Abstract
While protected areas are often considered strongholds for wildlife populations, recent research in protected areas has highlighted that both human activity (i.e. presence) and footprint (i.e. structures) can influence wildlife. To determine how human activity and structures affect the spatiotemporal activity of wildlife on the Apostle Islands National Lakeshore, Wisconsin, United States, we monitored the carnivore community for 5 years (2014–2018) using camera traps. We found that lighthouses had a negative impact on carnivore community richness, while historical sites had a positive impact. Responses of individual carnivore species to anthropogenic structures varied depending on structure type, with most of the canids and mustelids exhibiting negative associations with campgrounds. When examining the seasonal effects of human activity and footprint (i.e., when park visitation is relatively high or low), we found that carnivore richness was lower during the high human activity season, suggesting that seasonal variation in human activity influences carnivore activity. We also compared carnivore nocturnality along a gradient of anthropogenic activity, but our results indicate that the carnivore community did not become more nocturnal with increasing anthropogenic activity as expected. However, the carnivore community did display spatial avoidance of current anthropogenic structures, especially campgrounds. Our study indicates that human footprint in the form of structures and seasonal variation in human activity can influence wildlife activity within protected areas. Based on this study, species-specific research that includes multiple representations of potential human effects (i.e., including categories of human footprint and activity) will allow for a more nuanced and cohesive understanding of the impacts of humans on the spatial and temporal distributions of wildlife species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expansion of human influence has greatly affected wildlife around the world. In particular, human impacts on carnivores (species of the order Carnivora) have historically been detrimental (Karanth and Chellam 2009; Kellert et al. 1996; Ripple et al. 2014). This is partly because humans have traditionally viewed carnivores as pests or competitors for resources such as deer and other game species, leading to many instances of species persecution (Ripple et al. 2014). In recent decades, humans have made targeted efforts to conserve carnivore populations; however, human effects on carnivores, such as habitat loss due to development, are particularly strong relative to other wildlife groups since carnivores tend to occur at low population densities and require large home ranges to meet their nutritional needs (Ripple et al. 2014). In addition, both the increasing human population and the recent increase in outdoor recreation have intensified human impacts on carnivores in both urban and non-urban environments (Cordell et al. 2008; Gaynor et al. 2018; Ripple et al. 2014).
Human activity (i.e., presence) and human footprint (i.e., structures) are often considered analogous, but they can have differing impacts on wildlife (Nickel et al. 2020; Suraci et al 2021). This nuance is important to consider, as human activity and footprint could be having impacts on carnivore populations that thus far have not been considered or accounted for in wildlife management or human–wildlife conflict mitigation (e.g., Pelletier 2006; Reed and Merenlender 2011). Since risk associated with human footprint is likely perceived as spatially constant, human footprint is more likely than human activity to affect spatial patterns of carnivores. For example, some species, such as coyotes (Canis latrans) and red foxes (Vulpes vulpes) have been able to adapt their behavior to live within highly developed areas (Mueller et al. 2018), but other carnivores such as mountain lions (Puma concolor) and certain bear species tend to avoid establishing home ranges within highly developed areas (Beckmann and Lackey 2008; Gibeau et al. 2002; Riley et al. 2014). Human activity is more likely to affect temporal patterns of carnivores rather than their spatial patterns (Nickel et al. 2020). Urban carnivores that have adapted to highly altered areas tend to exhibit increased nocturnality compared to their rural counterparts (Gaynor et al. 2018). Though human activity and footprint typically have negative effects on wildlife, there are many species that have been able to take advantage of human activity (i.e., human shield effect; Moll et al. 2018; Suraci et al. 2019) or human footprint (i.e., urban dwellers; Fischer et al. 2015). Furthermore, it is likely that human activity influences wildlife perception of human footprint, and since human activity varies widely based on structure type (i.e., a suburban home versus a high-rise apartment building), wildlife likely do not perceive all structure types as equally rewarding or risky. Since human activity consistently follows daily, weekly, and seasonal temporal cycles, it is also likely that wildlife perception of different structure types may vary over time.
Protected areas, such as national and state parks, are often considered strongholds for wildlife populations, especially those that may be particularly susceptible to human impacts (Pacifici et al. 2020). Since protected areas are often managed under a dual mandate of conserving biodiversity and providing recreational opportunities, many protected areas have some degree of human footprint and human activity (Reed and Merenlender 2008). Similar to urban areas, human footprint in protected areas is likely perceived by wildlife as spatially constant while human activity is likely perceived as temporally constant. With respect to human footprint, carnivores in protected areas may avoid or be attracted to structures depending on their perceived risk or reward; though, avoidance or attraction is likely species-specific (Suraci et al. 2021). In contrast, carnivores in protected areas, where human activity is generally confined to daytime, can shift their daily activity patterns to avoid perceived risk associated with human activity (Farmer and Allen 2019; Nickel et al. 2020).
The Apostle Islands National Lakeshore has a long history of human activity and human footprint, and as part of the National Park Service (NPS), is a popular destination for recreational activities, including camping, hiking, and boating. Our objectives were to determine whether human activity and footprint (in the form of structures) affect the spatiotemporal activity of the carnivore community within the Apostle Islands. We hypothesized that species richness of the carnivore community and relative abundance of individual species will be positively related to distance from human structures, as carnivore species tend to spatially avoid human structures. We also expected that during the high human activity season, when there are substantial differences in the amount of human activity at different structure types, carnivores would more strongly avoid life estates, campgrounds, lighthouses, and Park Service buildings than historical structures, which receive much less human activity. In contrast, we expected no difference in avoidance between structure types during the low human use season as there is not a substantial difference in the amount of human activity between structure types. Finally, we hypothesized that the carnivore community would be more nocturnal on islands with greater human activity as they shift their daily activity patterns to avoid humans. Currently, there is minimal existing evidence concerning the impact of human activity and footprint on wildlife in remote protected areas.
Materials and methods
Study area
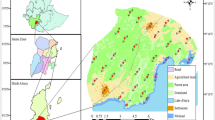
The Apostle Islands is an archipelago of twenty-one islands in lake Superior, Wisconsin, USA (Fig. 1). Beginning in the late 1600s, resource extraction from the islands included fur, fish, lumber, and sandstone, and companies built infrastructure for these activities, such as railroads and housing, on many of the islands. In 1970, Congress designated 21 of the islands in the archipelago as a National Lakeshore. Madeline Island was not included due to an already established year-round human population. Historical anthropogenic sites, which the National Park Service manages as cultural resources, include sites such as abandoned farms and quarries. Historical sites represent areas of human interest that are visited occasionally by park staff and infrequently by tourists. Current anthropogenic structures include campgrounds, lighthouses, and other structures that were built after 1970, as well as two life estates, one on Sand and one on Rocky Islands.
Location of camera traps and anthropogenic structures within the Apostle Islands National Lakeshore. Camera traps were placed as part of the carnivore monitoring project conducted from autumn 2014 to autumn 2018. Current structures include campgrounds, lighthouses, and Park Service buildings. Historical structures include those that were privately owned before inclusion in the national park system and are no longer maintained, such as quarries and farms. Private structures include the two life estates, one on Sand Island and one on Rocky Island. Figure created in ArcGIS version 10.5.1
Anthropogenic activity within the National Lakeshore includes recreational activities such as boating, camping, and hiking. Overnight camping on the islands is permitted through the National Park Service, which maintains individual campgrounds (maximum occupancy = 7) and group campgrounds (maximum occupancy = 21) on 18 of the islands and in the mainland unit, in addition to zone camping opportunities. Anthropogenic activity on the islands is dependent on accessibility, which varies throughout the year. During the warmer months, the islands are accessible by private boat, water taxi, or commercial boating operations, such as the Apostle Islands Cruises. The Apostle Islands Cruises, a NPS authorized concessioner, operates cruises that take day visitors to Raspberry and Michigan Island and campers to Stockton and Oak Island from June 28th until September 2nd.
Camera traps
Carnivores can be cryptic and difficult to monitor due to large home ranges and low population densities, but camera traps have proven to be an efficient technique for observing carnivore populations (Allen et al. 2018). To understand the effects of anthropogenic structures and activity on the carnivore community of the Apostle Islands, we used a grid of camera traps deployed across the island system to determine spatial and temporal activity patterns for the carnivore community and the individual carnivore species.
We deployed and maintained 164 camera traps on 19 islands from autumn 2014 to autumn 2018 (Fig. 1) using a systematic design for camera placement that maximized carnivore detections and camera independence. We identified potential camera sites as the center points of a 1 km2 grid superimposed over each island. We limited the potential sites to grid cells that contained at least 50% land area, and we adjusted the camera trap density on each island so that smaller islands had a higher density of cameras than larger islands using the power law equation:
where \(y\) is the number of camera traps on a specific island and \(x\) is island size (Allen et al. 2019). We adjusted camera trap density to ensure that every island received at least two cameras to offset potential camera failure, to purposefully under sample the largest islands due to logistical constraints, and to account for non-circular islands, which may result in relatively more grid cells with less than 50% land area. As a result, the smallest islands had a higher density of camera traps than the largest islands. Eagle Island only received one camera due to concerns over nesting shorebirds. To place each camera, we started at the center point of each grid cell and then identified a location that would maximize carnivore detections based on vegetation and signs of wildlife. After identifying an appropriate location, we placed a camera trap (HC600 Hyperfire™ High Output Covert, PC 800 Hyperfire Professional Semi-covert or HC500 Hyperfire Semi-covert cams; RECONYX, Inc., Holmen, WI, USA) on a nearby tree. We programmed each camera trap to capture a series of three pictures with no delay when activated and to include the date, time, and temperature with each picture.
Due to travel logistics for remote islands, we rotated camera traps between islands during the study period. We were unable to deploy cameras simultaneously on all of the islands due to a deployment period significantly limited by lake and weather conditions as well as staff availability. As such, during each deployment, we placed cameras on a subset of islands while ensuring equivalent sampling effort between islands. At either 6 months or 12 months intervals (depending on the island), we checked each camera trap to switch out batteries and SD cards. During the first deployment, we randomly selected half of the cameras on a given island to receive a scent lure placed within view of the camera using a commercially available predator lure (Caven’s Gusto, Minnesota Trapline Products Inc., Pennock MN). During the first camera check, camera traps that did not receive scent lure at first deployment received a scent lure, and then during subsequent camera checks, cameras were randomly selected for a scent lure. Camera traps on Eagle Island did not receive any scent lure due to concern over nesting shorebirds.
Statistical analyses
We performed all statistical analysis using R version 3.5.3 (R Core Team 2019) and ArcGIS version 10.5.1 (ESRI 2017). For all of the statistical analyses, we considered P < 0.05 to be significant.
To determine how anthropogenic structures and human activity affect the spatial activity patterns of the carnivore species, we used generalized linear mixed effects models to determine whether distance to nearest structure, type of nearest structure, and seasonal human activity levels predicted the richness of the carnivore community and the relative abundance of each carnivore species. We began by calculating the community richness as the number of carnivore species detected at each camera and a relative abundance index (RAI) for each carnivore species at each site using the equation:
where D is the number of detections of a given species at a given site and TN is the total number of trap nights that the camera trap at that site was active (Jenks et al. 2011; Farmer and Allen 2019). RAI is a more accurate indicator of both abundance (Palmer et al. 2018; Parsons et al. 2017) and site use (Sollmann 2018; though see Stewart et al. 2018) than using the raw number of photographs or occupancy (Parsons et al. 2017). However, detectability may vary between sites due to factors other than a species’ underlying distribution or activity patterns, such as viewshed obstructions (Moll et al. 2020). With regard to species richness, the longevity of the data collection period minimizes potential differences in detection probability between sites, which is an important consideration when using camera traps. Although RAI does not explicitly incorporate detectability, our camera placement framework, assuming that mechanisms underlying any potential differences in detection probability between sites are random relative to our variables of interest, mitigates potential differences in detection between sites as camera were placed based on a grid system which should minimize systematic detectability bias. To determine the number of detections, we first addressed possible psuedoreplication by consolidating multiple photographs of the same species that were taken within a span of 30 mins to be the same detection (Allen et al. 2019). To determine whether anthropogenic effects on the carnivore community are seasonal, we calculated a high use season RAI for each carnivore species using only data from June 28th until September 2nd of each year (i.e. the high-use season) (2014–2018) and a low use season RAI for each carnivore species using only data from September 3rd until June 27th. We selected these dates based on availability of public boat shuttles to the islands, which represents the period with the greatest accessibility.
We calculated Euclidean distance in kilometers from each camera station to the nearest anthropogenic structure using proximity analysis in ArcMap (Olson et al. 2012). We classified anthropogenic structures within the national lakeshore as ‘life estates’, ‘campground’, ‘historical site’, ‘lighthouse’, or ‘Park Service building’, which included ranger stations and visitor centers. We identified current and historic structures from NPS maps, and then determined exact GPS coordinates for all structures using satellite imagery from Google Earth (Version 7.3.1, Google Inc. 2018) (Fig. 1). Locations of life estates were provided by the NPS. We used a generalized linear mixed effects model with a negative binomial distribution and a log link in the lme4 package in R to test whether distance from the closest structure, the classification of the closest structure, and seasonal human activity affected carnivore community species richness and RAI of each individual carnivore species at each camera site (Bates et al. 2015). We used species richness or RAI of a carnivore as our dependent variable, distance from nearest structure, type of nearest structure, human use season (high or low), and an interaction between type of nearest structure and human use season as our fixed independent variables, and ‘island’ and ‘camera’ as random effects.
To determine how anthropogenic activities affected the temporal activity patterns of the carnivore community, we calculated risk ratios and built temporal activity overlap plots over a gradient of human activity. We calculated risk ratios, which measures the relationship between exposure to some treatment (in our case, different levels of human activity) and observed effects (in our case, nocturnal activity of carnivore species), for the carnivore community as a measure of the effect size of human activity. We also built temporal activity kernel density plots and estimated and compared activity levels for the carnivore community and individual species based on sites with differing levels of human activity. Based on potential maximum campground capacity and accessibility, we selected two islands and the mainland unit that represent a gradient anthropogenic activity: mainland unit (high), Stockton Island (moderate), and Outer Island (low). Both Stockton Island and Outer Island are approximately the same area but have vastly different potential maximum human use thresholds. Additionally, Stockton Island is accessible via the Apostle Islands cruises camping shuttle in addition to private vessels while Outer Island is only accessible via private vessels.
Using the timestamps on the camera trap photographs and the suntime function (‘overlap’, Meredith and Ridout 2017), we transformed each timestamp from ‘clock time’ to ‘sun time’. We then split the detections into Summer/Fall (June–November) and Winter/Spring (December–May) since anthropogenic activity and tourism is much higher during the summer and fall months. Next, we used the sun times to calculate the percentage of detections of carnivores for each island that occurred at night and the species-specific proportion of nocturnal events for each island. To measure effect size of anthropogenic activity, we calculated risk ratios with a 95% confidence interval using the proportion of nocturnal detections at each of the three sites and the fmsb package (Gaynor et al. 2018; Nakazawa 2018). Risk ratios are calculated by taking the ratio of two proportions, so we calculated risk ratios between island pairs during Summer/Fall and during Winter/Spring by dividing the proportion of nocturnal detections on the island with higher human activity by the proportion of nocturnal detections on the island with lower human activity. We calculated confidence intervals for the risk ratios using the equation:
where \(z\) is the z value of the desired confidence level, \(x\) is the number of nocturnal detections on a given island, \(n-x\) is the number of diurnal detections on a given island, and \(n\) is the total number of detections on a given island. We then took the antilog of the upper and lower limit to calculate the 95% confidence intervals. Since the null value for risk ratio confidence intervals is 1, confidence intervals that overlap 1 indicate that there is not sufficient evidence that the island pairs had statistically significant differences in nocturnal activity.
We also compared detections by building temporal activity kernel density plots for the entire carnivore community on each island. Due to low detection rates for most of the carnivores on the islands, we were unable to estimate and compare activity levels for each individual species. Instead, we built temporal activity kernel density plots for black bear and coyote, the only species with detections in all three focal areas. We then estimated activity levels for the carnivore community and for black bears and coyotes across all three sites by fitting a kernel density model to our timestamped detection data (Rowcliffe 2021). Finally, we applied a pairwise Wald test to determine whether there were any differences between activity level estimates across the three sites (Rowcliffe 2021).
Results
Summary statistics
Over the 5 year study period, we had 164 camera traps operating for 88,712 trap nights and documented 8869 independent detection events of 12 carnivore species. Detected carnivore species included: American marten (Martes americana; 11% of detections), black bear (Ursus americanus; 51% of detections), bobcat (Lynx rufus; 3% of detections), coyote (21% of detections), fisher (Martes pennanti; 4% of detections), gray fox (Urocyon cinereoargenteus; 3% of detections), gray wolf (2% of detections), mink (Neovision vision), raccoon (Procyon lotor), red fox (5% of detections), river otter (Lontra canadensis), and weasels (Mustela spp.). Six carnivore species (black bear, bobcat, coyote, fisher, grey fox, red fox, and American marten) were detected in all 5 years. We detected common raccoon and gray wolf in 4 out of 5 years; while weasels (Mustela spp.), otter, and mink were detected in three or fewer years and were not included in the spatial analyses due to low detection rates. For further details on the occupancy of specific islands, see Allen et al. (2018).
Human footprint
For carnivore community richness, historical sites and lighthouses both had a significant relationship with carnivore richness. Historical sites had a positive relationship (β = 0.46, P = 0.04), and lighthouses had a negative relationship with carnivore richness (β = −0.98, P < 0.01) (Table 1).
The RAI of canids (gray wolf, coyote, red fox, and grey fox), generally exhibited negative relationships with distance and campground (Table 1). Gray wolf, coyote, and red fox all had significant negative relationships with campgrounds (β = −2.56, P < 0.01, β = −0.92, P = 0.01, and β = −2.71, P < 0.01, respectively). Grey fox and red fox were the only carnivore species tested that had a significant negative relationship with distance to nearest structure (β = −0.01, P = 0.02 and β = −0.01, P = 0.03, respectively).
The RAI of mustelids (fisher and American marten) had a significant and negative relationship with campgrounds (β = −1.87, P < 0.01 and β = −2.55, P < 0.01, respectively). The RAI of black bears had a significant negative relationship with lighthouses (β = −1.86, P < 0.01), but a significant positive relationship with campgrounds (β = 0.85, P < 0.01). In contrast, bobcat RAI had a significant negative relationship with campgrounds (β = −1.97, P < 0.01) (Table 1).
After examining the effect of seasonal variation of human use of anthropogenic structures, there is some evidence that seasonality influences anthropogenic effects on the carnivore community. Season had a significant relationship with community richness (β = 0.95, P < 0.01), red fox RAI (β = 1.70, P = 0.01), and American marten RAI (β = 1.08, P = 0.03). Since high human activity was the reference level, and because the coefficients are positive, our results indicate greater carnivore richness and higher red fox and American marten RAI during the low human activity season.
Human activity
On both Stockton Island and the mainland unit, a majority of the total number of nocturnal detections were of black bears (55% of nocturnal detection on Stockton Island, 20% of nocturnal detections in the mainland unit) or coyotes (26% of nocturnal detections on Stockton Island, 53% of nocturnal detections in the mainland unit). In contrast, black bears and coyotes only made up 30% of the nocturnal detections on Outer Island while American martens accounted for over 60% of the nocturnal detections (Fig. 2A). Since Outer Island has relatively low species richness, a comparison of each individual species was not possible. When we compared nocturnality along a gradient of anthropogenic activity, we found there was no difference in nocturnality between the mainland unit, Stockton Island, and Outer Island. Risk ratio values for all the pairwise comparisons of the three units during the summer/fall and winter/spring seasons had confidence intervals that overlapped 1, which indicated no difference in temporal activity patterns associated with anthropogenic activity (Table 2). Temporal activity estimates for the entire carnivore community, black bear, and coyote provided further evidence that there was no difference in temporal activity distributions along the gradient of human activity as the p values for almost all of the pairwise comparisons were not significant (Table 3, Fig. 2B–D). The only significant comparisons were for the carnivore community between Outer Island and the Mainland (P < 0.01) and for coyote between Outer Island and the Mainland (P < 0.01) and Outer Island and Stockton Island (P < 0.01); however, the results for coyote activity may be due to low detection rates as Outer Island only had 16 coyote detections.
A Proportion of total nocturnal events by species with standard error bars captured by camera traps along a gradient of anthropogenic activity of the Apostle Islands National Lakeshore. Temporal activity overlap plots comparing the temporal activity of B the entire carnivore community, C coyotes, and D black bears along a gradient of human use in the Apostle Island National Lakeshore (WI, USA), where human activity was ranked as low, moderate, and high for Outer Island, Stockton Island, and the mainland, respectively. Figures created in R version 3.5.3 and then assembled in Powerpoint
Discussion
Our research in the Apostle Islands National Lakeshore demonstrated that current and historical human structures, as well as seasonal fluctuations in human activity, can influence carnivore community richness and behavior. Additionally, the carnivore community did not appear to display a community-wide shift to nocturnality in response to increasing human activity. Protected areas are often created with multiple objectives including, but not limited to, conserving biodiversity and provisioning recreational opportunities for the public. Although these areas are often considered strongholds for wildlife populations, there are human structures in many protected areas, and since wildlife tend to spatially avoid most forms of human footprint, including human structures, (Nickel et al. 2020; Pelletier 2006) it is important for managers to consider the potential impacts of human structures on wildlife within protected areas. Furthermore, due to the species- and structure-specific relationships, considering a more detailed categorization of human impacts may reveal relationships that may otherwise be concealed.
Since the magnitude and direction of the impacts from structures varied by species, it is especially important to consider potential impacts on each species, in addition to the community as a whole. All individual species, aside from gray fox, had significant relationships with campgrounds, and all of these relationships, aside from black bear, were negatively related. The difference between a large-bodied omnivore (black bears) and the rest of the carnivore species could be due to human food subsidies at campgrounds that draw in opportunistic wildlife, similar to urban food subsidies (Beckmann and Berger 2003; Hopkins et al. 2014). Across the United States, human–wildlife conflict is increasing and often caused by food-conditioned individuals (individuals that have learned to associate humans with food) (Baker and Timm 2017; Mazur 2010). Managers should determine whether positive associations between campgrounds and individual species are based on human food subsidies. This may be especially important with black bears, as they tend to be human-tolerant and are one of a few large carnivore species whose population is increasing (Ripple et al. 2014). Anecdotal evidence from the Apostle Islands, especially Stockton Island, Outer Island, and Oak Island, indicates that this may be the case for black bears.
Within protected areas, structure type is likely correlated to degree of human activity; however, it can be difficult to track human activity within protected areas due to the logistical difficulties involved with rigorously monitoring what are often large and minimally developed areas (Hadwen et al. 2007). For example, historical sites, which are visited occasionally by park staff and visitors, had a positive or no significant relationship with any of the carnivore species while lighthouses, many of which are visited by tour boats, had a negative or no significant relationship with any of the carnivore species. Though degree of human activity may be influencing the relationships between structure type and carnivore activity, it would be difficult to test conclusively as the park is not able to track visitation rates aside from camping permits and lighthouse tours. Similarly, shuttle services in protected areas may concentrate human activity at specific structures or specific structures themselves, such as campgrounds, may draw more human activity than other structures, resulting in a relationship between structure type and human activity that could explain the impacts of some structures on wildlife (Zeng et al. 2005). As such, new park infrastructure should take into account the impact of both the structures and the potential human activity on wildlife in the area.
As expected, seasonal fluctuations in human activity influenced carnivore community richness and the activity of red fox and American marten. The effect of season is likely related to accessibility and weather conditions (Hewer et al. 2017; Richardson and Loomis 2017). For example, Apostle Islands Cruises provides transportation between several of the islands and the mainland, which allows members of the public that don’t own boats to access the islands. Since the campground shuttle cruise service only operates from the end of June through the early September, the level of human activity within the island system will likely be different depending on whether the cruise service is operating. Accessibility is also a potential factor in other protected areas, as access is likely limited by road and trail networks (Walden-Schreiner et al. 2018). Similarly, campgrounds in protected areas are much more likely to be occupied during the summer months than during the winter months due to increased accessibility and weather conditions (Wilton and Wirjanto 1998). These results highlight the importance of accounting for seasonality of human activity, especially in remote regions with variable weather conditions.
Contrary to our hypotheses, the carnivore community exhibited no difference in nocturnality between areas with high, moderate, and low human activity. Even though carnivores generally show increased nocturnality in response to human activity and footprint, humans can have a differential effect on individual species (Crooks 2002; Gaynor et al. 2018). Body size and degree of specialization are often related to the magnitude of anthropogenic effect. As such, larger-bodied animals tend to be more negatively affected than smaller-bodied animals (Taylor and Knight 2003), and specialist species tend to be more negatively affected than generalists (Devictor et al. 2008). Since the carnivore community on the Apostle Islands ranges in body size and degree of specialization and because the proportion of nocturnal events for each species was not constant across the gradient of human activity, there may be a change in nocturnality for an individual species that is being masked by another, more common species. However, based on the temporal overlap plots for two of the largest carnivore species, which should be more affected than the smaller species (Gaynor et al. 2018; Farmer and Allen 2019), there was still no evidence of increased nocturnality based on human activity. Alternatively, carnivores within this system may be dependent on nocturnal prey, so there may be a temporal response to prey availability rather than to risk associated with human activity (Vilella et al. 2020).
Within a protected area, the carnivore community displayed spatial avoidance of specific human structures, though there was no detected difference in nocturnality along a gradient of human activity. Since season and type of structure are likely correlated with the level of human activity within protected areas, it is important to be able to accurately track the level of human activity to inform wildlife management within these areas. Understanding the mechanisms underlying species’ spatial and temporal distributions is necessary for effective and efficient conservation, especially concerning the expanding sphere of human influence and our changing climate. In this study, response to humans was both species- and structure-specific. Species-specific research that includes multiple representations of potential human effects (i.e., including categories of human footprint and activity) will allow for a much more nuanced and cohesive understanding of the impacts of humans on the spatial and temporal distributions of wildlife species. For example, had we not categorized the human structures, the pattern between campgrounds and almost all of the carnivore species may not have been evident due to the lack of relationship with many of the other structure types. Successful wildlife conservation within protected areas also requires mitigation of human–wildlife conflict. We demonstrate that campground management remains an important focal point for mitigation of human–wildlife conflict in protected areas. Future research should further examine the effects of human structures and activity on carnivore species fitness and population dynamics and how these effects may differ between species to better assess the conservation impacts of human footprint and activity.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to the state conservation status of the American marten, but are available from the corresponding author on reasonable request.
Code availability
The R code used during the current study is available from the corresponding author on reasonable request.
References
Allen ML, Farmer MJ, Clare JDJ, Olson ER, Van Stappen J, Van Deelen TR (2018) Is there anybody out there? occupancy of the carnivore guild in a temperate archipelago. Community Ecol 19:272–280. https://doi.org/10.1556/168.2018.19.3.8
Allen ML, Harris RE, Olson LO, Olson ER, Van Stappen J, Van Deelen TR (2019) Resource limitations and competitive interactions affect carnivore community composition at different ecological scales in a temperate island system. Mammalia 10:49–53. https://doi.org/10.1515/mammalia-2017-0162
Baker RO, Timm RM (2017) Coyote attacks on humans, 1970–2015: implications for reducing the risks. Hum–wildl Interact 11:120–132
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear-mixed effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beckmann JP, Berger J (2003) Rapid and ecological behavioural changes in carnivores: the responses of black bears (Ursus americanus) to altered food. J Zool 261:207–212. https://doi.org/10.1017/S0952836903004126
Beckmann JP, Lackey CW (2008) Carnivores, urban landscapes, and longitudinal studies: a case history of black bears. Hum Wildl Confl 2:168–174
Cordell HK, Betz CJ, Green GT (2008) Nature-based outdoor recreation trends and wilderness. Int J Wilderness 14:7–10
Crooks KR (2002) Relative sensitivities to mammalian carnivores to habitat fragmentation. Conserv Biol 16:488–502. https://doi.org/10.1046/j.1523-1739.2002.00386.x
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514. https://doi.org/10.1111/j.0030-1299.2008.16215.x
ESRI (2017) ArcGIS Desktop: Release 10. Environmental Systems Research Institute, Redlands, California, United States.
Farmer MJ, Allen ML (2019) Persistence in the face of change: effects of human recreation on coyote (Canis latrans) habitat use in an altered ecosystem. Urban Nat 29:1–14
Fischer JD, Schneider SC, Ahlers AA, Miller JR (2015) Categorizing wildlife responses to urbanization and conservation implications of terminology. Conserv Biol 29:1246–1248. https://doi.org/10.1111/cobi.12451
Gaynor KM, Hojnowski CE, Carter NH, Brashares JS (2018) The influence of human disturbance on wildlife nocturnality. Science 360:1232–1235. https://doi.org/10.1126/science.aar7121
Gibeau ML, Clevenger AP, Herrero S, Wierzchowski J (2002) Grizzly bear response to human development and activities in the bow river watershed, Alberta, Canada. Biol Conserv 103:227–236. https://doi.org/10.1016/S0006-3207(01)00131-8
Hadwen WL, Hill W, Pickering CM (2007) Icons under threat: why monitoring visitors and their ecological impacts in protected areas matters. Ecol Manage Restor. https://doi.org/10.1111/j.1442-8903.2007.00364.x
Hewer MJ, Scott DJ, Gough WA (2017) Differences in the importance of weather and weather-based decisions among campers in ontario parks (Canada). Int J Biometeorology 61:1805–1818
Hopkins JB III, Koch PL, Ferguson JM, Kalinowski ST (2014) The changing anthropogenic diets of American black bears over the past century in yosemite national park. Front Ecol Environ 12:107–114. https://doi.org/10.1890/130276
Jenks KE, Chanteap P, Damrongchainarong K, Cutter P, Cutter P, Redford T, Lynam AJ, Howard J, Leimgruber P (2011) Using relative abundance indices from camera-trapping to test wildlife conservation hypotheses—an example from Khao Yai national park, Thailand. Trop Conserv Sci 4:113–131. https://doi.org/10.1177/194008291100400203
Karanth KU, Chellam R (2009) Carnivore conservation at the crossroads. Oryx 43:1–2. https://doi.org/10.1017/S003060530843106X
Kellert SR, Black M, Rush CR, Bath AJ (1996) Human culture and large carnivore conservation in North America. Conserv Biol 10:977–990. https://doi.org/10.1046/j.1523-1739.1996.10040977.x
Mazur RL (2010) Does aversive conditioning reduce human–black bear conflict? J Wildl Manage 74:48–54. https://doi.org/10.2193/2008-163
Meredith M, Ridout M (2017) Overview of the overlap package. The Comprehensive R Archive Network. https://cran.r-project.org/web/packages/overlap/vignettes/overlap.pdf. Accessed 2 Oct
Moll RJ, Cepek JD, Lorch PD, Dennis PM, Robison T, Millspaugh JJ, Montgomery RA (2018) Humans and urban development mediate the sympatry of competing carnivores. Urban Ecosys 21:765–778. https://doi.org/10.1007/s11252-018-0758-6
Moll RJ, Ortiz-Calo W, Cepek JD, Lorch PD, Dennis PM, Robison T, Montgomery RA (2020) The effect of camera-trap viewshed obstruction on wildlife detection: implications for inference. Wildl Res 47:158–165. https://doi.org/10.1071/WR19004
Mueller MM, Drake D, Allen ML (2018) Coexistence of coyotes (Canis latrans) and red foxes (Vulpes vulpes) in an urban landscape. PLoS ONE. https://doi.org/10.1371/journal.pone.0190971
Nakazawa M (2018) fmsb: Functions for medical statistics book with some demographic data. R package version 0.6.3. The Comprehensive R Archive Network. https://CRAN.R-project.org/package=fmsb. Accessed 5 Oct
Nickel BA, Suraci JP, Allen ML, Wilmers CC (2020) Human presence and human footprint have non-equivalent effects on wildlife spatiotemporal habitat use. Biol Conserv. https://doi.org/10.1016/j.biocon.2019.108383
Olson ER, Ventura SJ, Zedler JB (2012) Merging geospatial and field data to predict the distribution and abundance of an exotic macrophyte in a large Wisconsin reservoir. Aquat Bot 96:31–41. https://doi.org/10.1016/j.aquabot.2011.09.007
Pacifici M, Di Marco M, Watson JEM (2020) Protected areas are now the last strongholds for many imperiled mammal species. Conserv Lett. https://doi.org/10.1111/conl.12748
Palmer MS, Swanson A, Kosmala M, Arnold T, Packer C (2018) Evaluating relative abundance indices for terrestrial herbivores from large-scale camera trap surveys. Afr J Ecol 56:791–803. https://doi.org/10.1111/aje.12566
Parsons AW, Forrester T, McShea WJ, Baker-Whatton MC, Millspaugh JJ, Kays R (2017) Do occupancy or detection rates from camera traps reflect deer density. J Mammal 98:1547–1557. https://doi.org/10.1093/jmammal/gyx128
Pelletier F (2006) Effects of tourist activities on ungulate behaviour in a mountain protected area. J Mt Ecol 8:15–19
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reed SE, Merenlender AM (2008) Quiet, nonconsumptive recreation reduces protected area effectiveness. Conserv Lett 1:146–154. https://doi.org/10.1111/j.1755-263X.2008.00019.x
Reed SE, Merenlender AM (2011) Effects of management of domestic dogs and recreation on carnivores in protected areas in Northern California. Conserv Biol 25:504–513. https://doi.org/10.1111/j.1523-1739.2010.01641.x
Richardson RB, Loomis JB (2017) Climate change and recreation benefits in an alpine national park. J Leis Res 37:307–320. https://doi.org/10.1080/00222216.2005.11950055
Riley SPD, Serieys LEK, Pollinger JP, Sikich JA, Dalbeck L, Wayne RK, Ernest HB (2014) Individual behaviors dominate the dynamics of an urban mountain lion population isolated by roads. Curr Biol 24:1989–1994. https://doi.org/10.1016/j.cub.2014.07.029
Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AR (2014) Status and ecological effects of the world’s largest carnivores. Science. https://doi.org/10.1126/science.1241484
Rowcliffe M (2021) activity: animal activity statistics. R package version 1.3.1. https://CRAN.R-project.org/package=activity
Sollmann R (2018) A gentle introduction to camera-trap data analysis. Afr J Ecol 56:740–749. https://doi.org/10.1111/aje.12557
Stewart FEC, Fisher JT, Burton AC, Volpe JP (2018) Species occurrence data reflect the magnitude of animal movements better than the proximity of animal space use. Ecosphere. https://doi.org/10.1002/ecs2.2112
Suraci JP, Clinchy M, Zanette LY, Wilmers CC (2019) Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol Lett 22:1578–1586. https://doi.org/10.1111/ele.13344
Suraci JP, Gaynor KM, Allen ML, Alexander P, Brashares JS, Cendejas-Zarelli S, Crooks K, Elbroch LM, Forrester T, Green AM, Haight J, Harris NC, Hebblewhite M, Isbell F, Johnston B, Kays R, Lendrum PE, Lewis JS, McInturff A, McShea W, Murphy TW, Palmer MS, Parsons A, Parsons MA, Pendergast ME, Pekins C, Pruch LR, Sager-Fradkin KA, Schuttler S, Şekercioğlu ÇH, Shepherd B, Whipple L, Whittington J, Wittemyer G, Wilmers CC (2021) Disturbance type and species life history predict mammal response to humans. Glob Change Biol 27:3718–3731. https://doi.org/10.1111/gcb.15650
Taylor AR, Knight RL (2003) Wildlife responses to recreation and associated visitor perceptions. Ecol Appl 13:951–963. https://doi.org/10.1890/1051-0761(2003)13[951:WRTRAA]2.0.CO;2
Vilella M, Ferrandiz-Rovira M, Sayol F (2020) Coexistence of predators in time: effects of season and prey availability on species activity within a mediterranean carnivore guild. Ecol Evol 10:11408–11422. https://doi.org/10.1002/ece3.6778
Walden-Schreiner C, Leung Y, Tateosian L (2018) Digital footprints: incorporating crowdsourced geographic information for protected area management. Appl Geogr 90:44–54. https://doi.org/10.1016/j.apgeog.2017.11.004
Wilton D, Wirjanto T (1998) An analysis of the seasonal variation in the national tourism indicators. Canadian Tourism Commission, Ottawa, Canada
Zeng H, Sui DZ, Wu XB (2005) Human disturbances on landscapes in protected areas: a case study of the wolong nature reserve. Ecol Appl 20:487–496. https://doi.org/10.1007/s11284-005-0065-6
Acknowledgements
We are grateful to the many students, employees, and volunteers that aided this research through camera trap maintenance and data input. We thank Dr. Kaitlyn Gaynor for supplying R code to build the temporal overlap figures.
Funding
Funding for this research was provided by the Apostle Islands National Lakeshore (GLNF CESU Agreement P14AC01180; J. Van Stappen), the Department of Natural Resources at Northland College (Sigurd Olson Professorship in the Natural Sciences, Morris O. Ristvedt Professorship in the Natural Sciences; E.R. Olson), NASA Earth and Space Science Fellowship (Grant number NNX16AO61H) and the Department of Forestry and Wildlife Ecology at the University of Wisconsin–Madison (Schorger Fund, Beers-Bascom Professorship in Conservation, T.R. Van Deelen).
Author information
Authors and Affiliations
Contributions
MJF: conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft, visualization. MLA: methodology, data curation, writing—review and editing. ERO: conceptualization, methodology, investigation, resources, writing—review & editing, funding acquisition. JVS: conceptualization, investigation, resources, writing—review and editing, project administration, funding acquisition. TRVD: conceptualization, methodology, investigation, resources, writing—review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
N/A.
Consent to participate
N/A.
Consent to publication
All authors have approved the manuscript before submission and consent to its publication.
Additional information
Communicated by Xiaoli Shen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farmer, M.J., Allen, M.L., Olson, E.R. et al. Anthropogenic activity and structures have varying effects on the activity of carnivores in a protected area in Wisconsin, United States. Biodivers Conserv 31, 3163–3178 (2022). https://doi.org/10.1007/s10531-022-02482-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02482-x