Abstract
One of the most important steps in identifying priority areas for conservation is the assessment of species richness and their extinction risks. While most species remain undescribed, the identification of cryptic lineages is frequent in phylogenetic and phylogeographic studies. This is particularly common in troglobites, exclusively subterranean organisms. The Jandaíra Formation, in the Brazilian semiarid, combines the occurrence of extensive karstic areas with hundreds of caves and subterranean aquifers in a region with intense paleoclimatic changes. This region is recognized for the richness of troglobitic species, some of which are widely distributed in heterogeneous areas. This suggests cryptic lineages that can be differentially exposed to anthropogenic threats, with distinct extinction risks regarding the nominal taxa of which they putatively belong. To test it, a large sampling was conducted and, by means of lineage delimitation analyses, the genetic structure of four troglobitic taxa, three aquatic and one terrestrial, was evaluated. In addition, the extinction risk of these lineages was assessed and priority areas for conservation were identified. The results indicated that while Cirolanidae sp. 1 (Isopoda) is a single species widely distributed, Cirolanidae sp. 2, Potiberaba porakuara (Amphipoda) and Kinnapotiguara troglobia (Hemiptera) present an extensive diversity of cryptic and endemic lineages, most of which are likely new threatened species. Furthermore, two priority areas for conservation of these lineages were identified. Thus, comparative phylogeography may represent a first step in the conservation of subterranean taxa, indicating areas that should be prioritized in a context of increasing threats and dwindling conservation resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rising anthropogenic threats to biodiversity make imperative conservation actions grounded in evolutionary patterns to safeguard endangered species (Smith et al. 2014; Nielsen et al. 2017). Within this framework, mitigating habitat loss presents a significant challenge, and the accurate assessments of species richness and endemism are essential to identify priority areas for conservation (Myers et al. 2000; Bickford et al. 2007). A pivotal step in this process involves assessing species’ risk of extinction, based on their geographic distribution and/or population dynamics, in addition to actual and potential threats they face (IUCN 2022).
Notwithstanding, most species remain undescribed, and efforts to catalog biodiversity need to be prioritized (Bickford et al. 2007). Species have been described based on the phenetic or morphological species concept (Mayden 1997). Nevertheless, speciation does not always result in significant morphological differences. Therefore, the identification of species complexes has important implications for conservation (Bickford et al. 2007), as these are often composed of allopatric lineages with restricted range and higher risk of extinction (Ralls et al. 2018). Furthermore, comparative analyses among taxa of the same region offer insights into geographic patterns, revealing shared evolutionary histories and endemism hotspots that substantiate the prioritization of conservation areas (Bickford et al. 2007).
There is a growing consensus that species are lineages that evolve separately (Padial et al. 2010), and can be delimited using the criteria of genetic isolation (Fišer et al. 2018). Thus, molecular lineage delimitation analyses, including the use of Operational Taxonomic Units (OTUs), are essential for guiding conservation efforts, especially for understudied groups and those in extreme environments, as subterranean habitats (Carstens et al. 2013; Fišer et al. 2018).
Molecular studies of exclusively subterranean organisms, so called troglobites, frequently revealed these species are strikingly diversified species complexes (Finston et al. 2007; Trontelj et al. 2009; Niemiller et al. 2012, 2013; Fišer et al. 2018). Troglobites generally exhibit morphological, physiological, and behavioral specializations that result from evolution in subterranean environments and absence of selective pressures typical of surface habitats (Culver and Pipan 2009). These specializations may result in lower dispersal capacity, favoring isolation and speciation. Therefore, troglobites often exhibit restricted distribution, sometimes confined to a single cave (Mammola et al. 2015). Despite their unique biodiversity and conservation significance, subterranean habitats are typically overlooked in global conservation efforts (Mammola et al. 2019). Protected areas generally fail to represent both terrestrial (Colado et al. 2023) and aquatic (Iannella et al. 2021; Premate et al. 2024) subterranean biodiversity. In Brazil, the risks also involve recent legislative changes which can lead to the destruction of the country’s most significant caves (Ferreira et al. 2022).
Studies in subterranean biology have historically focused on temperate regions, mainly in Europe and USA, however tropical regions of Australia and Brazil has uncovered notable troglobitic species richness (White et al. 2019). One of these is the northeastern Brazilian semiarid, with extensive karstic areas and large concentrations of caves in limestone outcrops of the Jandaíra Formation, deposited during Upper Cretaceous marine transgressions (Bezerra et al. 2007; Rabelo et al. 2020; CANIE/CECAV 2022). In the context of South America, the western region of this Formation is unique, hosting the only cave-restricted oceanic relicts currently known in Brazil. It has a great richness of troglobitic species associated to the aquifers and paleoclimatic changes (Ferreira et al. 2010; Fisĕr et al. 2013; Souza et al. 2018; Bento et al. 2021).
As seen in other regions of the world (Holsinger 2000), the majority of the over 70 known troglobitic species in this area exhibit restricted distributions (Bento et al. 2021). However, four taxa have relatively broader ranges, three of which are aquatic (also called stygobites) and one terrestrial: (i) two undescribed species of cirolanid isopods; (ii) the amphipod Potiberaba porakuara (Amphipoda: Mesogammaridae) (iii) and the troglobitic planthopper Kinnapotiguara troglobia (Hemiptera: Kinnaridae) (Ferreira et al. 2010; Fisĕr et al. 2013; Hoch and Ferreira 2013; Bento et al. 2021). These described species are categorized as vulnerable on the Brazilian list of endangered species (MMA 2022). Nevertheless, only a small portion of their distribution falls within protected areas, and most caves and karst springs are subjected to various anthropogenic impacts (Cruz et al. 2010; Ferreira et al. 2010).
These troglobitic arthropods are excellent models for exploring evolutionary patterns and diversification in subterranean aquatic and terrestrial environments. Several studies showed that intraspecific genetic diversity is spatially structured, with more diverse areas often coinciding across multiple species (Hewitt 1996), which results in genetic diversity hotspots (Carnaval et al. 2014). Nevertheless, few studies have incorporated comparative phylogeography in the identification of priority areas for conservation (Carvalho et al. 2017). Therefore, it is expected that troglobitic taxa with broad geographic ranges consist of multiple cryptic lineages. Consequently, these lineages may face distinct anthropogenic threats, having greater risks of extinction compared to their putative nominal taxa.
Using phylogenetic and phylogeographic analyses, integrated with systematic conservation planning (SCP) tools, we aimed to: (i) assess the patterns of genetic variation of the target taxa, investigating their potential cryptic diversity and delimiting OTUs; (ii) verify threats and extinction risks of these taxa; (iii) apply the International Union for Conservation of Nature (IUCN) categories and criteria method (IUCN 2022) for each OTU; and (iv) identify priority areas for conservation of these cryptic lineages, in order to address conservation strategies, even before their formal description.
This is the first comparative molecular phylogeographic analysis focusing on troglobitic invertebrates from the Caatinga biome. The broad sampling, which includes the entire known geographic distribution of the target taxa, allowed the investigation of patterns of diversification and the factors that influenced it. Thus, this study contributes not only to the understanding of the evolution but also for the conservation of this troglobitic fauna from the Brazilian semiarid.
Methods
Study area and sampling sites
The study area is in the Caatinga biome, unique to Brazil and the largest seasonally dry forest in South America (Silva et al. 2017). It is the result of several paleoclimatic changes that have occurred since the Miocene, responsible for the diversification and adaptation of several animal and plant taxa to an increasingly arid and strongly seasonal climate (Werneck 2011; Dupont et al. 2013; Costa et al. 2018; Silva and Souza 2018). Additionally, the northern Caatinga has experienced many sea level fluctuations since the upper Cretaceous, with significant events also in the Miocene (Rosseti et al. 2013; Bagni et al. 2020). Currently it presents a climate predominantly of BSh type on the Köppen classification, indicating a hot and dry (B) semiarid (S) climate in areas of low latitude and altitude (h) (Alvares et al. 2013). The average annual precipitation is around 800 mm (70% can fall in a single month), which leads to high evaporation rates and water deficit throughout the year (Silva et al. 2017).
The study encompassed 31 caves and karst springs in the Jandaíra Formation, situated in several municipalities across Rio Grande do Norte (RN) and Ceará (CE) states, northeastern Brazil (Table S1, Fig. S1). The Jandaíra Formation, extending over an area of over 13,000 km² in northern RN and northeastern CE, represents a carbonate ramp with outcrops along the emersed part of the Potiguar basin (Bezerra et al. 2007). There are also several intensely karstified limestone outcrops (locally known as lajedos) which host most of the more than 1,000 known caves (Cruz et al. 2010; Rabelo et al. 2020; CANIE/CECAV 2022).
The caves and karst springs are inserted in two hydrographic basins, the Apodi-Mossoró river basin and the coastal microbasins (Table S1, Fig. S1). A unique feature is the Crotes cave in Felipe Guerra (RN), which hosts an epikarstic drainage disconnected from the water table, located in the Arapuá stream microbasin, Apodi-Mossoró river basin (Fig. S1k).
Four caves lie within the Furna Feia National Park (FFNP) (Table S1, Fig. S1), established in 2012 to protect a significant aggregation of caves and the biodiversity of the Caatinga biome (Brasil 2012).
Invertebrate sampling
In addition to caves with target group records (Ferreira et al. 2010; Bento et al. 2021), collections were extended to other caves and karst springs within the study area. All potential habitats, such as wet areas with roots (for K. troglobia) or subterranean lakes, streams, roots and submerged organic matter (for aquatic invertebrates) were visually inspected. K. troglobia individuals were manually gathered using tweezers and brushes, while aquatic invertebrates were collected employing aquarium nets and traps baited with goat liver pieces, left submerged for 24 h. All invertebrates were preserved in 99.5% absolute ethanol and stored at -20o C.
DNA extraction, amplification and sequencing
Total DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen), following the manufacturer’s recommendations. Partial cox1 gene sequences were obtained and amplified by polymerase chain reaction (PCR) with LCO-1490 and HCO-2198 primers (Folmer et al. 1994). Despite being a single gene, cox1 is widely utilized as a marker across most animal phyla and serves as a well-established “barcoding” system for animal species identification (Hebert et al. 2003; Fišer et al. 2018). Numerous studies have demonstrated correspondence between cryptic lineages identified through mitochondrial DNA, typically using the cox1 marker, and groupings delineated from nuclear markers, including genomic data. Among Arthropoda, such studies have encompassed insects (Janzen et al. 2017; Kozlov et al. 2017; Dincă et al. 2013), as well as isopods (Guzik et al. 2019) and stygobitic amphipods (Zakšek et al. 2019). The primer sequences, PCR parameters and sequencing reactions are provided in Supplementary Material 2.
Data Analysis
Consensus sequences were assembled using Geneious Prime (https://www.geneious.com/prime/) and aligned using ClustalW (Thompson et al. 1994) using MEGA X (Kumar et al. 2018), followed by visual inspection.
In phylogenetic analyses, only single haplotypes were used, except when shared between different localities (to retain geographic distribution information), defined after haplotype distribution using DNAsp v.5 (Librado and Rozas 2009). It was also used to build a haplotype network, using TCS network, in PopART (Leigh and Bryant 2015). A spatial analysis of molecular variance (SAMOVA, Dupanloup et al. 2002) was carried out to evaluate structuring patterns and determine the optimal number of populations within each target taxon.
The nucleotide substitution model for each dataset was selected using JmodelTest 2 (Darriba et al. 2012). Applying the selected evolutionary model, Bayesian Inference (BI) was performed in BEAST 2 (Bouckaert et al. 2019). BI and divergence time analyses were performed using the ‘relaxed clock log normal’ option in addition to coalescent model with constant population. Additionally, maximum likelihood (ML) phylogenetic reconstructions were conducted using RAxML 8 (Stamatakis 2014), implemented within Geneious Prime.
To identify OTUs (herein interpreted as potential species), five distinct lineage delimitation methods were used. Exploratory methods, including ABGD (Puillandre et al. 2012), sGMYC and mGMYC (Fujisawa and Barraclough 2013), and structural approaches as bPTP (Zhang et al. 2013) and the Bayesian model-based clustering algorithm in BAPS 6.0 (Corander and Tang 2007), were used. ABGD analyses were performed on the ABGD web server (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html), while sGMYC and mGMYC (https://species.h-its.org/gmyc/) and bPTP (https://species.h-its.org/ptp/) were run on the Species Delimitation server (https://species.h-its.org/).
OTUs were determined from the agreement between most lineage delimitation methods and the geographic distribution of each lineage, indicating genetic and geographic isolation. Subsequently, intra (between individuals of the same OTU) and interspecific (between individuals of different OTUs) mean genetic distances were calculated with the K2P substitution model, using MEGA X.
Analysis of molecular variance (AMOVA) was performed in Arlequin 3.5 (Excoffier and Lischer 2010) to test the hypotheses of genetic structuring of the target taxa according to their distribution in the different tributaries (microbasins) and limestone outcrops. Arlequin 3.5 was also employed to calculate genetic diversity (haplotypic diversity) of the distinct populations identified within each lineage. Further methodological details can be found in Supplementary Material 2.
Threats identification
Concurrently with invertebrate collection, anthropogenic threats in the caves and surroundings were identified. This assessment was supplemented by satellite image observations using Google Earth Pro 7.3 and information on impacting activities in the published literature.
Furthermore, we conducted a survey of advanced-stage mining rights polygons in the study area, using the Mining Geographic Information System (Sigmine) provided by the National Mining Agency (Sigmine 2023). Such polygons represent potential threats to subterranean environments.
Risk of extinction assessment
The assessment of extinction risk followed the International Union for Conservation of Nature (IUCN 2022) categories and criteria method. Assessments were performed on four target taxa and also on their identified cryptic lineages (OTUs). The OTUs were evaluated as formally described species. These assessments were then compared, in order to highlight the differences and their implications for conservation. As the IUCN method only considers formally described taxa, lineages or cryptic species are not amenable to evaluation. This can lead to scenarios that are not consistent with the real extinction risk when evaluating species complexes as single species.
Species are categorized as threatened when they meet one of five criteria designed to assess the level of extinction risk. These criteria include: (A) population reduction (past, present, and/or projected); (B) restricted geographic distribution and showing fragmentation, decline, or fluctuations; (C) small population with fragmentation, decline, or fluctuations; (D) very small population or very restricted distribution; and (E) quantitative extinction risk analysis. Most criteria have subcriteria to determine placement within a specific category (Table S2). Given the lack of population data required for the application of criteria A, C, and E, the risk of extinction was assessed based only on criteria B and D.
Applying these criteria requires the calculation of the Extent of Occurrence (EOO) and/or Area of Occupancy (AOO), in addition to the number of locations. The EOO is the area that covers all sites of a taxon’s current presence, and it’s calculated by measuring the area of a Minimum Convex Polygon (MCP) that includes all occurrence points. The AOO is the total area a species occupies within its EOO, and it’s computed by summing the areas occupied by the species in 2 km × 2 km grid cells across its range (IUCN 2022). These calculations were performed in GPS Trackmaker Pro 4.9 (https://www.trackmaker.com/main/pt/). The number of locations was calculated based on the number of distinct areas in which an impact would have to occur to affect the entire species (IUCN 2022). Since the location size depends on the area covered by the threat, and may include part of one or more subpopulations, the populations of each taxon or OTU (defined by SAMOVA) and their geographic distributions were taken into consideration.
OTUs that meet the requirements to be categorized as Critically Endangered (CR), Endangered (EN) or Vulnerable (VU) were considered as threatened with extinction. Other possible categories include Near Threatened (NT) (when not meeting the previous criteria but are close to doing so or likely to in the near future), Least Concern (LC) (when criteria are not met) or Data Deficient (DD) (when there’s insufficient information for a proper application of the criteria). The detailed methodology can be found in Supplementary Material 2.
Identification of Priority Areas
Priority areas for the conservation of genetic diversity of target groups were identified using the software Zonation 4 (Moilanen et al. 2014). The study area was divided into a grid with square cells measuring 1 km on each side, and only the cells that overlapped with the conservation targets were selected.
The selected cells received scores according to the presence of conservation targets (the studied troglobite OTUs) and weights related to their risk of extinction and endemism. Each OTU present in the cell received a value corresponding to the population genetic diversity (PopGenDiv), calculated as detailed in the Methods - Data Analysis section. Given that genetic diversity values range from 0 to 1, to ensure Zonation didn’t interpret populations with only one haplotype (PopGenDiv = 0) as absences, a value of 1 was added to all PopGenDiv values. This value was multiplied by the weight corresponding to the risk of extinction of the OTU (CR = 4, EN = 3, VU = 2 and LC = 1) plus an additional 0.25 in case of endemic OTU (those occurring only in one cell). The weights related to the extinction risk of OTUs aimed to prioritize areas that harbor more threatened lineages in relation to those with OTUs at lower risk of extinction or classified as LC (Possingham and Wilson 2005).
We used the Additive Benefit Function removal rule, which sums all the conservation values of all targets in a given cell and the result of the sum will define the order of cell removal. After removing the cell with the lowest value, the algorithm is run repeatedly with the remaining cells until the last cell (the one with the highest conservation value) is removed. For the graphical representation of spatial prioritization, the final values of the cells were divided into quartiles: maximum (red), high (orange), medium (dark green) and low (light green).
Results
Individuals were collected and sequenced from all known localities, in addition to new occurrences recorded that resulted in the expansion of their distributions (Table S1).
Partial 593 bp cox1 sequences were extracted from 67 Cirolanidae sp. 1 individuals, revealing 32 haplotypes in nine caves; 18 individuals of Cirolanidae sp. 2 (nine haplotypes) from three caves. For P. porakuara, 588 bp cox1 sequences were obtained from 64 individuals (23 haplotypes) in nine caves and two karst springs. Furthermore, 659 bp cox1 sequences were generated from 126 K. troglobia individuals (21 haplotypes) from 20 caves (Supplementary Material 3).
Phylogenetic analysis, lineage delimitation and population structure
Only one OTU was delimited in Cirolanidae sp.1. Although BI identifies three clades, with two displaying high posterior probability values, this division lacked support from both ML and lineage delimitation methods. The latter typically indicated two or a single grouping across all localities, regardless of hydrography (Fig. 1a). Haplotype sharing was common among individuals from proximate caves in the same microbasin, but also between individuals in caves from adjacent microbasins or different watersheds (Fig. 1a1).
Time calibrated phylogenetic trees resulting from Bayesian Inference, plus the results of lineage delimitation methods and location of the caves and karst springs (with indication of anthropic threats) with occurrence of Cirolanidae sp. 1 (A) and Cirolanidae sp. 2 (B). The haplotype networks (A1 and B1) and the categorization of the extinction risk of the taxa and OTUs (A2 and B2) are also presented. At each node in the trees the posterior probabilities (above left), bootstrap values (resulting from the maximum likelihood phylogenetic reconstructions, above right), and the estimated mean age (below) are shown. The purple bars represent the 95% HPD intervals for the estimated ages. For purposes of graphical presentation, outgroups have been removed from the phylogeny
In Cirolanidae sp. 2, a distinct scenario emerged, supported by both BI and ML, with unanimous agreement from all lineage delimitation methods. Three OTUs were delimited, corresponding to three monophyletic clades, each corresponding to separate caves and drainages (Fig. 1b). Notably, the separation of lineage in the geographically distant Pedra Lisa cave (PDL) was evident, as well as distinct lineages in the closely situated Três Lagos (TLG) and Crotes (CRO) caves, both located along the Apodi-Mossoró river. It’s worth noting that the habitat in CRO is epikarstic and does not connect to the water table. This structuring was further supported by the haplotype network, showing unique haplotypes from each cave separated by several mutational steps from those belonging to other allopatric OTUs (Fig. 1b1).
Five OTUs were delimited in P. porakuara, with agreement between the observed clades and the drainage patterns. Robust support from BI and ML was further substantiated by agreement from five lineage delimitation methods in clades exclusively composed of specimens from Olho d’Água da Onça (ODO) and PDL, which belong to different microbasins. Although with lower support in BI and ML, the clades formed exclusively by the localities FFA and CRO (epikarstic habitat) are distinct lineages according to four out of five methods (sGMYC, mGMYC, bPTP and BAPS). There is no agreement between BI and ML, as well as between the lineage delimitation methods, for clades encompassing the remaining localities, including the type locality of P. porakuara (TLG), suggesting a species with a broader distribution (Fig. 2a). Haplotype sharing was evident among nearby localities in the same or adjacent microbasins. However, unique haplotypes were also observed for each locality, separated by several substitutions from the others, usually belonging to other OTUs (Fig. 2b).
Time calibrated phylogenetic trees resulting from Bayesian Inference, plus the results of lineage delimitation methods and location of the caves and karst springs (with indication of anthropic threats) with occurrence of the Potiberaba porakuara (A). The haplotype network (B) and the current and proposed categorization of the extinction risk of the taxon and OTUs (C) are also presented. At each node in the trees the posterior probabilities (above left), bootstrap values (resulting from the maximum likelihood phylogenetic reconstructions, above right), and the estimated mean age (below) are shown. The purple bars represent the 95% HPD intervals for the estimated ages. For purposes of graphical presentation, outgroups have been removed from the phylogeny
Eight OTUs were delimited in K. troglobia, supported by both BI and ML. These clades generally exhibited a distribution consistent with the caves of the different limestone outcrops, corroborated by the results of lineage delimitation methods and the haplotype network (Fig. 3a, B). Based on molecular clock BI estimates, the deepest divergence occurred at the end of the Miocene (as observed in Cirolanidae sp.2 and P. porakuara), between lineages from opposite banks of the Apodi-Mossoró River. The six OTUs from localities on the left bank of the river, including the type locality (Troglóbios cave), diverged around the same period (middle Pleistocene, approximately 1.5 Ma) (Fig. 3a).
Time calibrated phylogenetic trees resulting from Bayesian Inference, plus the results of lineage delimitation methods and location of the caves (with indication of anthropic threats) with occurrence of the Kinnapotiguara troglobia (A). The haplotype network (B) and the current and proposed categorization of the extinction risk of the taxon and OTUs (C) are also presented. At each node in the trees the posterior probabilities (above left), bootstrap values (resulting from the maximum likelihood phylogenetic reconstructions, above right), and the estimated mean age (below) are shown. The purple bars represent the 95% HPD intervals for the estimated ages. For purposes of graphical presentation, outgroups have been removed from the phylogeny
These findings are supported by SAMOVA analysis, which identified optimal scenarios of subdivision as follows: seven populations for Cirolanidae sp.1 (explaining 49.36% of the genetic variation), eight for P. porakuara (86.67%), and 13 for K. troglobia (99.32%). These subdivisions concur with the identified OTUs, as they can include one or more populations and populations belonging to more than one OTU have not been delimited. The only feasible scenario for Cirolanidae sp.2 (k = 2) was not statistically significant (Table S3). Haplotypic diversity ranged from 0.67 to 1 in Cirolanidae sp. 1, and from 0.5 to 0.9 in Cirolanidae sp. 2. In P. porakuara, it ranged from 0 to 1, generally lower in the newly identified OTUs. In K. troglobia, it ranged from 0 to 0.67, being generally low in populations of all OTUs (Table S3).
There is an agreement with AMOVA outcomes, as the distribution of localities based on tributaries (microbasins) explains 43.22% (p = 0.049) of the genetic variation in Cirolanidae sp. 1 and 86.67% (p < 0.0001) for P. porakuara. Due to the distribution and limited number of localities for Cirolanidae sp. 2, the only possible distribution for performing AMOVA (in watersheds) did not yield significant results. No significant results were obtained when considering the distribution of localities with occurrences of aquatic taxa according to different limestone outcrops (Table S4). For K. troglobia, the Apodi-Mossoró river and its tributaries seem to be a biogeographic barrier, explaining 77.07% of the observed genetic variation (p < 0.0001). However, the distribution of caves according to limestone outcrops better explains (93.07%) the variation (p < 0.0001) (Table S4).
Genetic distances
Intraspecific genetic distances (within the same OTU) were 0.74% for Cirolanidae sp. 1, ranged from 0.08 to 0.37% for Cirolanidae sp. 2, from 0 to 0.93% for P. porakuara and from 0 to 0.8% for K. troglobia (Table 1).
Aquatic taxa exhibited lower genetic distances between OTUs than terrestrial one, although with distinct patterns. While Cirolanidae sp. 1 showed low mean genetic distance values among all caves, Cirolanidae sp. 2 showed higher values among OTUs from different localities (2.08% between the geographically close TLG and CRO, and from 9.17 to 9.54% between these and PDL; Table 1).
P. porakuara smallest mean genetic distances (1.74%) occurred between OTU 2 (P. porakuara) and OTU 3 (lineage occurring in the CRO epikarstic drainage) (Table 1). Larger values were found among the other OTUs, with emphasis on OTU 5 (ODO karstic spring), in the Rio do Carmo microbasin, ranging from 6.14 to 7.57%. Notably, OTUs 1 (FUF) and 4 (PDL), which are geographically close but situated in distinct microbasins, exhibited high genetic distances among themselves (4.14%) and among all other localities (Table 1), highlighting the barrier role of the drainages.
K. troglobia exhibited the highest mean genetic distances among OTUs, spanning from 3.00 to 14.72%, with higher values among OTUs from opposite banks of the Apodi-Mossoró River (Table 1). Even among OTUs on the same bank, genetic distances were high. OTUs on the right bank of the river showed a genetic distance of 7.12%, and those on the left bank ranged from 3.41 to 11.96%, irrespective of geographical distance.
Threats
Seven anthropogenic threats affecting subterranean environments in the study areas were identified: deforestation, agriculture, irregular mining, oil exploration, livestock, disorderly visitation, and groundwater level lowering. Additionally, two potential threats were recognized: mining rights polygons and disorderly visitation. The most widespread threat is deforestation, impacting all four taxa and 11 out of the 17 OTUs.
All taxa and half of the OTUs occurs in areas with mining rights polygons at an advanced stage, posing future threats from mining developments. Agriculture affects all taxa and six OTUs, while irregular limestone mining impacts three taxa and seven OTUs. Currently, disorderly visitation is limited to the TLG and Poço Feio cave (PFE) but is a potential threat to OTUs in the Crotes cave, affecting all taxa and five OTUs. Other threats are more localized, and only the caves in the FFNP remain unaffected by anthropogenic threats (Figs. 1 and 2; Table 2, S1).
IUCN categorization
The EOO was calculated as 1,395.2 km² for Cirolanidae sp. 1, 62.11 km² for Cirolanidae sp. 2, 2,004.37 km² for P. porakuara, and 50.06 km² for K. troglobia. The AOO was calculated as 32 km² for Cirolanidae sp. 1, 12 km² for Cirolanidae sp. 2, 36 km² for P. porakuara, and 28 km² for K. troglobia. The number of locations was seven, three, eight, and thirteen, respectively (Table S3). Given the prevalence of anthropogenic activities impacting nearly the entire distribution range of these taxa, it is evident that habitat quality is steadily declining. Consequently, Cirolanidae sp. 1 and P. porakuara (in agreement with their current status on the list of threatened species, MMA 2022) can be categorized as Vulnerable (VU). Cirolanidae sp. 2 qualifies as EN, according to criterion B1 and subcriteria ab(iii) (Figs. 1 and 2; Table 2).
The number of locations for K. troglobia (13) slightly exceeds the threshold for VU (10) categorization, and it can be categorized as NT (Fig. 3; Table 2). Nevertheless, categorization as DD is preferable because of the lack of knowledge, for example, of how current threats may affect different populations on the same outcrop. K. troglobia is currently in the official list of threatened species as VU, because the new occurrences identified herein weren’t considered.
It’s important to highlight that the categorization of the different OTUs has yielded distinct scenarios, with more threatened units and higher extinction risk, since most cryptic lineages are exposed to threats and exhibit lower EOO, AOO and number of locations (Figs. 1, 2 and 3; Table 2). Only OTUs with occurrence restricted to the FFNP are not threatened.
Priority areas for conservation
The spatial prioritization considered 26 cells that overlapped with the conservation targets. A minimum of 13 cells (including seven maximum priority and six high priority cells) should be protected to avoid the loss of OTUs, ensuring that each OTU is present in at least one cell of the solution (Table 3; Fig. 4).
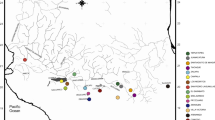
Priority areas for conservation of cryptic and threatened species of troglobitic arthropods in the Brazilian semiarid. Pizza charts have a diameter proportional to the amount of OTUs in different microbasins and limestone outcrops, also indicating the amount of lineages in each category of threat - Critically Endangered (CR, red), Endangered (EN, orange), Vulnerable (VU, yellow) and Least Concern (LC, green)
Among the nine microbasins where troglobitic taxa occur in the study area, the Arapuá Stream microbasin contains the largest number of locations in priority areas. This includes the Rosário outcrop, which houses a significant portion of the genetic variability for all the target taxa, hosting five OTUs, three of which are endemic and categorized as CR (Table 3; Fig. 4).
Abreu Stream microbasin comes next, mainly due to the Três Lagos Cave (TLG), which houses three OTUs, one endemic and one CR. The Cote Stream microbasin also stands out, and the Angicos outcrop houses three OTUs, one endemic and one CR. The Rio do Carmo microbasin has one priority area, while the Bom Sucesso and Saco microbasins have areas of lower priority. The microbasins with localities in the FFNP have the lowest conservation priority because they are already in an integral protection conservation unit and harbor mainly LC OTUs (Table 3; Fig. 4).
Given the proximity of the Rosário outcrop with the Abreu Stream microbasin, it is feasible to connect them into a contiguous area that concentrates the localities with the highest conservation priority for the targeted taxa. The second area of highest priority encompasses the cluster of localities within the Cote Stream microbasin (Table 3; Fig. 4).
Discussion
Lineage diversity
Except for Cirolanidae sp. 1, the other taxa exhibited distinct cryptic lineages, suggesting troglobitic species complexes. These findings corroborate the pattern of a high number of allopatric troglobitic species in a highly fragmented karstic area (Bickford et al. 2007; Niemiller et al. 2012; Fišer et al. 2018).
Stygobites generally have wider distributions than terrestrial troglobites, attributed to their presumed higher dispersal capacity and groundwater connectivity. However, they may encompass populations arranged according to hydrographic boundaries (Finston et al. 2007; Trontelj et al. 2009; Niemiller et al. 2012, 2013), or even involve species complexes (Trontelj et al. 2009; Fišer et al. 2018; Recknagel et al. 2023). Therefore, the existence of different OTUs within Cirolanidae sp. 2 and P. porakuara across different microbasins, as well as in the epikarstic drainage of Crotes cave, was expected. Regarding K. troglobia, recent molecular studies have revealed similar distribution patterns for several troglobitic species, with lineages exclusive to one or a few nearby caves (Wessel et al. 2013; Mammola et al. 2015; Gómez et al. 2016; Allegrucci et al. 2017).
The estimated divergence times between the different OTUs align with paleoclimatic events (Werneck 2011; Dupont et al. 2013; Herbert et al. 2016; Silva and Souza 2018) that shaped the current cave faunas around the world (White et al. 2019). The oldest lineages of Cirolanidae sp. 2 and P. porakuara would have diverged at the end of the Miocene. This might be due to the isolation after oceanic regressions that occurred in that period, which is regionally and locally well-documented (Rosseti et al. 2013) and corresponds to global eustatic levels (Haq et al. 1987). Sea level fluctuations have been invoked to explain the origin and distribution of various stygobites in coastal regions (Alvarez et al. 2015; Humphreys 2019; Delić et al. 2020), and the Jandaíra Formation harbors an oceanic relict community comprising cirolanids (Ferreira et al. 2010), amphipods (Fisĕr et al. 2013), and flatworms (Souza et al. 2018). For K. troglobia, the earliest divergence between lineages on opposite riverbanks likely occurred at the end of the Miocene, corresponding with current estimates for the geological fault that formed the river channel (Bagni et al. 2020).
The global cooling at the end of the Miocene was followed by a period of aridification in the tropics (Rosseti et al. 2013; Herbert et al. 2016). While glaciations are credited with isolating ancestral lineages in subterranean environments and giving rise to troglobitic species in the Northern Hemisphere (White et al. 2019), the establishment of arid climates played a similar role in tropical regions (Finston et al. 2007; Guzik et al. 2011; Humphreys 2019). Similar to Cirolanidae sp. 2 and certain OTUs of P. porakuara, many stygobites in Australia have limited distributions within individual aquifers or drainages (Guzik et al. 2011; Bradford et al. 2013). Comparable phylogeographic patterns among unrelated taxa provide strong evidence for a shared spatio-temporal history (Carstens et al. 2005).
The Jandaíra Formation has been undergoing late exhumation since the Miocene, resulting in intensive karstification processes that gave rise to subterranean environments (Bagni et al. 2020). The origin and expansion of these subterranean habitats occurred during alternating dry and wet periods (glacial and interglacial), contributing to the expansion of semiarid conditions and the dispersion and diversification of organisms adapted to seasonally dry tropical forests, such as Caatinga (Werneck 2011; Dupont et al. 2013; Costa et al. 2018). Therefore, the combination of abundant subterranean habitats and challenging external conditions likely played a pivotal role in the diversification of these troglobitic arthropods.
Most of the OTUs delimited in this study exhibited average genetic distances compared to other OTUs that were either higher or within the divergence limits for cox1 reported in prior studies (Brower 1994; Witt et al. 2006; Stokkan et al. 2018). However, the genetic distances between OTUs 2 and 3 of P. porakuara (1.74%) and between OTUs 2 and 3 of Cirolanidae sp.2 (2.08%) fall below the limits proposed by Witt et al. (2006) and Stokkan et al. (2018), yet they are in proximity to the 2.3% threshold advocated by Brower (1994). Both OTUs are found within Crotes Cave, in an epikarst drainage not linked to the water table. Apart from being geographically isolated, these OTUs display signs of genetic isolation and establish well-supported monophyletic groups, aligning with the phylogenetic species concept and suggesting independent evolutionary trajectories (de Queiroz 2007). Hence, as with other OTUs, their delimitation results from the integration of genetic data, geographic distribution and evolutionary history, forming more robust species hypotheses (Padial et al. 2010). Thus, the OTUs delimited are presented as species hypotheses and further studies are recommended to test them. If confirmed, studies are needed to formally describe them based on an integrative taxonomy approach.
Unlike the other studied taxa, genetic distances in Cirolanidae sp. 1 across its distribution are consistent with low intraspecific variability in Isopoda (Rodcharoen et al. 2016), indicating a single widely distributed species. A similar pattern occurs in other regions, as Europe, where macrostygobites with broad distributions are found, particularly among isopods (Henry and Magniez 2003). Additional research is required to comprehend whether this condition results from higher dispersal capacity and/or lower ecological specificity, enabling dispersion through inaccessible aquifers to other taxa and maintaining gene flow between localities, maybe intermittently and during wetter periods (Silva and Souza 2018).
Genetic diversity and conservation
Haplotype networks offer insights into the identified lineages. Cirolanidae sp. 1 exhibits an expansion pattern with radiations of rare, unique singletons originating from central dominant haplotypes. A similar but less distinct pattern can be observed in P. porakuara (OTU 2) and OTU 5 of K. troglobia, possibly resulting from population bottlenecks followed by expansion (Avise 1994). It has also been identified in beetles (Guzik et al. 2009) and stygobitic amphipods in Australia (Guzik et al. 2011; Bradford et al. 2013). Furthermore, some populations of Cirolanidae sp. 1 (CAG and PDL) and P. porakuara showed high haplotype diversity, indicating stable populations with long evolutionary history (Guzik et al. 2011).
Most lineages, including OTUs of all taxa, exhibit low genetic diversity, to the extent that the majority or all individuals share the same haplotype. This indicates small, genetically isolated populations with limited or nonexistent gene flow, resulting in significant differentiation (Holsinger and Weir 2009; Recknagel et al. 2023). This pattern is common among subterranean organisms, affecting both terrestrial (Mammola et al. 2015) and aquatic species (Finston et al. 2007; Recknagel et al. 2023). Loss of genetic diversity, often driven by stochastic processes, reduces the adaptability of these cryptic lineages, leading to compromised population growth and a heightened risk of extinction when facing anthropogenic impacts (Smith et al. 2014).
This is of particular concerning because, except for the FFNP OTUs, all others are exposed to anthropogenic impacts and can be considered threatened. The most prevalent threat is deforestation, which can disrupt subsurface hydrological systems and alter nutrient inputs, potentially leading to population decline or lineage extinction (Mammola et al. 2019). K. troglobia planthoppers are phytophagous (Hoch and Ferreira 2013), and individuals of P. porakuara in some localities (like ODF, PFE, and ODT) are always found associated with roots, indicating a clear dependence on surface vegetation.
Alongside the deforestation impact, the expansion and intensification of agriculture contribute to increased evapotranspiration (Destouni et al. 2013), diminishing water runoff and aquifer recharge (Ceballos et al. 2015). Moreover, groundwater and karst aquifers are vulnerable to contamination from pesticides and fertilizers, as they typically have limited renewal potential and a high likelihood of retaining pollutants (Ford and Williams 2007). The overexploitation of groundwater for irrigation can also lower the water table, recognized as one of the primary threats to groundwater environments (Mammola et al. 2019).
Another substantial impact on subterranean lineages in the study area is irregular limestone mining, often linked to the quicklime industry. In addition to the impacts on native vegetation by the use of firewood as fuel, which indirectly affects the subterranean environment, limestone mining can lead to intense disturbance of the epikarstic and associated fauna and cave suppression (Ferreira et al. 2010).
Disorderly visitation, though limited to a few caves, impacts multiple OTUs. These impacts encompass trampling, soil compaction, and trophic changes caused by improper waste disposal (Ferreira et al. 2010). Furthermore, the entry of visitors into the water bodies can lead to trampling of individuals and modifications of water characteristics, changing turbidity and destroying microhabitats (Ferreira et al. 2010). Currently, three caves in the FFNP (including Furna Feia and Furna Nova) and another three caves in the Rosário outcrop (including Crotes cave) are going under feasibility analysis for tourism use. The data collected here will be valuable for the management of these caves.
Expectedly, newly discovered cryptic lineages often fall into different threat categories compared to the complexes they are part of, typically indicating a higher risk of extinction. Cryptic lineages have a more restricted geographic ranges and are more likely to be threatened (Bickford et al. 2007; Niemiller et al. 2013). Moreover, cryptic lineages may face distinct levels of threats, necessitating diverse conservation strategies (Niemiller et al. 2013; Recknagel et al. 2023). This is particularly concerning when cryptic lineages are components of already threatened nominal species (Bickford et al. 2007), as is the case with P. porakuara and K. troglobia.
Although areas with rare and endemic species are often considered in the prioritization process for biodiversity conservation, not all are of equal importance. It’s essential to prioritize areas that harbor more threatened species over those with species classified as LC (Possingham and Wilson 2005). Therefore, further discussion is needed regarding the constraints for extinction risk assessment, often focused only on formally described species despite the IUCN methodology may be applied to any taxonomic unit at or below the species level. Subspecies, subpopulations, or forms that are not yet fully described can be evaluated in exceptional circumstances, particularly if there is a clear conservation benefit (IUCN 2022). Recent studies have pointed out that undescribed vertebrate species have higher extinction risk than known species (Liu et al. 2022), and this should be even more worrying for invertebrates. In this context, a possible assessment of the conservation status of delimited OTUs from molecular data would enable the adoption of conservation actions and minimize the negative impacts of taxonomic impediments on these taxa’s conservation.
This discussion aligns with the recommendations of Premate et al. (2024), which underscore the vulnerability of undescribed subterranean species and advocate for prioritizing the formal description of lineages deemed high priority. The authors also recommend conducting additional IUCN assessments and updating the statuses of species and subspecies that have undergone DNA analyses since the last assessment (Premate et al. 2024). This is particularly relevant in Brazil, where the National Biodiversity Policy mandates that public authorities must implement effective measures to prevent environmental degradation in cases where there is substantial scientific evidence of a significant and irreversible threat to biological diversity, including genetic variability (Brasil 2002). Despite providing important information on evolutionary processes necessary for conserving multiple levels of biodiversity, molecular data are rarely used to guide spatial conservation decision-making (Nielsen et al. 2023).
As almost all OTUs identified, most subterranean species have restricted distributions (Gibert and Deharveng 2002; Trontelj et al. 2009), and even small-ranged threats are more likely to cause extinctions (Mammola et al. 2019). In addition, troglobites often exhibit high longevity and low metabolism and fecundity, resulting in slow population growth and instability due to catastrophic or stochastic events (Mammola et al. 2019). Additionally, simple communities with few species and low tolerances to changes in abiotic conditions results in low ecological resilience and increased disturbance vulnerability (Mammola et al. 2019). Consequently, endemic subterranean taxa are at greater risk of ecological imbalances and habitat loss (Cardoso et al. 2010). Moreover, such species frequently represent relicts, as is the case with the taxa in this study, further underscoring their importance for conservation efforts (Nitzu et al. 2018).
If all OTUs outside the FFNP are threatened, which ones should be prioritized? On the one hand, genetic diversity is important for conservation because it can increase population resilience, and is the raw material of natural selection (Lande and Shannon 1996). In addition to the importance of high genetic diversity, populations that are not genetically isolated are potentially more resilient and resistant. On the other hand, low genetic diversity can make lineages more susceptible to inbreeding depression, and possibly extinction. The conservation value of such lineages lies in their uniqueness and rarity (Nielsen et al. 2017; Recknagel et al. 2023). Recommended conservation measures should include preserving genetic diversity and evolutionary relationships within and between species to maintain community-level evolutionary patterns (Carvalho et al. 2017; Nielsen et al. 2017). Priority should be given to areas that not only house rare and endemic species but also support more threatened ones (Possingham and Wilson 2005).
Felipe Guerra (RN) region, particularly the Rosário outcrop, harbors a considerable part of the genetic variability of the studied taxa. It houses five OTUs, including three endemic and CR species, along with two populations of other lineages with lower extinction risk. Conservation efforts should also target nearby areas in the Abreu Stream microbasin, which holds more than half of the identified OTUs (10, eight of which are exclusive) in a small 15 square kilometer region. Such concentration is in line with studies that indicated that areas of higher genetic diversity are often coincident among several species (Hewitt 1996), resulting in hotspots of genetic diversity or concentrations of phylogeographic endemism (Carnaval et al. 2014). The second priority area includes localities in the Cote Stream microbasin. This region faces significant threats, such as irregular mining, deforestation linked to quicklime production, and disorderly visitation.
It’s worth noting that the areas identified as conservation priorities align with two official initiatives focused on biodiversity and speleological heritage conservation. Firstly, they correspond to the CA063 area (Chapada do Apodi) in the update of priority areas for the conservation, utilization, and benefit sharing of Caatinga Biodiversity (MMA 2018). This area is regarded as having extremely high biological importance and is a priority for establishing integral protection conservation units. Secondly, these areas are also in line with the priority areas for the conservation of the Brazilian speleological heritage (ICMBio/CECAV 2018), falling within the priority category 1 polygons. This designation emphasizes actions related to creating or expanding conservation units, strengthening enforcement and monitoring, and fostering environmental and heritage education (Fig. S2).
Conservation efforts targeting subterranean biodiversity offer benefits that extend beyond safeguarding endemic and relict lineages. The anthropic activities in the study area endanger its own sustainability. Therefore, conservation and planning initiatives can yield both social and environmental advantages. Preserving groundwater species and habitats brings about numerous biodiversity benefits (Premate et al. 2024). For instance, subterranean aquatic fauna plays a role in various ecosystem services, including nutrient recycling and sediment oxygenation (Abell and Harrison 2020). Furthermore, safeguarding forest remnants in karst areas, vital for maintaining the ecological equilibrium of subterranean environments, contributes to the protection of surface and subterranean water sources. This, in turn, enhances the availability and conservation of water resources in regions where their supply is limited, particularly in semiarid areas (Farrick and Branfireun 2015).
Conclusions
Subterranean environments are often overlooked in conservation efforts, yet they host extraordinary life forms and unique adaptations. These habitats, in addition to offering essential ecosystem services, present a frontier for scientific exploration (Mammola et al. 2019).
Even though accessible subterranean habitats, like caves, are challenging to study and require specialized techniques (Ficetola et al. 2019), there is an even larger, mostly inaccessible subterranean network that may serve as the main habitat for many species (Howarth 1983). The full extent of this subterranean world remains largely unknown. In light of these challenges and uncertainties, practical measures for protecting subterranean environments need to be considered (Mammola et al. 2019). Thus, comparative phylogeography can unveil distribution patterns and factors influencing them, highlighting subterranean connectivity and areas beyond caves that need conservation focus.
Molecular data isn’t always available for all species in conservation planning and may be constrained by the use of less informative markers like mtDNA (Nielsen et al. 2023), as in this study. While these data are crucial for effective conservation planning, waiting for more data could lead to worse biodiversity outcomes when immediate action is needed (Grantham et al. 2009; Liu et al. 2022). This is even more valid when considering that the overall integration of molecular data into conservation planning remains similar when using older or newer sequencing techniques (Nielsen et al. 2023).
These approaches can represent a first step towards safeguarding subterranean environments, highlighting areas and taxa that should be prioritized. This is especially crucial in a context of increasingly scarce resources for conservation, especially in Brazil, where caves face unprecedented threats (Ferreira et al. 2022).
Data availability
All data generated during and/or analysed during the current study are available from the authors upon request.
References
Abell R, Harrison IJ (2020) A boost for freshwater conservation. Science. https://doi.org/10.1126/science.abe3887
Allegrucci G, Ketmaier V, Di Russo C, Rampini M, Sbordoni V, Cobolli M (2017) Molecular phylogeography of Troglophilus cave crickets (Orthoptera, Rhaphidophoridae): A combination of vicariance and dispersal drove diversification in the East Mediterranean region. J Zool Syst Evol Res. https://doi.org/10.1111/jzs.12172
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol. Z. https://doi.org/10.1127/0941-2948/2013/0507
Alvarez F, Iliffe TM, Benitez S, Brankovits D, Villalobos JL (2015) New records of anchialine fauna from the Yucatan Peninsula, Mexico. Check List 11(1):1–10. https://doi.org/10.15560/11.1.1505
Bagni FL, Bezerra FH, Balsamo F, Maia RP, Dall’Aglio M (2020) Karst dissolution along fracture corridors in an anticline hinge, Jandaíra Formation, Brazil: implications for reservoir quality. Mar Pet Geol 115:104249. https://doi.org/10.1016/j.marpetgeo.2020.104249
Bento DM, Souza-Silva M, Vasconcellos A, Bellini BC, Prous X, Ferreira RL (2021) Subterranean oasis in the Brazilian semiarid region: neglected sources of biodiversity. Biodivers Conserv. https://doi.org/10.1007/s10531-021-02277-6
Bezerra FHR, Takeya MK, Sousa MOL, Nascimento AF (2007) Coseismic reactivation of the Samambaia fault. Brazil Tectonophysics. https://doi.org/10.1016/j.tecto.2006.10.007
Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2006.11.004
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A et al (2019) BEAST 2.5: an advanced software platform for bayesian evolutionary analysis. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1006650
Bradford TM, Adams M, Guzik MT, Humphreys WF, Austin AD, Cooper SJ (2013) Patterns of population genetic variation in sympatric chiltoniid amphipods within a calcrete aquifer reveal a dynamic subterranean environment. Heredity 111:77–85. https://doi.org/10.1038/hdy.2013.22
Brasil (2002) Decreto no. 4.339, de 22 de agosto de 2002. Institui princípios e diretrizes para a implementação da Política Nacional da Biodiversidade. Diário Oficial [da] República Federativa do Brasil: seção 1, Brasília, DF, n. 163, 23 ago
Brasil, Decreto de 5 de junho de 2012 (jun. 2012) Dispõe sobre a criação do Parque Nacional Da Furna Feia, nos municípios de Baraúna e Mossoró, Estado do Rio Grande do Norte. Diário Oficial [da] República Federativa do Brasil, Brasília, DF, p 06
Brower AVZ (1994) Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. PNAS 91(14):6491–6495. https://doi.org/10.1073/pnas.91.14.6491
CANIE/CECAV (2022) Cadastro Nacional de Informações Espeleológicas. Available in: https://www.gov.br/icmbio/pt-br/assuntos/centros-de-pesquisa/cecav/cadastro-nacional-de-informacoes-espeleologicas/canie. Accessed 30 December 2022
Cardoso P, Borges PAV, Triantis KA, Ferrández MA, Martín HL (2010) Adapting the IUCN Red List criteria for invertebrates. Biol Conserv. https://doi.org/10.1016/j.biocon.2011.06.020
Carnaval AC et al (2014) Prediction of phylogeographic endemism in an environmentally complex biome. Proc. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rspb.2014.1461
Carstens BC, Brunsfeld SJ, Demboski JR, Good JM, Sullivan J (2005) Investigating the evolutionary history of the Pacific Northwest mesic forest ecosystem: hypothesis testing within a comparative phylogeographic framework. Evolution 59(8):1639–1652. https://doi.org/10.1111/j.0014-3820.2005.tb01815.x
Carstens BC, Pelletier TA, Reid NM, Salter JD (2013) How to fail at species delimitation. Mol Ecol. https://doi.org/10.1111/mec.12413
Carvalho SB, Velo-Antón G, Tarroso P, Portela AP, Barata M, Carranza S, Possingham HP (2017) Spatial conservation prioritization of biodiversity spanning the evolutionary continuum. Nat Ecol Evol. https://doi.org/10.1038/s41559-017-0151
Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM (2015) Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci Adv. https://doi.org/10.1126/sciadv.1400253
Colado R, Abellán P, Pallarés S, Mammola S, Milione R, Faille A, Fresneda J, Sánchez-Fernández D (2023) A dark side of conservation biology: protected areas fail in representing subterranean biodiversity. Insect Conserv Divers 16(5):674–683. https://doi.org/10.1111/icad.12666
Corander J, Tang J (2007) Bayesian analysis of population structure based on linked molecular information. Math Biosci. https://doi.org/10.1016/j.mbs.2006.09.015
Costa WJ, Amorim PF, Mattos JLO (2018) Synchronic historical patterns of species diversification in seasonal aplocheiloid killifishes of the semi-arid Brazilian Caatinga. PLoS ONE. https://doi.org/10.1371/journal.pone.0193021
Cruz JB, Bento DM, Bezerra FHR, Freitas JIM, Campos UP (2010) Diagnóstico Espeleológico do Rio Grande do Norte. Revista Brasileira De Espeleologia 1(1):1–24
Culver DC, Pipan T (2009) The biology of caves and other subterranean habitats. Library of Congress Cataloging in Publication Data. Oxford University Press, Oxford
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics andparallel computing. Nat Methods. https://doi.org/10.1038/nmeth.2109
de Queiroz K (2007) Species concepts and species delimitation. Syst Biol 56(6):879–886. https://doi.org/10.1080/10635150701701083
Delić T, Stoch F, Borko Š, Flot JF, Fišer C (2020) How did subterranean amphipods cross the Adriatic Sea? Phylogenetic evidence for dispersal–vicariance interplay mediated by marine regression–transgression cycles. J Biogeogr. https://doi.org/10.1111/jbi.13875
Destouni G, Jaramillo F, Prieto C (2013) Hydroclimatic shifts driven by human water use for food and energy production. Nat Clim Change. https://doi.org/10.1038/nclimate1719
Dincă V, Wiklund C, Lukhtanov VA, Kodandaramaiah U, Norén K, Dapporto L, Friberg M (2013) Reproductive isolation and patterns of genetic differentiation in a cryptic butterfly species complex. J Evol Biol 26(10):2095–2106. https://doi.org/10.1111/jeb.12211
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Mol Ecol. https://doi.org/10.1046/j.1365-294X.2002.01650.x
Dupont LM, Rommerskirchen F, Mollenhauer G, Schefuß E (2013) Miocene to Pliocene changes in South African hydrology and vegetation in relation to the expansion of C4 plants. Earth Planet Sci Lett. https://doi.org/10.1016/j.epsl.2013.06.005
Excoffier L, Lischer HL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. https://doi.org/10.1111/j.1755-0998.2010.02847.x
Farrick KK, Branfireun BA (2015) Flowpaths, source water contributions and water residence times in a Mexican tropical dry forest catchment. J Hydrol. https://doi.org/10.1016/j.jhydrol.2015.08.059
Ferreira RL, Prous X, Bernardi LFO, Souza-Silva M (2010) Fauna subterrânea do Estado do Rio Grande do Norte: Caracterização E impactos. Revista Brasileira De Espeleologia 1:25–51
Ferreira RL et al (2022) Brazilian cave heritage under siege. Science. https://doi.org/10.1126/science.abo1973
Ficetola GF, Canedoli C, Stoch F (2019) The Racovitzan impediment and the hidden biodiversity of unexplored environments. Conserv Biol. https://doi.org/10.1111/cobi.13179
Finston T, Johnson M, Humphreys W, Eberhard SM, Halse SA (2007) Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape. Mol Ecol. https://doi.org/10.1111/j.1365-294X.2006.03123.x
Fisĕr C, Zagmajster M, Ferreira RL (2013) Two new Amphipod families recorded in South America shed light on an old biogeographical enigma. Syst Biodivers. https://doi.org/10.1080/14772000.2013.788579
Fišer C, Robinson CT, Malard F (2018) Cryptic species as a window into the paradigm shift of the species concept. Mol Ecol. https://doi.org/10.1111/mec.14486
Folmer M, Black W, Hoeh R, Lutz L, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3(5):294–299
Ford D, Williams P (2007) Karst hydrogeology and geomorphology. Wiley, New York
Fujisawa T, Barraclough TG (2013) Delimiting species using single-locus data and the generalized mixed Yule Coalescent approach: a revised method and evaluation on simulated data sets. Syst Biol. https://doi.org/10.1093/sysbio/syt033
Gómez RA, Reddell J, Will K, Moore W (2016) Up high and down low: molecular systematics and insight into the diversification of the ground beetle genus Rhadine LeConte. Mol Phylogenet Evol. https://doi.org/10.1016/j.ympev.2016.01.018
Grantham HS et al (2009) Delaying conservation actions for improved knowledge: how long should we wait? Ecol Lett. https://doi.org/10.1111/j.1461-0248.2009.01287.x
Guzik MT, Cooper SJB, Humphreys WF, Austin AD (2009) Fine-scale comparative phylogeography of a sympatric sister species triplet of subterranean diving beetles from a single calcrete aquifer in Western Australia. Mol Ecol. https://doi.org/10.1111/j.1365-294X.2009.04296.x
Guzik MT, Cooper SJB, Humphreys WF, Ong S, Kawakami T, Austin AD (2011) Evidence for population fragmentation within a subterranean aquatic habitat in the western Australian desert. Heredity 107:215–230. https://doi.org/10.1038/hdy.2011.6
Guzik MT, Stringer DN, Murphy NP, Cooper SJ, Taiti S, King RA, Austin AD (2019) Molecular phylogenetic analysis of Australian arid-zone oniscidean isopods (Crustacea: Haloniscus) reveals strong regional endemicity and new putative species. Invertebrate Syst 33(3):556–574. https://doi.org/10.1071/IS18070
Haq BU, Hardenbol J, Vail PR (1987) Chronology of fluctuating sea levels since the Triassic. Science. https://doi.org/10.1126/science.235.4793.1156
Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc B: Biol Sci 270(1512):313–321. https://doi.org/10.1098/rspb.2002.2218
Henry JP, Magniez G (2003) Isopodes aselloïdes stygobies d’Espagne, III–Le genre Proasellus: B–espèces anophtalmes. Beaufortia 53(6):129–157
Herbert TD, Lawrence KT, Tzanova A, Peterson LC, Caballero-Gill R, Kelly CS (2016) Late miocene global cooling and the rise of modern ecosystems. Nat Geosci. https://doi.org/10.1038/ngeo2813
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc. https://doi.org/10.1111/j.1095-8312.1996.tb01434.x
Hoch H, Ferreira RL (2013) Potiguara Troglobia gen. n., sp. n.–first record of a troglobitic Kinnaridae from Brazil (Hemiptera: Fulgoromorpha). Deut Entomol. https://doi.org/10.1002/mmnd.201300003
Holsinger JR (2000) Ecological derivation, colonization, and speciation. In: Winkens H, Culver DC, Humphreys WF (eds) Ecosystems of the world: 30, subterranean ecosystems. Elsevier Science, Amsterdam, pp 399–415
Holsinger KE, Weir BS (2009) Genetics in geographically structured populations: defining, estimating and interpreting FST. Nat Rev Genet. https://doi.org/10.1038/nrg2611
Humphreys WF (2019) Biodiversity patterns in Australia. 109–126. In: White WB and Culver DC (eds), Encyclopedia of Caves, 3nd Edition. Academic Press, San Diego
Iannella M, Fiasca B, Di Lorenzo T, Di Cicco M, Biondi M, Mammola S, Galassi DM (2021) Getting the ‘most out of the hotspot’for practical conservation of groundwater biodiversity. Glob Ecol Conserv 31:e01844. https://doi.org/10.1016/j.gecco.2021.e01844
ICMBio/CECAV (2018) Áreas prioritárias para conservação do patrimônio espeleológico Brasileiro: primeira aproximação. Brasília-DF. https://www.gov.br/icmbio/pt-br/assuntos/centros-de-pesquisa/cecav/publicacoes/areas_prioritarias_patrimonio_espeleologico_2018.pdf. Accessed 20 January 2023
IUCN Standards and Petitions Committee (2022) Guidelines for Using the IUCN Red List Categories and Criteria. Version 15.1. Prepared by the Standards and Petitions Committee. https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf. Accessed 30 November 2022
Janzen DH, Burns JM, Cong Q, Hallwachs W, Dapkey T, Manjunath R, Grishin NV (2017) Nuclear genomes distinguish cryptic species suggested by their DNA barcodes and ecology. PNAS 114(31):8313–8318. https://doi.org/10.1073/pnas.1621504114
Kozlov MV, Mutanen M, Lee KM, Huemer P (2017) Cryptic diversity in the long-horn moth Nemophora degeerella (Lepidoptera: Adelidae) revealed by morphology, DNA barcodes and genome-wide ddRAD-seq data. Syst Entomol 42:329–346. https://doi.org/10.1111/syen.12216
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. https://doi.org/10.1093/molbev/msy096
Lande R, Shannon S (1996) The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50:434–437. https://doi.org/10.2307/2410812
Leigh JW, Bryant D (2015) Popart: full-feature software for haplotype network construction. Methods Ecol Evol. https://doi.org/10.1111/2041-210x.12410
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. https://doi.org/10.1093/bioinformatics/btp187
Liu J, Slik F, Zheng S, Lindenmayer DB (2022) Undescribed species have higher extinction risk than known species. Conserv Lett 15:e12876. https://doi.org/10.1111/conl.12876
Mammola S, Isaia M, Arnedo MA (2015) Alpine endemic spiders shed light on the origin and evolution of subterranean species. PeerJ 3:e1384. https://doi.org/10.7717/peerj.1384
Mammola S et al (2019) Scientists’ warning on the conservation of subterranean ecosystems. Bioscience. https://doi.org/10.1093/biosci/biz064
Mayden RL (1997) A hierarchy of species concepts: the denoument in the saga of the species problem. In: Claridge MF, Dawah HA, Wilson MR (eds) Species: the units of diversity. Chapman and Hall, London, pp 381–423
MMA – Ministério do Meio Ambiente (2018) Portaria Nº 463, de 18 de dezembro de 2018. Áreas Prioritárias para a Conservação, Utilização e Repartição de Benefícios da Biodiversidade Brasileira ou Áreas Prioritárias para a Biodiversidade. Diário Oficial da União. Brasília/DF, seção 1, n. 243, p. 160, 19 dez. 2018
MMA, Ministério do Meio Ambiente (2022) Portaria Nº 148, de 7 de junho de 2022. Altera os Anexos Da Portaria nº 443, de 17 de dezembro de 2014, da portaria nº 444, de 17 de dezembro de 2014, E Da Portaria nº 445, de 17 de dezembro de 2014, referentes à atualização Da Lista Nacional De Espécies Ameaçadas De Extinção. Diário Oficial Da União Bras–lia/DF(se–o 1, n 108, 74):2022
Moilanen A et al (2014) Zonation—spatial conservation planning methods and software. Version 4. User Manual. 290 (C-BIG Conservation Biology Informatics Group, Univ. Helsinki, 2014)
Myers N, Mittermeir RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature. https://doi.org/10.1038/35002501
Nielsen ES, Beger M, Henriques R, Selkoe KA, von der Heyden S (2017) Multispecies genetic objectives in spatial conservation planning. Conserv Biol. https://doi.org/10.1111/cobi.12875
Nielsen ES et al (2023) Molecular ecology meets systematic conservation planning. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2022.09.006
Niemiller ML, Near TJ, Fitzpatrick BM (2012) Delimiting species using multilocus data: diagnosing cryptic diversity in the southern cavefish Typhlichthys subterraneus (Teleostei: Amblyopsidae). Evolution. https://doi.org/10.1111/j.1558-5646.2011.01480.x
Niemiller ML et al (2013) Doomed before they are described? The need for conservation assessments of cryptic species complexes using an amblyopsid cavefish (Amblyopsidae: Typhlichthys) as a case study. Biodivers Conserv. https://doi.org/10.1007/s10531-013-0514-4
Nitzu E, Vlaicu M, Giurginca A, Meleg IN, Popa I, Nae A, Baba Ş (2018) Assessing preservation priorities of caves and karst areas using the frequency of endemic cave-dwelling species. Int J Speleol. https://doi.org/10.5038/1827-806X.47.1.2147
Padial JM, Miralles A, la Riva De I, Vences M (2010) The integrative future of taxonomy. Front Zool 7:16. https://doi.org/10.1186/1742-9994-7-16
Possingham HP, Wilson KA (2005) Turning up the heat on hotspots. Nature. https://doi.org/10.1038/436919a
Premate E, Zagmajster M, Fišer C (2024) Evaluating the overlap of surface protected areas with different facets of groundwater biodiversity: Glass half empty or half full? Biol Conserv 289:110392. https://doi.org/10.1016/j.biocon.2023.110392
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. https://doi.org/10.1111/j.1365-294X.2011.05239.x
Rabelo JG, Maia RP, Bezerra FHR, Silva CCN (2020) Karstification and fluid flow in carbonate units controlled by propagation and linkage of mesoscale fractures, Jandaíra formation, Brazil. Geomorphology 357:107090. https://doi.org/10.1016/j.geomorph.2020.107090
Ralls K et al (2018) Call for a paradigm shift in the genetic management of fragmented populations. Conserv Lett. https://doi.org/10.1111/conl.12412
Recknagel H, Zakšek V, Delić T, Gorički Š, Trontelj P (2023) Multiple transitions between realms shape relict lineages of Proteus cave salamanders. Mol Ecol. https://doi.org/10.1111/mec.16868
Rodcharoen E, Bruce NL, Pholpunthin P (2016) Description of four new species of the Cirolana ‘parva group’ (Crustacea: Isopoda: Cirolanidae) from Thailand, with supporting molecular (COI) data. J Nat Hist. https://doi.org/10.1080/00222933.2016.1180718
Rosseti DF, Bezerra FHR, Dominguez JML (2013) Late oligocene–miocene transgressions along the equatorial and eastern margins of Brazil. Earth Sci Rev. https://doi.org/10.1016/j.earscirev.2013.04.005
Sigmine (2023) Sistema de Informações Geográficas da Mineração. https://geo.anm.gov.br/portal/apps/webappviewer/index.html?id=6a8f5ccc4b6a4c2bba79759aa952d908. Accessed 30 january 2023
Silva AC, Souza AF (2018) Aridity drives plant biogeographical sub regions in the Caatinga, the largest tropical dry forest and woodland block in South America. PLoS ONE. https://doi.org/10.1371/journal.pone.0196130
Silva JMC, Leal I, Tabarelli M (2017) Caatinga: the largest tropical dry forest region in South America, first ed. Springer, New York
Smith TB, Kinnison MT, Strauss SY, Fuller TL, Carroll SP (2014) Prescriptive evolution to conserve and manage biodiversity. Ann Rev Ecol Evol Syst. https://doi.org/10.1146/annurev-ecolsys-120213-091747
Souza S, Riutort M, Ferreira RL, Leal-Zanchet A (2018) An integrative taxonomic approach reveals the first marine triclad (Platyhelminthes) trapped in a cave from a semiarid neotropical environment. Invertebr Syst. https://doi.org/10.1071/IS17062
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. https://doi.org/10.1093/bioinformatics/btu033
Stokkan M, Jurado-Rivera JA, Pedro O, Juan C, Jaume D, Pons J (2018) Species delimitation and mitogenome phylogenetics in the subterranean genus Pseudoniphargus (Crustacea: Amphipoda). Mol Phylogenetics Evol 127:988–999. https://doi.org/10.1016/j.ympev.2018.07.002
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positionspecific gap penalties and weight matrix choice. Nucleic Acids Res. https://doi.org/10.1093/nar/22.22.4673
Trontelj P, Douady CJ, Fiser C, Gibert J, Goricki S, LeFebure T, Sket B, Zaksˇek V (2009) A molecular test for cryptic diversity in ground water: how large are the ranges of macro-stygobionts? Freshw Biol 54(4):727–744. https://doi.org/10.1111/j.1365-2427.2007.01877.x
Werneck FP (2011) The diversification of eastern south American open vegetation biomes: historical biogeography and perspectives. Quat Sci Rev. https://doi.org/10.1016/j.quascirev.2011.03.009
Wessel A et al (2013) Founder effects initiated rapid species radiation in Hawaiian cave planthoppers. PNAS 110(23):9391–9396. https://doi.org/10.1073/pnas.1301657110
White WB, Culver DC, Pipan T (2019) Encyclopedia of caves, 3rd edn. Academic
Witt JDS, Threloff DL, Hebert PDN (2006) DNA barcoding reveals extraordinary cryptic diversity in an amphipod genus: implications for desert spring conservation. Mol Ecol 15(10):3073–3082. https://doi.org/10.1111/j.1365-294X.2006.02999.x
Zakšek V, Delić T, Fišer C, Jalžić B, Trontelj P (2019) Emergence of sympatry in a radiation of subterranean amphipods. J Biogeogr 46(3):657–669. https://doi.org/10.1111/jbi.13514
Zhang J, Kapli P, Pavlidis P, Stamatakis A (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics. https://doi.org/10.1093/bioinformatics/btt499
Acknowledgements
The authors would like to thank Programa de Pós-Graduação em Sistemática e Evolução (PPGSE/UFRN), Laboratório de Ictiologia Sistemática e Evolutiva (LISE/UFRN), Centro de Estudos em Biologia Subterrânea (CEBS/UFLA), Instituto Tecnológico Vale (ITV-DS) and Centro Nacional de Pesquisa e Conservação de Cavernas (ICMBio/Cecav) for providing infrastructure for the development of this study; to Marconi Silva, Iatagan Freitas, Daniel Mendonça, Matheus Rocha, Valéria Vale, Carol Puppin, Nathália Kaluana, Ana Bennemann, Yasmin Costa, Yuri Abrantes, Lindsey Hellmann and Geilson Góis for helping with the collections; We also thank Dr. Antonio Mateo Solé Cava, Dra. Lívia Medeiros Cordeiro, Dr. Sandro Santos and Dra. Christiane Ramos Donato for contributions to previous versions of the manuscript. The collection permit 54334 was granted by Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). The access to genetic heritage was duly registered in the Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado (SISGEN) under the number AA22F38.
Funding
This research was funded by Term of Commitment of Speleological Compensation (TCCE) 01/2018, signed between ICMBio and Vale S.A. and with resource management by the Instituto Brasileiro de Desenvolvimento e Sustentabilidade (IABS). Authors RLF and SMQL are grateful to the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the grant provided (CNPq n. 302925/2022-8 and 312066/2021-0, respectively). Part of the analyses were made possible through support by the Instituto Tecnológico Vale (ITV-DS, project “Cavidades - RBRS000603.84”).
Author information
Authors and Affiliations
Contributions
D.M.B., R.L.F. and S.M.Q.L. contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by DMB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Nigel Stork.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Medeiros Bento, D., Ferreira, R.L., Vasconcelos, S. et al. Before it’s too late: priority areas for conservation of cryptic and threatened species of troglobitic arthropods in the Brazilian semiarid. Biodivers Conserv 33, 1985–2010 (2024). https://doi.org/10.1007/s10531-024-02833-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-024-02833-w