Abstract
Understanding the ecological factors influencing African forest elephant (Loxodonta cyclotis) abundance and distribution is crucial for their conservation management in Central Africa. Dung surveys have been conducted at the landscape scale and confirmed the overwhelming impact of anthropogenic activities on forest elephants. We present results from a small-scale survey in a pristine protected area without anthropogenic activities to elucidate the ecological factors influencing forest elephant density. We conducted a line transect dung survey in a small study area (110 km2) around Mbeli Bai, a natural forest clearing in the Nouabalé-Ndoki National Park, Republic of Congo, and compare results with a landscape survey conducted during the same period. We used habitat specific dung decay data collected on site to estimate elephant density using distance sampling. We fitted Generalized Additive Models to elephant dung encounter rate using explanatory variables collected during the transect survey and from geospatial data. The small-scale survey revealed a precise estimate of forest elephant density that was twice as high as the result from a landscape survey with higher density in mixed species forest for the small-scale survey. We could not find an impact of the proximity of forest clearings and proximity to rivers at the small scale. Fine-scale habitat features, e.g. degree of canopy and understorey closure, had little explanatory power for elephant dung encounter rate. Small-scale dung surveys are a useful method to reveal spatio-temporal variation in forest elephant density and distribution which can inform conservation practitioners in a timely manner. Combining monitoring methods at various spatial scales improves our knowledge and conservation efforts of forest elephants. Nouabalé-Ndoki National Park is a stronghold for forest elephants and of global importance for their conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat destruction, overexploitation, and climate change has led to a global decline in many wildlife populations (McCallum 2015; Young et al. 2016; Benítez-López et al. 2017). Central Africa has the second largest continuous rainforest on earth, which is of global priority for biodiversity conservation (Kamdem-Toham et al. 2003; Malhi et al. 2013). However, these ecosystems are rapidly changing due to deforestation, habitat degradation, small-scale agriculture, and infrastructure development, most notably logging roads (Tyukavina et al. 2018; Kleinschroth et al. 2019b; Molinario et al. 2020). Consequently, intact forest landscapes free from human activities are becoming increasingly rare even in the most remote regions (Potapov et al. 2017; Kleinschroth et al. 2019a; Morgan et al. 2019; Shapiro et al. 2021). Wildlife in Central Africa is under threat due to the loss of suitable habitat, unsustainable hunting, and commercial poaching (Blake et al. 2007; Junker et al. 2012; Abernethy et al. 2013). Particularly forest elephants (Loxodonta cyclotis) are declining dramatically throughout their range due to poaching activities triggered by international ivory demand (Blake et al. 2007; Maisels et al. 2013; Poulsen et al. 2017). Poaching and population declines of forest elephants can have far reaching consequences for ecological processes, people´s livelihoods, and on carbon storage capacities (Breuer et al. 2016; Poulsen et al. 2017; Berzaghi et al. 2019).
Identifying and monitoring factors associated with species´ population density is crucial to understanding how ecological factors and anthropogenic threats impact mammal abundance and distribution in space and time (Ives 1995; Balmford et al. 2003; Nichols and Williams 2006; Lindenmayer and Likens 2010). The natural variation in wildlife abundance and distribution is related to ecological variability, most often related to food availability in different habitat types. Such information contributes to the improvement of conservation planning, for example, through the identification of high conservation value forest (Elith and Leathwick 2009; Holbrook et al. 2017; Tédonzong et al. 2018; Ives 1995; Lindenmayer and Likens 2010). Surveying mammals at differential scales offers the potential to provide insightful information for conservation monitoring and species specific habitat requirements (Chapman and Chapman 1999; Cromsigt et al. 2009; Mayor et al. 2009; Bortolamiol et al. 2014). For example, where larger landscape surveys can reveal important insights into the human activities, such as logging, hunting or infrastructure development impacting forest elephant distribution, small scale studies, particularly within pristine sites, can help to elucidate the environmental factors that impact the natural distribution, abundance, and habitat choice of forest elephants.
There are various approaches to investigate the ranging behaviour, habitat choice, and population density of elephants. For herd-living elephants, such as Asian (Elephas spp.) and African savanna elephants (Loxodonta africana) a common method is to radio collar a few members of an elephant herd to reveal habitat use and provide local density estimates (Bastille-Rousseau et al. 2020; Evans et al. 2020; Wilson et al. 2021). However, given that forest elephants do not occur in large herds (Fishlock et al. 2008; Fishlock and Lee 2013; Schuttler et al. 2014; Goldenberg et al. 2021), radio-collared forest elephants show strong individual differences in ranging patterns that make it difficult to assess population relevant patterns of habitat use (Blake et al. 2008; Momont et al. 2015; Beirne et al. 2020; Molina-Vacas et al. 2020). Additionally, aerial surveys and more recently high-resolution satellite imagery are used to count savanna elephants (Duporge et al. 2020; Lamprey et al. 2020). Most mammal species in the African rainforest, including forest elephants, are difficult to survey via direct counts due to their elusive behaviour and the dense inaccessible rainforest (Plumptre 2000). Therefore, indirect sign left by forest dwelling wildlife (e.g. dung, tracks, acoustic cues, …) is often used to assess their status (Walsh and White 1999; Plumptre 2000; Hedges 2012b; Head et al. 2013; Starkey et al. 2014; Wrege et al. 2017). Dung count distance sampling along line transects (Buckland et al. 2001, 2015) is the most widely used method to estimate forest elephant density and abundance (Merz 1986; Barnes and Jensen 1987; Hedges and Lawson 2006; Strindberg 2012; Hedges 2012a). Dung surveys often remain the only approach that allows comparisons with results from decades ago (Thouless et al. 2016).
Elephant survey methods need to be adjusted according to objectives and available resources (Hedges 2012a; Strindberg 2012). On a large scale forest elephant abundance and distribution in human-modified landscapes is largely determined by anthropogenic factors (Barnes et al. 1991; Beyers 2008; Maisels et al. 2013). Landscape surveys demonstrate that proximity to roads is a strong correlate of the density and distribution of forest elephants and a driver of poaching pressure by providing access to remote areas (Blom et al. 2004; Laurance et al. 2006; Blake et al. 2008; Yackulic et al. 2011; Vanthomme et al. 2013). Consequently, higher densities of wildlife are found inside protected areas and certified logging concessions and with increasing distance from roads and human settlements (Barnes et al. 1991; Clark et al. 2009; Stokes et al. 2010; Remis and Kpanou 2011; Maisels et al. 2013; N´Goran et al. 2016; Lhoest et al. 2020). The forests in and around Nouabalé-Ndoki National Park (NNNP) are mainly composed of mixed species forest on terra firma and monodominant Gilbertiodendron dewevrei forest and to some extent swampy forest. While the landscape surveys confirmed a general preference of forest elephants for mixed species forest, results were confounded by anthropogenic impacts (Stokes et al. 2010). The forests of northern Congo also contain mineral rich natural forest clearings, called “bais”, a prominent feature in most of these forests. Bais can attract many different large mammal species, that consume mineral rich soil, water, and vegetation (Klaus et al. 1998; Magliocca and Gautier-Hion 2002; Gessner et al. 2014). Hundreds of different forest elephants can visit bais (Vanleeuwe et al. 1997; Fishlock 2010; Turkalo et al. 2013) potentially leading to high local forest elephant density at a small scale. Bais appear to positively influence forest elephant density at the large landscape scale (Clark et al. 2009; Stokes et al. 2010). Results from small scale studies within areas without human activities are largely lacking. In north-eastern Gabon distance to village was a strong correlate of wildlife abundance but not for elephants (Koerner et al. 2017).
In this study, we investigated forest elephant density and habitat preference within a pristine rainforest in northern Congo without human activities (neither hunting nor logging). The forests of northern Congo contain one of the largest remaining populations of forest elephants, and the largest remaining populations of western lowland gorillas (Gorilla gorilla gorilla) and central chimpanzees (Pan troglodytes troglodytes) (Maisels et al. 2013; Strindberg et al. 2018). Particularly, NNNP in the Republic of Congo, which is part of the Sangha Trinational World Heritage Site (https://whc.unesco.org/en/list/1380), is considered as one of the most pristine remaining rainforest in Central Africa, as there are no roads within NNNP, it has never been logged (Morgan et al. 2019), and has extremely low levels of illegal human activities (Stokes et al. 2010; Maisels et al. 2012).
To shed light on possible associations of ecological factors influencing forest elephant density and distribution at a small scale (~ 100 km2), we present results from a line transect elephant dung survey conducted (at the same time period as a larger landscape survey) around Mbeli Bai, a natural forest clearing in the south west of NNNP. A site specific dung decay rate was used to convert dung density and abundance to forest elephant density and abundance. We expected high local elephant densities in this pristine protected area compared to the overall landscape survey results. We predicted forest elephant abundance to be higher 1) in mixed species forest compared to monodominant Gilbertiodendron forest, and 2) closer to the Mbeli Bai clearing. We additionally included other habitat features, such as proximity to rivers, in the analysis, but expected little to no influence on elephant distribution within the study site. Furthermore, we investigated whether proximity of the national park boundary influences elephant distribution, given that human impacts are expected to be higher near the boundary of NNNP.
We compare our small-scale elephant survey results with findings from our landscape surveys conducted at the same time using the same dung decay from our site and same defecation rate estimates. We discuss the implications for forest elephant monitoring programs and elephant conservation.
Methods
Study area
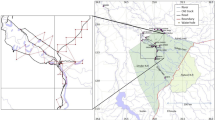
Our survey area (114.3 km2) was located around Mbeli Bai (N2 14.400 E16 23.862), the largest (0.127 km2) of several natural forest clearings along the tributaries of the Ndoki River that forms the south-western border of NNNP with an altitude between 300-500 m (Fig. 1). The vegetation in NNNP is typically semi-deciduous rainforest with a dry season from December to March and a rainy season from April to November (with peaks around September and October). Annual rainfall averages 1,694 mm with a minimum and maximum mean monthly temperature of 21.1 ºC and 26.6 ºC, respectively. The forest in NNNP has never been logged and is one of the most pristine protected areas in Central Africa and an important stronghold for forest elephants, great apes, and other large mammals (IUCN 2014). NNNP has extremely low levels of illegal hunting activities (Stokes et al. 2010). NNNP is surrounded by various logging concession with the FSC-certified Kabo logging concessions bordering the study area to the west (see also Morgan et al. 2019). Mbeli Bai was a major poaching area before the establishment of our study (Fay and Agnagna 1991) and since 1993 has been free of poaching until a poaching event in 2017 in the bai (Stephan et al. 2020). Mbeli Bai and several other forest clearings in the Sangha Trinational are regularly visited by forest elephants who feed on mineral rich water and soil as well as aquatic vegetation (Fishlock et al. 2008; Turkalo et al. 2013; Gessner et al. 2014; Metsio Sienne et al. 2014; Maisels et al. 2015b).
Survey design and data collection
In May 2006, we conducted a three-day pilot line transect survey (total transect distance covered = 12.5 km along five 2.5 km transect) to estimate elephant dung pile encounter rate and to get familiar with the survey techniques revealing an encounter rate of 3.5 dung piles per km which we used to inform our study design.
We used line transect distance sampling to estimate the densities of elephant dung piles (Buckland et al. 2001). We systematically placed 61 line transects (1 km long and 1 km equidistant) with a random start within the survey area using Distance 5.0 (release 2) (Thomas et al. 2010). We placed transects perpendicular to the Ndoki River in order to account for the potential influence of this border river. Our survey was conducted between May and July 2006, which coincides with the start of the rainy season. We walked along straight transect lines and collected data for all elephant dung that was not considered to have decayed based on the same state of decomposition as in our elephant dung decay study (Breuer and Hockemba 2007). For each dung pile we recorded the perpendicular distance between the transect and the centre of the sign and the distance along the transect using standard procedures (Hedges 2012a).
We recorded the forest type, as well as whether the canopy and understorey were open or closed, and the composition of the understory (liana dominated, mixed vegetation, Marantaceae or open understorey). The changes in forest type along the transect were noted using a Forest Suppliers Hip Chain® distance measurer. The three main forest types that occur in the study are: (i) Monodominant Gilbertiodendron dewevrei forest, which is either found along watercourses or occasionally occurs in large monodominant inland stands. The understorey in this monodominant forest is generally open and the canopy closed, but many light gaps exist due to regular tree falls (hence causing a closed understorey); (ii) Mixed species forest on terra firma soil, which is the dominant forest type in NNNP with high diversity; the most common tree species come from the families Annonaceae, Ebenaceae, Irvingiaceae, Leguminoseae, Meliaceae, and Sterculiaceae (Moutsamboté et al. 1994; Harris et al. 2008). Mixed species forest can show larger patches of broken canopy with dense understorey of terrestrial herbaceous vegetation (monocotyledons) from the families Marantaceae or Commelinaceae; (iii) Swamp forest that can include bais, which are almost always associated with watercourses (such as the Ndoki River) at low altitude and often show lower canopy height, many vines, and are dominated by the families: Annonaceae, Euphorbiaceae, and Rubiaceae.
Data analysis
Density estimates
We estimated elephant density using Distance 7.3 (Thomas et al. 2010). We considered several detection function models with different combinations of the key function (half-normal, hazard rate, and uniform) and adjustment terms (cosine, simple polynomial, hermite polynomial) with 0%, 5%, and 10% right truncation of the data for each of those models. We selected the best model based on the lowest Akaike’s Information Criterion (Burnham et al. 2011), the model fit on or close to the line, and the associated coefficient of variation. To convert dung density into elephant density, two conversion rates are needed: elephant dung defecation rate and dung decay rate. Defecation rates come from several studies following elephants through the forest (Tchamba 1992; Theuerkauf and Ellenberg 2000; Nchanji et al. 2008; Theuerkauf and Gula 2010). Dung density was converted into elephant density using a defecation rate of 19.77 (SE = 0.23) dung piles (Tchamba 1992). Elephant dung decay was obtained from published data from the same study area of 51.30 days (SE = 2.81) (Breuer and Hockemba 2007). We present elephant density estimates of mixed species and Gilbertiodendron forests using forest type specific decay rates (mixed species forest: 57.60 days (SE = 1.179); Gilbertiodendron forest: 53.07 days (SE = 0.466)) (Breuer and Hockemba 2007) and forest type specific effective strip half-widths (ESWs).
Testing covariables of elephant density distribution with generalized additive models.
We next investigated which covariables explained elephant density distribution (measured by dung encounter rate). The explanatory variables considered, included both those based on data recorded in the field along the transects and some data layers extracted using ArcGIS. The former included the total distance along the transect during which the following key habitat features were observed: 1) forest type, 2) open/closed canopy, 3) open/closed understorey, and 4) type of understorey vegetation). The GIS-based variables included distance to key habitat features (5) Mbeli Bai, 6) closest other bai, 7) nearest river, 8) and the distance to the national park border.
We fitted Generalized Additive Models (GAMs) (Hastie and Tibshirani 1990) to the elephant dung count data by means of the mgcv library (Wood 2017) in the R statistical software (R Core Team 2020) using a Tweedie distribution (Jørgensen 1987) to correctly account for the large proportion of zero counts (see Fig. 1). A log link was used and during model fitting an iterative search was applied to estimate the power parameter of the Tweedie distribution. The transect length was included as an offset term in the models to account for observer effort, thus effectively modelling dung encounter rate. The correlation between all explanatory variables was considered and highly correlated variables (with the absolute value of the correlation coefficient > 0.7) were not included in the same model to facilitate interpretation of the results. Models were ranked by Akaike’s Information Criterion (AIC) (Anderson and Burnham 2002).
Results
Habitat types
We walked a total of 52.4 km along the transects. Transects passed through a mixture of forest types. Mixed species forest was the most common forest type (61.7% of distance walked along transects), followed by monodominant Gilbertiodendron forest (34.4%) and swamp forest (4%). In total, 54.6% was closed canopy forest compared to 45.4% open canopy forest. Monodominant Gilbertiodendrion forest contained a larger proportion of closed canopy forest (65.7%), whereas swamp forest contained more open canopy forest (59.5%). Mixed species forest had almost equal proportions of closed (49.2%) and open canopy forest (50.8). The understorey was either open (59.5%) or closed with lianas (8.7%) or Marantaceae species (31.8%). Mixed species forest contained 52.4% open understorey and 11.8% closed understorey dominated by liana species and 35.9% understorey dominated by Marantaceae species. Monodominant Gilbertiodendron forest was dominated by open understorey (71.6%), and a much smaller portion of closed understorey (Marantaceae: 27.2%: lianas: 1.2%). Swamp forest either had open understorey (64.1%) or closed understory dominated by lianas (35.9%).
Sampling effort and encounter rates
We found no sign of illegal human activity during the survey. We counted 324 elephant dung piles along the transects, giving a dung encounter rate of 6.2 dung piles/km. Elephant dung was encountered on every transect (average 6.00 ± 4.38 SD; range 1—22). The total percent coefficient of variation (%CV) of the elephant density estimate (see next paragraph) was 12.2%. Encounter rate made up the biggest portion of the variation in density (53.2%), followed by detection probability (25.8%), dung decay rate (20%) and dung production rate (0.9%).
Forest elephant density, and the effect of forest type, Mbeli Bai, rivers and park border
The best selected model (uniform cosine with 10% truncation) revealed an overall density of 0.946 (0.743–1.203) elephants/km2 (%CV = 12.2%; ESW = 2.91 m; the two next best models had very similar AIC values and revealed very similar density estimates). Density estimates from Gilbertiodendron forests (fg; 0.496 (0.351–0.701) elephants/km2; %CV = 17.4% of which 82% was due to encounter rate variation; ESW = 4.20 m) were half of those from mixed species forest (fm; 0.977 (0.781 – 1.233) elephants/km2; %CV = 11.3 of which 87% was due to encounter rate variation; ESW = 2.85 m).
When considering elephant dung counts along the transects as a response variable the most parsimonious, highest ranked models according to AIC value were single variable models that included either the explanatory variable Gilbertiodendron forest (p = 0.017) or mixed forest (p = 0.026). With increasing amounts of Gilbertiodendron and mixed species forest along the transect, the encounter rate of elephant dung piles decreased and increased, respectively (Fig. 2). None of the other explanatory variables (understorey closure, distance to Mbeli Bai, …) had significant predictive power, although there was some (non-significant) indication that the encounter rate of elephant dung piles was lower, or higher with increasing amounts of open and closed canopy, respectively. There was also a non-significant increase in the encounter rate of elephant dung piles with increasing distance to rivers and to the park border.
Elephant dung encounter rate for key habitat variables, namely Gilbertiodendron forest and mixed forest included in single variable GAMs. The amount of the particular habitat along the transect line (in meters) is shown on the x axis, the y axis (dung encounter rate) is on the scale of the response, and estimates (solid lines) with confidence intervals (dashed lines) and a rug plot are shown
Discussion
Density estimates and habitat choice
The forest elephant dung encounter rate revealed in our study was one of the highest recorded in Central Africa (Maisels et al. 2013). Our density estimates are high, but lower than those from protected areas that contain large amounts of closed understorey forest with high density of terrestrial herbaceous vegetation in the understorey (i.e., Marantaceae forest) (Blake 2002; Blake et al. 2007; Beyers 2008; Maisels et al. 2013). Forest elephant density around Mbeli Bai was twice as high as the result from the landscape survey conducted during the same period and very similar to density estimates from before the creation of NNNP in the same area (Fay and Agnagna 1991; Stokes et al. 2010). This likely reflects the pristine state of the study area providing a genuine estimate of forest elephant density in an intact environment in the absence of poaching. While we could not find a direct effect of the proximity of Mbeli Bai on elephant dung encounter rate within the study site, the natural clearing might still draw elephants from outside the study area given its nutritional importance for forest elephants explaining the high local elephant density.
Density estimates in mixed species terra firma forest were twice as high as those from Gilbertiodendron forests. Mixed species forest in NNNP (as elsewhere) contains higher diversity and density of fruit bearing tree species (Blake 2002; Sunderland and Balinga 2005). Forest elephants prefer terra firma mixed species over monodominant Gilbertiodendron forest, as confirmed by landscape surveys (e.g. Stokes et al. 2010). The local availability of high-quality fruit resources can strongly affect forest elephant ranging behaviour and can lead to temporally high local elephant abundance (White 1994; Blake 2002; Morgan and Lee 2007; Cardoso et al. 2019; Beirne et al. 2020). Our survey period did fall at the start of the fruiting season, so it is likely that in the peak season, local elephant density might be even higher, which is also in line with higher elephant visitation rates to Mbeli Bai (Mbeli Bai long-term data). Higher forest elephant encounter rates in monodominant Gilbertiodendron forest (Melletti et al. 2009) might be due to mast-fruiting events of Gilbertiodendron (Blake and Fay 1997), which attract forest elephants, as the germinating shoots are highly favoured by them.
Contrary to other studies, (Barnes et al. 1991; Blake 2002; Beyers 2008) we could not find a preference for closed understorey that is indicative of high densities of herbaceous vegetation. However, we limited the habitat analysis to encounter rate and could not take micro habitat related variation in effective strip width into account. Thus, while high density of these herbs might be prevalent in logged forest due to removal of canopy trees, we did not find evidence that forest elephants prefer this habitat feature, albeit larger stands of these thick impenetrable understorey were absent at our site. While well managed logged forest might help to maintain high densities of forest elephants (Barnes et al. 1991; Clark et al. 2009; Poulsen et al. 2011), it remains speculative whether logging (in the absence of poaching) promotes high elephant densities. Further, studies are needed to investigate the long-term effect of removing large trees, including important fruiting species, on elephant abundance and whether this effect is compensated by the short-term increase in terrestrial herbaceous vegetation in forests with disturbed canopy closure. In a recent study, climate driven decline of fruit availability is leading to poorer physical condition in forest elephants (Bush et al. 2020). As an alternative explanation, elephants might feed less on terrestrial herbaceous vegetation in the vicinity of the clearing as the bai provides a superabundant source of aquatic herbs.
Proximity to Mbeli Bai, swamp use, scale of the study, and anthropogenic impacts
Despite high overall density, we could not find an effect of the proximity of Mbeli Bai (or other bais, or rivers) on elephant distribution in the study area, compared to the significant impact of bais (but not rivers) on forest elephant distribution within the landscape (Clark et al. 2009; Stokes et al. 2010). Several explanations for such a lack of effect are possible. Given the wide-ranging nature of forest elephants it is possible that all transects in the study area are in easy walking distance to the bai and only if one moves further out into the landscape the effect of bais becomes evident. Given the existence of a large elephant trail system around Mbeli Bai and elsewhere around bais (Vanleeuwe and Gautier-Hion 1998; Blake and Inkamba-Nkulu 2004) it is likely that elephants that walk several kilometres within a day (Blake 2002; Mills et al. 2018) move easily in and out of the study area. Alternatively, Mbeli Bai is indeed not an elephant concentration and it is possible that such small-scale effects are more visible for elephants at so called “elephant bais” with bare soil, whereas Mbeli Bai and its floating mass of vegetation is rather considered a typical gorilla bai (Maisels and Breuer 2015). The north of NNNP contains a series of such important elephant bais (Blake 2002). Further studies are needed to see whether there are small scale impacts on elephant abundance related to these elephant bais.
Due to small sample size we could not investigate habitat preference for swamps as dung deposited in swamps and water has a very quick decay rate (Breuer and Hockemba 2007). The importance of swamps for forest elephants is poorly known. For other large mammals, such as gorillas, forests can produce important fruits, such as Grewia and Nauclea (Doran-Sheehy et al. 2004) and lead to peak activities at Mbeli Bai (Walsh et al. 2007). While we could not find a significant effect of proximity to rivers (likely because we surveyed at the beginning of the fruiting season when elephants were not limited by water), elephant density in NNNP might be higher closer to rivers in the dry season (Blake 2002) further indicating how local density varies with environmental factors and seasonality.
We could not find a significant effect of the national park boundary on elephant distribution in our small-scale study, while these effects are more prominent in landscape surveys (Stokes et al. 2010). An absence of the effect of the national park border might be related to the location of our study side within the park borders and far away from the nearest village as within the landscape survey such effects are more prominent. Our study site was more than 20 km from the nearest village and during the study period there was no poaching or logging activity that might have influenced forest elephant ranging behaviour as shown elsewhere (Yackulic et al. 2011).
Recommendations for monitoring mammals in Afrotropical forests
Dung surveys remain an important monitoring method for forest elephants. Our survey results indicate that line transect dung counts were a suitable survey method for our small-scale study over a short survey period. Dung decay varies substantially between study sites in relation to a variety of factors, particularly rainfall, forest type, degree of canopy closure, and exposure to sunshine (White 1995; Barnes et al. 1997, 2006). Thus, when comparing survey results, variation in these conversion rates between study sites and periods might lead to a bias in elephant population estimates, particularly when using dung decay rates from other sites (Barnes 2001). Nchanji and Plumptre 2001; Breuer and Hockemba 2007; Vanleeuwe and Probert 2014). Since we used on-site dung decay and were consistent in assigning elephant dung age classes, as this was done by the same observers, we removed this significant source of error. Nevertheless, more recent work indicates that the accuracy of the estimates needs to be examined carefully. For example a recent genetic survey in Gabon revealed much smaller estimates compared to dung surveys (Brand et al. 2020). Direct observations of elephants along open line transects revealed only half the estimated density during the same period in the same area (Poulsen et al. 2011) which is not surprising given that these observations were done during the day, whereas elephants are also very active during the night.
Visibility (as expressed through the estimated effective strip width) varies with the degree of understorey closure as reflected by habitat type making simple encounter rates unreliable indicators of abundance with additional variability caused by seasonality and between observer differences (Breuer and Breuer-Ndoundou Hockemba 2012), unless changes in visibility are accounted for by the survey method, as is the case when detectability is estimated as part of a distance sampling analysis. Sampling effort is correlated with forest elephant dung encounter rate, and precision (expressed as the Coefficient of Variation (CV)) is related to variance in (i) the encounter rate, (ii) the detectability, and (iii) the conversion factors (Walsh and White 1999; Plumptre 2000; Walsh et al. 2001; Strindberg 2012). Our transect survey revealed precise estimates for forest elephants, which could be refined using habitat specific dung decay estimates. Standing crop line transects methods assume that elephant dung density is in a steady-state (Ahrestani et al. 2018), which might not hold due to elephant movements. This is particularly problematic during long-lasting landscape surveys. To avoid the steady-state assumption altogether retrospective on site dung decay estimates or rainfall/sunshine models should be applied particularly during landscape surveys (Barnes and Dunn 2002; Laing et al. 2003; Hedges and Lawson 2006).
Our small-scale survey might be particularly useful to complement landscape surveys that are generally repeated every five years, particularly to detect short-term changes in animal abundance related to anthropogenic threats and conservation efforts. Surveys results might then be used to inform law enforcement patrol effort (i.e., more surveys in mixed species forest; more rapid detection of changes in elephant abundance and poaching pressure). More specific survey designs, such as repeated surveys combined with botanical inventories to reveal the impact of fruit availability (Furuichi et al. 1997; Balcomb et al. 2000) are needed to understand spatial and temporal variation in elephant distribution due to environmental factors (Hedges 2012a; Strindberg 2012). For example, in the case of bais, radial walks around the clearing during different seasons might be able to detect the effects of bais on elephant abundance and distribution (Maisels et al. 2015a). Other methods, such as camera trapping, acoustic recording units, genetic methods or participatory approaches are increasingly applied in the rain forest environment (Buij et al. 2007; Eggert et al. 2014; Jathanna et al. 2015; Wrege et al. 2017; Bruce et al. 2018; Bourgeois et al. 2019; Nuñez et al. 2019; Bessone et al. 2020; Brand et al. 2020; Brittain et al. 2020; Laguardia et al. 2021).
Acoustics monitoring or camera traps might be of particular use in remote areas to reduce disturbance to wildlife. On the other hand more participation of indigenous and local communities in biomonitoring is needed in areas near human settlements outside strictly protected areas given the importance of involving local stakeholders in the management of multiple use conservation landscapes (Beirne et al. 2019; Brittain et al. 2020). Surveys alone cannot explain all the interactions of a species with the environment. Information from radio-collared forest elephants (Blake et al. 2001, 2008; Mills et al. 2018; Molina-Vacas et al. 2020) combined with these survey data will lead to more insights in the specific habitat preferences and species interactions in a rapidly changing landscape.
Conservation implications
Long-term research sites such as the Mbeli Bai Study act as an early warning system against poaching and are also a successful deterrent against illegal human activities (Campbell et al. 2011; Tagg et al. 2015). As poaching at other sites has and continues to reduce elephant populations (Maisels et al. 2013; N´Goran et al. 2016; Poulsen et al. 2017), NNNP and the larger Sangha Trinational area with its large tracts of intact forest is one of the last strongholds and refuge areas for these mega-herbivores and other wildlife (Stokes et al; 2010; Breuer et al. in press). Unfortunately, these last remaining pristine forests are increasingly threatened by human encroachment and extractive industries (Kleinschroth et al. 2019b; Morgan et al. 2019). It is possible that even here the elephants are experiencing anthropogenic threats and are being compressed into NNNP (Breuer et al. 2016) as happens elsewhere (Beyers 2008). Forest elephants exhibit slow population growth rates (Turkalo et al. 2017) making it difficult to recover populations that have been impacted by poaching. It is paramount to protect the remaining forest elephants in this stronghold of global importance.
Data availability
The data of our line transect survey are stored as Distance 7.3 projects and available from TB. The raw data can be obtained from the first author upon reasonable request.
References
Abernethy KA, Coad L, Taylor G et al (2013) Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. Philos Trans R Soc Lond B Biol Sci 368:20120303. https://doi.org/10.1098/rstb.2012.0303
Ahrestani FS, Kumar NS, Vaidyanathan S et al (2018) Estimating densities of large herbivores in tropical forests: Rigorous evaluation of a dung-based method. Ecol Evol 8:7312–7322. https://doi.org/10.1002/ece3.4227
Anderson KP, Burnham DA (2002) Model Selection and Multi-Model Inference : A Practical Information-Theoretic Approach, 2nd edn. Springer-Verlag, New York
Balcomb SR, Chapman CA, Wrangham RW (2000) Relationship between chimpanzee (Pan troglodytes) density and large, fleshy-fruit tree density: Conservation implications. Am J Primatol 51:197–203. https://doi.org/10.1002/1098-2345(200007)51:3%3c197::AID-AJP4%3e3.0.CO;2-C
Balmford A, Green RE, Jenkins M (2003) Measuring the changing state of nature. Trends Ecol Evol 18:326–330. https://doi.org/10.1016/S0169-5347(03)00067-3
Barnes RFW (2001) How reliable are dung counts for estimating elephant numbers? Afr J Ecol 39:1–9. https://doi.org/10.1046/j.1365-2028.2001.00266.x
Barnes RFW, Dunn A (2002) Estimating forest elephant density in Sapo National Park (Liberia) with a rainfall model. Afr J Ecol 40:159–163. https://doi.org/10.1046/j.1365-2028.2002.00362.x
Barnes RFW, Jensen KL (1987) How to count elephants in forests. IUCN African Elephant Rhino Spec Gr Tech Bull 1:1–9
Barnes RFW, Barnes KL, Alers MPT, Blom A (1991) Man determines the distribution of elephants in the rain forests of northern Gabon. Afr J Ecol 29:54–63. https://doi.org/10.1111/j.1365-2028.1991.tb00820.x
Barnes RFW, Asamoah-Boateng B, Naada Majam J, Agyei-Ohemeng J (1997) Rainfall and the population dynamics of elephant dung-piles in the forests of southern Ghana. Afr J Ecol 35:39–52. https://doi.org/10.1111/j.1365-2028.1997.061-89061.x
Barnes RFW, Majam JN, Asamoah-Boateng B, Agyei-Ohemeng J (2006) The survival of elephant dung piles in relation to forest canopy and slope in Southern Ghana. Pachyderm 41:37–43
Bastille-Rousseau G, Wall J, Douglas-Hamilton I et al (2020) Landscape-scale habitat response of African elephants shows strong selection for foraging opportunities in a human dominated ecosystem. Ecography (cop) 43:149–160. https://doi.org/10.1111/ecog.04240
Beirne C, Meier AC, Mbele AE et al (2019) Participatory monitoring reveals village-centered gradients of mammalian defaunation in central Africa. Biol Conserv 233:228–238. https://doi.org/10.1016/j.biocon.2019.02.035
Beirne C, Meier AC, Brumagin G et al (2020) Climatic and resource determinants of forest elephant movements. Front Ecol Evol 8:1–14. https://doi.org/10.3389/fevo.2020.00096
Benítez-López A, Alkemade R, Schipper AM et al (2017) The impact of hunting on tropical mammal and bird populations. Science 356:180–183. https://doi.org/10.1126/science.aaj1891
Berzaghi F, Longo M, Ciais P et al (2019) Carbon stocks in central African forests enhanced by elephant disturbance. Nat Geosci 12:725–729. https://doi.org/10.1038/s41561-019-0395-6
Bessone M, Kühl HS, Hohmann G et al (2020) Drawn out of the shadows: Surveying secretive forest species with camera trap distance sampling. J Appl Ecol Early View: https://doi.org/10.1111/1365-2664.13602
Beyers R (2008) Natural and anthropogenic influences on elephants and other ungulates in the Congo forest. Dissertation, University of British Columbia
Blake S (2002) The Ecology of Forest Elephant Distribution and its Implications for Conservation. Dissertation, University of Edinburgh
Blake S, Fay JM (1997) Seed production by Gilbertiodendron dewevrei in the Nouabalé-Ndoki National Park, Congo, and its implications for large mammals. J Trop Ecol 13:885–891
Blake S, Inkamba-Nkulu C (2004) Fruit, minerals, and forest elephant trails: do all roads lead to Rome? Biotropica 36:392–401. https://doi.org/10.1646/03215
Blake S, Douglas-Hamilton I, Karesh WB (2001) GPS telemetry of forest elephants in Central Africa: results of a preliminary study. Afr J Ecol 39:178–186. https://doi.org/10.1046/j.1365-2028.2001.00296.x
Blake S, Strindberg S, Boudjan P et al (2007) Forest elephant crisis in the Congo Basin. PloS Biol. https://doi.org/10.1371/journal.pbio.0050111
Blake S, Deem SL, Strindberg S et al (2008) Roadless wilderness area determines forest elephant movements in the Congo Basin. PLoS ONE 3:e3546. https://doi.org/10.1371/journal.pone.0003546
Blom A, Van Zalinge R, Mbea E et al (2004) Human impact on wildlife populations within a protected Central African forest. Afr J Ecol 42:23–31. https://doi.org/10.1111/j.0141-6707.2004.00441.x
Bortolamiol S, Cohen M, Potts K et al (2014) Suitable habitats for endangered frugivorous mammals: Small-scale comparison, regeneration forest and chimpanzee density in Kibale National Park. Uganda Plos One 9:e102177. https://doi.org/10.1371/journal.pone.0102177
Bourgeois S, Kaden J, Senn H et al (2019) Improving cost-efficiency of faecal genotyping: New tools for elephant species. PLoS ONE 14:e0210811. https://doi.org/10.1371/journal.pone.0210811
Brand CM, Johnson MB, Parker LD et al (2020) Abundance, density, and social structure of African forest elephants (Loxodonta cyclotis) in a human-modified landscape in southwestern Gabon. PLoS ONE 15:e0231832. https://doi.org/10.1371/journal.pone.0231832
Breuer T, Breuer-Ndoundou Hockemba M (2012) Intrasite variation in the ability to detect tropical forest mammals. Afr J Ecol 50:335–342. https://doi.org/10.1111/j.1365-2028.2012.01331.x
Breuer T, Hockemba MN (2007) Forest elephant dung decay in Ndoki Forest, northern Congo. Pachydem 43:43–51
Breuer T, Maisels F, Fishlock V (2016) The consequences of poaching and anthropogenic change for forest elephants. Conserv Biol 30:1019–1026. https://doi.org/10.1111/cobi.12679
Brittain S, Bata MN, De Ornellas P et al (2020) Combining local knowledge and occupancy analysis for a rapid assessment of the forest elephant Loxodonta cyclotis in Cameroon’s timber production forests. Oryx 54:90–100. https://doi.org/10.1017/S0030605317001569
Bruce T, Amin R, Wacher T et al (2018) Using camera trap data to characterise terrestrial larger-bodied mammal communities in different management sectors of the Dja Faunal Reserve, Cameroon. Afr J Ecol 56:759–776. https://doi.org/10.1111/aje.12574
Buckland ST, Anderson DR, Burnham KP et al (2001) Introduction to Distance sampling: estimating abundance of biological populations. Oxford University Press, Oxford
Buckland ST, Rexstad EA, Marques TA, Oedekoven CS (2015) Distance sampling: methods and applications. Methods in statistical ecology. Springer International Publishing, New York
Buij R, McShea WJ, Campbell P et al (2007) Use of patch-occupancy models to study forest elephants Loxodonta cyclotis movements in an industrial corridor in Gabon. Biol Conserv 135:189–201. https://doi.org/10.1016/j.biocon.2006.10.028
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. https://doi.org/10.1007/s00265-010-1029-6
Bush ER, Whytock RC, Bahaa-el-din L et al (2020) Long-term collapse in fruit availability threatens Central African forest megafauna. Science. https://doi.org/10.1126/science.abc7791
Campbell G, Kuehl H, Diarrassouba A et al (2011) Long-term research sites as refugia for threatened and over-harvested species. Biol Lett 7:723–726. https://doi.org/10.1098/rsbl.2011.0155
Cardoso AW, Malhi Y, Oliveras I et al (2019) The role of forest elephants in shaping tropical forest–savanna coexistence. Ecosystems 23:602–616. https://doi.org/10.1007/s10021-019-00424-3
Chapman CA, Chapman LJ (1999) Implications of small scale variation in ecological conditions for the diet and density of red colobus monkeys. Primates 40:215–231. https://doi.org/10.1007/BF02557712
Clark CJ, Poulsen JR, Malonga R, Elkan PW (2009) Logging concessions can extend the conservation estate for central African tropical forests. Conserv Biol 23:1281–1293. https://doi.org/10.1111/j.1523-1739.2009.01243.x
Cromsigt JPGM, Van Rensburg SJ, Etienne RS, Olff H (2009) Monitoring large herbivore diversity at different scales: Comparing direct and indirect methods. Biodivers Conserv 18:1219–1231. https://doi.org/10.1007/s10531-008-9506-1
Doran-Sheehy DM, Greer D, Mongo P, Schwindt D (2004) Impact of ecological and social factors on ranging in western gorillas. Am J Primatol 64:207–222. https://doi.org/10.1002/ajp.20075
Duporge I, Isupova O, Reece S et al (2020) Using very-high-resolution satellite imagery and deep learning to detect and count African elephants in heterogeneous landscapes. Remote Sens Ecol Conserv Early View. https://doi.org/10.1002/rse2.195
Eggert LS, Buij R, Lee ME et al (2014) Using genetic profiles of African forest elephants to infer population structure, movements, and habitat use in a conservation and development landscape in Gabon. Conserv Biol 28:107–118. https://doi.org/10.1111/cobi.12161
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697. https://doi.org/10.1146/annurev.ecolsys.110308.120159
Evans LJ, Goossens B, Davies AB et al (2020) Natural and anthropogenic drivers of Bornean elephant movement strategies. Glob Ecol Conserv. https://doi.org/10.1016/j.gecco.2020.e00906
Fay JM, Agnagna M (1991) A population survey of forest elephants (Loxodonta africana cyclotis) in northern Congo. Afr J Ecol 29:177–187
Fishlock VL (2010) Bai use in forest elephants (Loxodonta africana cyclotis). Dissertation, University of Stirling
Fishlock V, Lee PC (2013) Forest elephants: fission-fusion and social arenas. Anim Behav 85:357–363. https://doi.org/10.1016/j.anbehav.2012.11.004
Fishlock V, Lee PC, Breuer T (2008) Quantifying forest elephant social structure in Central African bai environments. Pachyderm 44:17–26
Furuichi T, Inagaki H, Angoue-Ovono S (1997) Population density of chimpanzees and gorillas in the Petit Loango Reserve, Gabon: Employing a new method to distinguish between nests of the two species. Int J Primatol 18:1029–1046. https://doi.org/10.1023/A:1026356432486
Gessner J, Buchwald R, Wittemyer G (2014) Assessing species occurrence and species-specific use patterns of bais (forest clearings) in Central Africa with camera traps. Afr J Ecol 52:59–68. https://doi.org/10.1111/aje.12084
Goldenberg SZ, Turkalo AK, Wrege PH et al (2021) Entry and aggregation at a Central African bai reveal social patterns in the elusive forest elephant Loxodonta cyclotis. Anim Behav. https://doi.org/10.1016/j.anbehav.2020.11.008
Harris DJ, Wortley AH, Moutsambote JM et al (2008) Liste des arbres du Parc National de Nouabalé-Ndoki, République du Congo / Draft checklist to the trees and shrubs of Nouabalé-Ndoki National Park. Republic of Congo, UK Darwin Initiative
Hastie T, Tibshirani R (1990) Generalized Additive Models. Chapman Hall & CRC, Boca Raton
Head JS, Boesch C, Robbins MM et al (2013) Effective sociodemographic population assessment of elusive species in ecology and conservation management. Ecol Evol 3:2903–2916. https://doi.org/10.1002/ece3.670
Hedges S (2012a) Estimating elephant population density and abundance from dung pile density: theoretical concepts. In: Hedges S (ed) Monitoring elephant populations and assessing threats: a manual for researchers, managers and conservationists. Universities Press, Himayatnagar, Hyderabad, pp 61–111
Hedges S (ed) (2012b) Monitoring elephant populations and assessing threats: a manual for researchers, managers and conservationists. Universities Press, Himayatnagar, Hyderabad
Hedges S, Lawson D (2006) Monitoring the Illegal Killing of Elephants. Dung Survey Standards for the Mike Programme. CITES MIKE Program
Holbrook JD, Squires JR, Olson LE et al (2017) Understanding and predicting habitat for wildlife conservation: The case of Canada lynx at the range periphery. Ecosphere 8:e01939. https://doi.org/10.1002/ecs2.1939
IUCN (2014) Regional action plan for the conservation of western lowland gorillas and central chimpanzees. IUCN SSC Primate Specialist Group, Gland
Ives AR (1995) Measuring Resilience in Stochastic Systems. Ecol Monogr 65:217–233. https://doi.org/10.2307/2937138
Jathanna D, Karanth KU, Kumar NS et al (2015) Reliable monitoring of elephant populations in the forests of India: Analytical and practical considerations. Biol Conserv 187:212–220. https://doi.org/10.1016/j.biocon.2015.04.030
Jørgensen B (1987) Exponential dispersion models. J R Stat Soc Ser B 49:127–162. https://doi.org/10.1111/j.2517-6161.1987.tb01685.x
Junker J, Blake S, Boesch C et al (2012) Recent decline in suitable environmental conditions for African great apes. Divers Distrib 18:1077–1091. https://doi.org/10.1111/ddi.12005
Kamdem-Toham A, Adeleke AW, Burgess ND et al (2003) Forest conservation in the Congo Basin. Science. https://doi.org/10.1126/science.299.5605.346
Klaus G, Klaus-Hugi C, Schmid BG (1998) Geophagy by large mammals at natural salt licks in the rain forest of the Dzanga National Park, Central African Republic. J Trop Ecol 14:829–839. https://doi.org/10.1017/S0266467498000595
Kleinschroth F, Garcia C, Ghazoul J (2019a) Reconciling certification and intact forest landscape conservation. Ambio 48:153–159. https://doi.org/10.1007/s13280-018-1063-6
Kleinschroth F, Laporte N, Laurance WF et al (2019b) Road expansion and persistence in forests of the Congo Basin. Nat Sustain 2:628–634. https://doi.org/10.1038/s41893-019-0310-6
Koerner SE, Poulsen JR, Blanchard EJ et al (2017) Vertebrate community composition and diversity declines along a defaunation gradient radiating from rural villages in Gabon. J Appl Ecol 54:805–814. https://doi.org/10.1111/1365-2664.12798
Laguardia A, Gobush KS, Bourgeois S et al (2021) Assessing the feasibility of density estimation methodologies for African forest elephant at large spatial scales. Glob Ecol Conserv 27:e01550. https://doi.org/10.1016/j.gecco.2021.e01550
Laing SE, Buckland ST, Burn RW et al (2003) Dung and nest surveys: Estimating decay rates. J Appl Ecol 40:1102–1111. https://doi.org/10.1111/j.1365-2664.2003.00861.x
Lamprey R, Pope F, Ngene S et al (2020) Comparing an automated high-definition oblique camera system to rear-seat-observers in a wildlife survey in Tsavo, Kenya: Taking multi-species aerial counts to the next level. Biol Conserv 241:108243. https://doi.org/10.1016/j.biocon.2019.108243
Laurance WF, Croes BM, Tchignoumba L et al (2006) Impacts of roads and hunting on Central African rainforest mammals. Conserv Biol 20:1251–1261. https://doi.org/10.1111/j.1523-1739.2006.00420.x
Lhoest S, Fonteyn D, Daïnou K et al (2020) Conservation value of tropical forests: Distance to human settlements matters more than management in Central Africa. Biol Conserv 241:108351. https://doi.org/10.1016/j.biocon.2019.108351
Lindenmayer DB, Likens GE (2010) The science and application of ecological monitoring. Biol Conserv 143:1317–1328. https://doi.org/10.1016/j.biocon.2010.02.013
Magliocca F, Gautier-Hion A (2002) Mineral content as a basis for food selection by western lowland gorillas in a forest clearing. Am J Primatol 57:67–77. https://doi.org/10.1002/ajp.10034
Maisels F, Breuer T (2015) What is a bai? In: Fishlock V, Breuer T (eds) Studying forest elephants. Neuer Sportverlag, Stuttgart, pp 12–13
Maisels F, Nishihara T, Strindberg S et al (2012) Great ape and human impact monitoring training, surveys, and protection in the Ndoki-Likouala Landscape. Bomassa, Congo
Maisels F, Strindberg S, Blake S et al (2013) Devastating decline of forest elephants in central Africa. PLoS ONE 8:e59469. https://doi.org/10.1371/journal.pone.0059469
Maisels F, Fishlock V, Greenway K et al (2015a) Detecting threats and measuring change at bais: a monitoring framework. In: Fishlock V, Breuer T (eds) Studying forest elephants. Neuer Sportverlag, Stuttgart, pp 144–155
Maisels F, Turkalo A, Breuer T (2015b) Elephants at bais: conservation, research and vulnerability. In: Fishlock V, Breuer T (eds) Studying forest elephants. Neuer Sportverlag, Stuttgart, pp 6–9
Malhi Y, Adu-Bredu S, Asare RA et al (2013) The past, present and future of Africa’s rainforests. Philos Trans R Soc B Biol Sci 368:20120293. https://doi.org/10.1098/rstb.2012.0293
Mayor SJ, Schneider DC, Schaefer JA, Mahoney SP (2009) Habitat selection at multiple scales. Ecoscience 16:238–247. https://doi.org/10.2980/16-2-3238
McCallum ML (2015) Vertebrate biodiversity losses point to a sixth mass extinction. Biodivers Conserv 24:2497–2519. https://doi.org/10.1007/s10531-015-0940-6
Melletti M, Mirabile M, Penteriani V, Boitani L (2009) Habitat use by forest mammals in Dzanga-Ndoki National Park, Central Africa Republic. Afr J Ecol 47:797–800. https://doi.org/10.1111/j.1365-2028.2008.01010.x
Merz G (1986) Counting elephants (Loxodonta africana cyclotis) in tropical rain forests with particular reference to the Tai National Park, Ivory Coast. Afr J Ecol 24:61–68
Metsio Sienne J, Buchwald R, Wittemyer G (2014) Differentiation in mineral constituents in elephant selected versus unselected water and soil resources at Central African bais (forest clearings). Eur J Wildl Res 60:377–382
Mills EC, Poulsen JR, Michael Fay J et al (2018) Forest elephant movement and habitat use in a tropical forest-grassland mosaic in Gabon. PLoS ONE 13:e0199387. https://doi.org/10.1371/journal.pone.0199387
Molinario G, Hansen M, Potapov P et al (2020) Contextualizing landscape-scale forest cover loss in the democratic Republic of Congo (DRC) between 2000 and 2015. Land 9:1–1. https://doi.org/10.3390/land9010023
Molina-Vacas G, Muñoz-Mas R, Martínez-Capel F et al (2020) Movement patterns of forest elephants (Loxodonta cyclotis Matschie, 1900) in the Odzala-Kokoua National Park, Republic of Congo. Afr J Ecol 58:23–33. https://doi.org/10.1111/aje.12695
Momont L, Breuer T, Schuttler S (2015) Forest elephant ranging and habitat use. In: Fishlock V, Breuer T (eds) Studying forest elephants. Neuer Sportverlag, Stuttgart, pp 132–139
Morgan BJ, Lee PC (2007) Forest elephant group composition, frugivory and coastal use in the Réserve de Faune du Petit Loango, Gabon. Afr J Ecol 45:519–526. https://doi.org/10.1111/j.1365-2028.2007.00762.x
Morgan D, Strindberg S, Winston W et al (2019) Impacts of selective logging and associated anthropogenic disturbance on intact forest landscapes and apes of northern Congo. Front for Glob Chang 2:28. https://doi.org/10.3389/ffgc.2019.00028
Moutsamboté J-M, Yumoto T, Mitani M et al (1994) Vegetation and list of plant species identified in the Nouabale-Ndoki forest, Congo. Tropics 3:277–293
N´Goran KP, Nzooh Dongma ZL, Le-Duc YS (2016) The status of forest elephant and great apes in Central Africa priority sites. WWF Biomonitoring report. Yaoundé
Nchanji AC, Plumptre AJ (2001) Seasonality in elephant dung decay and implications for censusing and population monitoring in south-western cameroon. Afr J Ecol 39:24–32. https://doi.org/10.1046/j.1365-2028.2001.00265.x
Nchanji AC, Forboseh PF, Powell JA (2008) Estimating the defaecation rate of the African forest elephant (Loxodonta cyclotis) in Banyang-Mbo Wildlife Sanctuary, south-western Cameroon. Afr J Ecol 46:55–59. https://doi.org/10.1111/j.1365-2028.2007.00808.x
Nichols JD, Williams BK (2006) Monitoring for conservation. Trends Ecol Evol 21:668–673. https://doi.org/10.1016/j.tree.2006.08.007
Nuñez CL, Froese G, Meier AC et al (2019) Stronger together: comparing and integrating camera trap, visual, and dung survey data in tropical forest communities. Ecosphere 10:e02965. https://doi.org/10.1002/ecs2.2965
Plumptre AJ (2000) Monitoring mammal populations with line transect techniques in African forests. J Appl Ecol 37:356–368. https://doi.org/10.1046/j.1365-2664.2000.00499.x
Potapov P, Hansen MC, Laestadius L et al (2017) The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci Adv 3:e160082. https://doi.org/10.1126/sciadv.1600821
Poulsen JR, Clark CJ, Bolker BM (2011) Decoupling the effects of logging and hunting on an Afrotropical animal community. Ecol Appl 21:1819–1836. https://doi.org/10.1890/10-1083.1
Poulsen JR, Koerner SE, Moore S et al (2017) Poaching empties critical Central African wilderness of forest elephants. Curr Biol 27:R134–R135. https://doi.org/10.1016/j.cub.2017.01.023
R Core Team (2020) R: A Language and Environment for Statistical Computing. Austria, Vienna
Remis MJ, Kpanou JB (2011) Primate and ungulate abundance in response to multi-use zoning and human extractive activities in a Central African Reserve. Afr J Ecol 49:70–80. https://doi.org/10.1111/j.1365-2028.2010.01229.x
Schuttler SG, Philbrick JA, Jeffery KJ, Eggert LS (2014) Fine-scale genetic structure and cryptic associations reveal evidence of kin-based sociality in the African forest elephant. PLoS ONE 9:e88074. https://doi.org/10.1371/journal.pone.0088074
Shapiro A, Grantham HS, Aguilar-Amuchastegui N et al (2021) Forest condition in the Congo Basin for the assessment of ecosystem conservation status. Ecol Indic. https://doi.org/10.1016/j.ecolind.2020.107268
Starkey M, Scholtz O, Taylor G (2014) Wildlife monitoring practices and use in Central Africa. Wildlife Conservation Society/ IUCN, Pretoria
Stephan C, Bahamboula JJD, Brncic TM (2020) Responses to a poached conspecific in wild forest elephants (Loxodonta africana cyclotis). Behaviour 157:823–833. https://doi.org/10.1163/1568539X-bja10025
Stokes EJ, Strindberg S, Bakabana PC et al (2010) Monitoring great ape and elephant abundance at large spatial scales: measuring effectiveness of a conservation landscape. PLoS ONE 5:e10294. https://doi.org/10.1371/journal.pone.0010294
Strindberg S (2012) Distance sampling along line transects: statistical concepts and analysis options. In: Hedges S (ed) Monitoring elephant populations and assessing threats: a manual for researchers, managers and conservationists. Universities Press, Himayatnagar, Hyderabad, pp 26–60
Strindberg S, Maisels F, Williamson EA et al (2018) Guns, germs, and trees determine density and distribution of gorillas and chimpanzees in Western Equatorial Africa. Sci Adv. https://doi.org/10.1126/sciadv.aar2964
Sunderland TCH, Balinga MPB (2005) A Preliminary vegetation assessment of the Nouabale-Ndoki National Park and support zone, Congo. Smithsonian Institution, Herbier National de Gabon, Forests, Resources and People, WCS, and Limbe Botanical Gardens, Libreville
Tagg N, Willie J, Duarte J et al (2015) Conservation research presence protects: A case study of great ape abundance in the Dja region, Cameroon. Anim Conserv 18:489–498. https://doi.org/10.1111/acv.12212
Tchamba M (1992) Defecation by the African forest elephant Loxodonta africana cyclotis in the Santchou Reserve, Cameroon. Mammalia 56:155–158
Tédonzong LRD, Willie J, Keuko AMP et al (2018) Using abundance and habitat variables to identify high conservation value areas for threatened mammals. Biodivers Conserv 27:1115–1137. https://doi.org/10.1007/s10531-017-1483-9
Theuerkauf J, Ellenberg H (2000) Movements and defaecation of forest elephants in the moist semi-deciduous Bossematie Forest Reserve, Ivory Coast. Afr J Ecol 38:258–261. https://doi.org/10.1046/j.1365-2028.2000.00240.x
Theuerkauf J, Gula R (2010) Towards Standardisation of Population Estimates: Defecation Rates of Elephants Should be Assessed using a Rainfall Model. Ann Zool Fennici 47:398–402. https://doi.org/10.5735/086.047.0603
Thomas L, Buckland ST, Rexstad EA et al (2010) Distance software: Design and analysis of distance sampling surveys for estimating population size. J Appl Ecol 47:5–14. https://doi.org/10.1111/j.1365-2664.2009.01737.x
Thouless C, Dublin HT, Blanc J, et al (2016) African Elephant Status Report 2016: An update from the African Elephant Database. IUCN, Nairobi
Turkalo AK, Wrege PH, Wittemyer G (2013) Long-Term monitoring of dzanga bai forest elephants: Forest clearing use patterns. PLoS ONE 8:e85154. https://doi.org/10.1371/journal.pone.0085154
Turkalo AK, Wrege PH, Wittemyer G (2017) Slow intrinsic growth rate in forest elephants indicates recovery from poaching will require decades. J Appl Ecol 54:153–159. https://doi.org/10.1111/1365-2664.12764
Tyukavina A, Hansen MC, Potapov P et al (2018) Congo Basin forest loss dominated by increasing smallholder clearing. Sci Adv. https://doi.org/10.1126/sciadv.aat2993
Vanleeuwe H, Gautier-Hion A (1998) Forest elephant paths and movements at the Odzala National Park, Congo: The role of clearings and Marantaceae forests. Afr J Ecol 36:174–182. https://doi.org/10.1046/j.1365-2028.1998.00123.x
Vanleeuwe H, Probert J (2014) Decay rate of elephant dung in Conkouati-Douli national park, Republic of Congo. Pachyderm 55:89–91
Vanleeuwe H, Gautier-Hion A, Cajani S (1997) Forest clearings and the conservation of elephants (Loxodonta africana cyclotis) north-east Congo Republic. Pachyderm 24:46–52
Vanthomme H, Kolowski J, Korte L, Alonso A (2013) Distribution of a community of mammals in relation to roads and other human disturbances in Gabon, Central Africa. Conserv Biol 27:281–291. https://doi.org/10.1111/cobi.12017
Walsh PD, White LJT (1999) What it will take to monitor forest elephant populations. Conserv Biol 13:1194–1202. https://doi.org/10.1046/j.1523-1739.1999.98148.x
Walsh PD, White LJT, Mbina C et al (2001) Estimates of forest elephant abundance: Projecting the relationship between precision and effort. J Appl Ecol 38:217–228. https://doi.org/10.1046/j.1365-2664.2001.00578.x
Walsh PD, Breuer T, Sanz C et al (2007) Potential for Ebola transmission between gorilla and chimpanzee social groups. Am Nat 169:684–689. https://doi.org/10.1086/513494
White LJTT (1994) Sacoglottis gabonensis fruiting and the seasonal movements of elephants in the Lopé Reserve, Gabon. J Trop Ecol 10:121–125. https://doi.org/10.1017/S0266467400007768
White LJT (1995) Factors affecting the duration of elephant dung piles in rain forest in the Lopé Reserve, Gabon. Afr J Ecol 33:142–150. https://doi.org/10.1111/j.1365-2028.1995.tb00789.x
Wilson G, Gray RJ, Radinal R et al (2021) Between a rock and a hard place: rugged terrain features and human disturbance affect behaviour and habitat use of Sumatran elephants in Aceh. Biodivers Conserv early view, Sumatra, Indonesia
Wood SN (2017) Generalized additive models: An introduction with R, 2nd edn. Chapman and Hall/CRC, Boca Raton
Wrege PH, Rowland ED, Keen S, Shiu Y (2017) Acoustic monitoring for conservation in tropical forests: examples from forest elephants. Methods Ecol Evol 8:1292–1301. https://doi.org/10.1111/2041-210X.12730
Yackulic CB, Strindberg S, Maisels F, Blake S (2011) The spatial structure of hunter access determines the local abundance of forest elephants (Loxodonta africana cyclotis). Ecol Appl 21:1296–1307. https://doi.org/10.1890/09-1099.1
Young HS, McCauley DJ, Galetti M, Dirzo R (2016) Patterns, Causes, and Consequences of Anthropocene Defaunation. Annu Rev Ecol Evol Syst 47:333–358. https://doi.org/10.1146/annurev-ecolsys-112414-054142
Acknowledgements
We thank the Ministry of Sustainable Development, Forest Economy and Environment and the Ministry of Scientific Research of the Republic of Congo for granting permission to conduct the study. We are grateful to the Wildlife Conservation Society, Congo, particularly to Emma J. Stokes for proving crucial logistical and administrative support and advice throughout the study. We are particularly thankful to our data collectors especially Ella Emeline Bamona and Ricky, as well as the local assistants. Funding for our long-term monitoring at Mbeli Bai was provided by Brevard Zoo, The Columbus Zoo and Aquarium, Cincinnati Zoo and Botanical Garden, Cleveland Metroparks Zoo, Cologne Zoo, Disney Worldwide Conservation Fund, Dublin Zoo, Houston Zoo, Sea World and Busch Gardens Conservation Fund, Toronto Zoo, Wildlife Conservation Society, and Woodland Park Zoo. We particularly thank the Pittsburgh Zoo that provided a specific grant for this study.
Funding
Brevard Zoo, The Columbus Zoo and Aquarium, Cincinnati Zoo and Botanical Garden, Cleveland Metroparks Zoo, Cologne Zoo, Disney Worldwide Conservation Fund, Dublin Zoo, Houston Zoo, Pittsburgh Zoo, Sea World and Busch Gardens Conservation Fund, Toronto Zoo, Wildlife Conservation Society, and Woodland Park Zoo.
Author information
Authors and Affiliations
Contributions
TB conceived the study, analysed the data, and wrote the manuscript. MB-NH collected the data. SS, conceived the study, analysed the data, wrote sections of and proof read the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. Data, distance analysis projects, and R code are available upon reasonable request from corresponding author.
Additional information
Communicated by Xiaoli Shen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Breuer, T., Breuer-Ndoundou Hockemba, M. & Strindberg, S. Small-scale dung survey reveals high forest elephant density and preference for mixed species forest in an intact protected area. Biodivers Conserv 30, 2671–2688 (2021). https://doi.org/10.1007/s10531-021-02214-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02214-7