10.1016/0920-5861(89)85047-3
Carpentier W, Sandra K, Smet ID, Brigé A, Smet LD, Beeumen JV (2003) Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl Environ Microbiol 69:3636–3639. https://doi.org/10.1128/AEM.69.6.3636-3639.2003
Ceccarelli M, Galluzzi L, Migliazzo A, Magnani M (2014) Detection and characterization of Leishmania (Leishmania) and Leishmania (Viannia) by SYBR green-based real-time PCR and high resolution melt analysis targeting kinetoplast minicircle DNA. PLoS ONE 9:e88845. https://doi.org/10.1371/journal.pone.0088845
Crans DC, Smee JJ, Gaidamauskas E, Yang L (2004) The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 104:849–902. https://doi.org/10.1021/cr020607t
Danilova OV, Suzina NE, Van De Kamp J, Svenning MM, Bodrossy L, Dedysh SN (2016) A new cell morphotype among methane oxidizers: a spiral-shaped obligately microaerophilic methanotroph from northern low-oxygen environments. ISME J 10:2734–2743. https://doi.org/10.1038/ismej.2016.48
de Godoi FC, Rodriguez-Castellon E, Guibal E, Beppu MM (2013) An XPS study of chromate and vanadate sorption mechanism by chitosan membrane containing copper nanoparticles. Chem Eng J 234:423–429. https://doi.org/10.1016/j.cej.2013.09.006
Ensafi AA, Amini MK, Mazloum M (1999) Spectrophotometric reaction rate method for the determination of trace amounts of vanadium(V) by its catalytic effect on the oxidation of Nile blue with bromate. Anal Lett 32:1927–1937. https://doi.org/10.1080/00032719908542943
Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MMM, Schreiber F, Dutilh BE, Zedelius J, de Beer D, Gloerich J, Wessels HJCT, van Alen T, Luesken F, Wu ML, van de Pas-Schoonen KT, Op den Camp HJM, Janssen-Megens EM, Francoijs K-J, Stunnenberg H, Weissenbach J, Jetten MSM, Strous M (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548. https://doi.org/10.1038/nature08883
Ettwig KF, Zhu B, Speth D, Keltjens JT, Jetten MSM, Kartal B (2016) Archaea catalyze iron-dependent anaerobic oxidation of methane. PNAS 113:12792–12796. https://doi.org/10.1073/pnas.1609534113
Feng Y, Xu Y, Yu Y, Xie Z, Lin X (2012) Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol Biochem 46:80–88. https://doi.org/10.1016/j.soilbio.2011.11.016
Gupta CK, Krishnamurthy N (1992) Extractive metallurgy of vanadium (process metallurgy). Elsevier, Duivendrecht
Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan Z, Tyson GW (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500:567–570. https://doi.org/10.1038/nature12375
Keränen A, Leiviskä T, Salakka A, Tanskanen J (2015) Removal of nickel and vanadium from ammoniacal industrial wastewater by ion exchange and adsorption on activated carbon. Desalin Water Treat 53:2645–2654. https://doi.org/10.1080/19443994.2013.868832
Kim BK, Pihl TD, Reeve JN, Daniels L (1995) Purification of the copper response extracellular proteins secreted by the copper-resistant methanogen Methanobacterium bryantii BKYH and cloning, sequencing, and transcription of the gene encoding these proteins. J Bacteriol 177:7178–7185. https://doi.org/10.1128/jb.177.24.7178-7185.1995
Kits KD, Klotz MG, Stein LY (2015) Methane oxidation coupled to nitrate reduction under hypoxia by the gammaproteobacterium Methylomonas denitrificans, sp. nov. type strain FJG1. Environ Microbiol 17:3219–3232. https://doi.org/10.1111/1462-2920.12772
Lai C-Y, Yang X, Tang Y, Rittmann BE, Zhao H-P (2014) Nitrate shaped the selenate-reducing microbial community in a hydrogen-based biofilm reactor. Environ Sci Technol 48:3395–3402. https://doi.org/10.1021/es4053939
Lai C-Y, Wen L-L, Shi L-D, Zhao K-K, Wang Y-Q, Yang X, Rittmann BE, Zhou C, Tang Y, Zheng P, Zhao H-P (2016a) Selenate and nitrate bioreductions using methane as the electron donor in a membrane biofilm reactor. Environ Sci Technol 50:10179–10186. https://doi.org/10.1021/acs.est.6b02807
Lai C-Y, Zhong L, Zhang Y, Chen J-X, Wen L-L, Shi L-D, Sun Y-P, Ma F, Rittmann BE, Zhou C, Tang Y, Zheng P, Zhao H-P (2016b) Bioreduction of chromate in a methane-based membrane biofilm reactor. Environ Sci Technol 50:5832–5839. https://doi.org/10.1021/acs.est.5b06177
Lai C-Y, Dong Q-Y, Chen J-X, Zhu Q-S, Yang X, Chen W-D, Zhao H-P, Zhu L (2018a) Role of extracellular polymeric substances in a methane based membrane biofilm reactor reducing vanadate. Environ Sci Technol 52:10680–10688. https://doi.org/10.1021/acs.est.8b02374
Lai C-Y, Dong Q-Y, Rittmann BE, Zhao H-P (2018b) Bioreduction of antimonate by anaerobic methane oxidation in a membrane biofilm batch reactor. Environ Sci Technol 52:8693–8700. https://doi.org/10.1021/acs.est.8b02035
Lai C-Y, Lv P-L, Dong Q-Y, Yeo SL, Rittmann BE, Zhao H-P (2018c) Bromate and nitrate bioreduction coupled with poly-β-hydroxybutyrate production in a methane-based membrane biofilm reactor. Environ Sci Technol 52:7024–7031. https://doi.org/10.1021/acs.est.8b00152
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. https://doi.org/10.1038/nbt.2676
Liu D, Dong HL, Bishop ME, Wang HM, Agrawal A, Tritschler S, Eberl DD, Xie SC (2011) Reduction of structural Fe(III) in nontronite by methanogen Methanosarcina barkeri. Geochim Cosmochim Acta 75:1057–1071. https://doi.org/10.1016/j.gca.2010.11.009
Lu Y-Z, Fu L, Ding J, Ding Z-W, Li N, Zeng RJ (2016) Cr(VI) reduction coupled with anaerobic oxidation of methane in a laboratory reactor. Water Res 102:445–452. https://doi.org/10.1016/j.watres.2016.06.065
Luo Y-H, Chen R, Wen L-L, Meng F, Zhang Y, Lai C-Y, Rittmann BE, Zhao H-P, Zheng P (2015) Complete perchlorate reduction using methane as the sole electron donor and carbon source. Environ Sci Technol 49:2341–2349. https://doi.org/10.1021/es504990m
Lv P-L, Shi L-D, Wang Z, Rittmann B-E, Zhao H-P (2019) Methane oxidation coupled to perchlorate reduction in a membrane biofilm batch reactor. Sci Total Environ 667:9–15. https://doi.org/10.1016/j.scitotenv.2019.02.330
Maeda H, Fujimoto C, Haruki Y, Maeda T, Kokeguchi S, Petelin M, Arai H, Tanimoto I, Nishimura F, Takashiba S (2003) Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol Med Microbiol 39:81–86. https://doi.org/10.1016/S0928-8244(03)00224-4
Martinez-Cruz K, Leewis M-C, Herriott IC, Sepulveda-Jauregui A, Anthony KW, Thalasso F, Leigh MB (2017) Anaerobic oxidation of methane by aerobic methanotrophs in sub-Arctic lake sediments. Sci Total Environ 607–608:23–31. https://doi.org/10.1016/j.scitotenv.2017.06.187
Meyerdierks A, Kube M, Kostadinov I, Teeling H, Glöckner FO, Reinhardt R, Amann R (2010) Metagenome and mRNA expression analyses of anaerobic methanotrophic archaea of the ANME-1 group. Environ Microbiol 12:422–439. https://doi.org/10.1111/j.1462-2920.2009.02083.x
Milucka J, Ferdelman TG, Polerecky L, Franzke D, Wegener G, Schmid M, Lieberwirth I, Wagner M, Widdel F, Kuypers MMM (2012) Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 491:541–546. https://doi.org/10.1038/nature11656
Modin O, Fukushi K, Yamamoto K (2007) Denitrification with methane as external carbon source. Water Res 41:2726–2738. https://doi.org/10.1016/j.watres.2007.02.053
Myers JM, Antholine WE, Myers CR (2004) Vanadium(V) reduction by Shewanella oneidensis MR-1 requires menaquinone and cytochromes from the cytoplasmic and outer membranes. Appl Environ Microbiol 70:1405–1412. https://doi.org/10.1128/AEM.70.3.1405-1412.2004
Naeem A, Westerhoff P, Mustafa S (2007) Vanadium removal by metal (hydr)oxide adsorbents. Water Res 41:1596–1602. https://doi.org/10.1016/j.watres.2007.01.002
Nriagu JO (1998) Vanadium in the environment (Part 1, Chemistry and biochemistry). Advances in environmental science and technology. Wiley, New York
Ortiz-Bernad I, Anderson RT, Vrionis HA, Lovley DR (2004) Vanadium respiration by geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl Environ Microbiol 70:3091–3095. https://doi.org/10.1128/AEM.70.5.3091-3095.2004
Patel B, Haswell SJ, Grzeskowiak R (1989) Flow injection flame atomic absorption spectrometry system for the pre-concentration of vanadium(V) and characterisation of vanadium(IV) and -(V) species. J Anal At Spectrom 4:195–198. https://doi.org/10.1039/JA9890400195
Raghoebarsing AA, Pol A, van de Pas-Schoonen KT, Smolders AJP, Ettwig KF, Rijpstra WIC, Schouten S, Damsté JSS, den Camp HJMO, Jetten MSM, Strous M (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440:918. https://doi.org/10.1038/nature04617
Rittmann BE, McCarty PL (2012) Environmental biotechnology: principles and applications. Tata McGraw-Hill Education, New Delhi
Rotaru A-E, Shrestha PM, Liu F, Markovaite B, Chen S, Nevin KP, Lovley DR (2014) Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl Environ Microbiol 80:4599–4605. https://doi.org/10.1128/AEM.00895-14
Safavi A, Hormozi Nezhad MR, Shams E (2000) Highly selective and sensitive kinetic spectrophotometric determination of vanadium(IV) in the presence of vanadium(V). Anal Chim Acta 409:283–289. https://doi.org/10.1016/S0003-2670(99)00794-1
Sheehan R, McCarver AC, Isom CE, Karr EA, Lessner DJ (2015) The Methanosarcina acetivorans thioredoxin system activates DNA binding of the redox-sensitive transcriptional regulator MsvR. J Ind Microbiol Biotechnol 42:965–969. https://doi.org/10.1007/s10295-015-1592-y
Siniscalchi LAB, Leite LR, Oliveira G, Chernicharo CAL, de Araújo JC (2017) Illumina sequencing-based analysis of a microbial community enriched under anaerobic methane oxidation condition coupled to denitrification revealed coexistence of aerobic and anaerobic methanotrophs. Environ Sci Pollut Res 24:16751–16764. https://doi.org/10.1007/s11356-017-9197-9
Standardization Administration of People’s Republic of China (2006) Standard for drinking water quality: GB5749-2006. Standards Press of China, Beijing
Stock D, Leslie AGW, Walker JE (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286:1700–1705. https://doi.org/10.1126/science.286.5445.1700
Wright MT, Belitz K (2010) Factors controlling the regional distribution of vanadium in groundwater. Groundwater 48:515–525. https://doi.org/10.1111/j.1745-6584.2009.00666.x
Xie W, Wang F, Guo L, Chen Z, Sievert SM, Meng J, Huang G, Li Y, Yan Q, Wu S, Wang X, Chen S, He G, Xiao X, Xu A (2011) Comparative metagenomics of microbial communities inhabiting deep-sea hydrothermal vent chimneys with contrasting chemistries. ISME J 5:414–426. https://doi.org/10.1038/ismej.2010.144
Xu X, Xia S, Zhou L, Zhang Z, Rittmann BE (2015) Bioreduction of vanadium(V) in groundwater by autohydrogentrophic bacteria: mechanisms and microorganisms. J Environ Sci 30:122–128. https://doi.org/10.1016/j.jes.2014.10.011
Yoo JS (1998) Metal recovery and rejuvenation of metal-loaded spent catalysts. Catal Today 44:27–46. https://doi.org/10.1016/S0920-5861(98)00171-0
Yu Y, Lee C, Kim J, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89:670–679. https://doi.org/10.1002/bit.20347
Zhang J, Dong H, Zhao L, McCarrick R, Agrawal A (2014) Microbial reduction and precipitation of vanadium by mesophilic and thermophilic methanogens. Chem Geol 370:29–39. https://doi.org/10.1016/j.chemgeo.2014.01.014
Zhang BG, Qiu R, Lu L, Chen X, He C, Lu JP, Ren ZJ (2018) Autotrophic vanadium(V) bioreduction in groundwater by elemental sulfur and zerovalent iron. Environ Sci Technol 52:7434–7442
Zhang B, Wang S, Diao M, Fu J, Xie M, Shi J, Liu Z, Jiang Y, Cao X, Borthwick AGL (2019a) Microbial community responses to vanadium distributions in mining geological environments and bioremediation assessment. J Geophys Res Biogeosci 124:601–615. https://doi.org/10.1029/2018JG004670
Zhang BG, Cheng YT, Shi JX, Xing X, Zhu YL, Xu N, Xia JX, Borthwick AGL (2019b) Insights into interactions between vanadium (V) bio-reduction and pentachlorophenol dechlorination in synthetic groundwater. Chem Eng Sci 375:121965
Zhao H-P, Van Ginkel S, Tang Y, Kang D-W, Rittmann B, Krajmalnik-Brown R (2011) Interactions between perchlorate and nitrate reductions in the biofilm of a hydrogen-based membrane biofilm reactor. Environ Sci Technol 45:10155–10162. https://doi.org/10.1021/es202569b
Acknowledgements
Authors greatly thank the “Natural Science Funds for Distinguished Young Scholar of Zhejiang Province (LR17B070001)”, “National Natural Science Foundation of China (Grant Nos. 21577123, 51878596)” and the “National Key Technology R&D Program (2018YFC1802203)” for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Z., Shi, LD., Lai, CY. et al. Methane oxidation coupled to vanadate reduction in a membrane biofilm batch reactor under hypoxic condition. Biodegradation 30, 457–466 (2019). https://doi.org/10.1007/s10532-019-09887-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-019-09887-6