Abstract
The global decline in biodiversity has invigorated the field of conservation biology, leading to investigation of species at risk of extinction in hopes of generating effective conservation strategies. Some highly diverse taxa, such as lichens, have received considerably less conservation attention, compared to plants and vertebrates. Here we add present the results of a comprehensive demographic survey and IUCN risk assessment of Cladonia submitis, a conspicuous macrolichen endemic to the Mid-Atlantic coast of eastern North America, across the core of its range. While it was found at several new locations, we found the species had disappeared from many locations where it once occurred. This decline, in conjunction with its restricted range, supports a status of Endangered under IUCN guidelines. While fire and sea level rise likely pose threats to the species, the most immediate threat is urbanization and alteration of coastal dunes. This evaluation does not consider collections from Japan and Sakhalin Island which have been assigned as C. submitis, due to differences in range, habitat and morphology that suggest this identification is inaccurate. In the absence of a proper taxonomic assessment or phylogenetic study to answer this question of identity, Japanese specimens could not be considered in this assessment. Altogether, this study provides a basis for effective management strategies of this charismatic species whose core range consists of the densely populated region between the American cities of Boston and Washington, D.C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global decline of biodiversity due to anthropogenic factors, such as habitat destruction, invasive species, and climate change, is well-documented and the subject of considerable study (Thomas et al. 2004; Clavero and García-Berthou 2005; Wake and Vredenburg 2008; Ceballos et al. 2015). This widespread loss is so significant that the Anthropocene epoch is now widely considered to mark a sixth mass extinction event (Wake and Vredenburg 2008; Dunn et al. 2009; Bellard et al. 2012; Ceballos et al. 2015). The severity of this downward trend has drawn considerable attention to at-risk and declining species, motivating study of their current status and conservation needs with increasing urgency (Barnosky et al. 2011; Ceballos et al. 2015; Forest et al. 2015). Conservation entities and legislation like the International Union for the Conservation of Nature (IUCN) and the United States Endangered Species Act (ESA) currently assess and track the statuses of such species, and recommend or enact tactics and practices to protect them, if deemed necessary. These and other organizations have set criteria species must meet to be granted protection. Using these criteria, researchers have gathered critical data for a staggering number of species, which have been instrumental in protecting vulnerable species, and in some cases, even shepherding them towards recovery (Rodrigues et al. 2006; Farrier et al. 2007; Lotze et al. 2011).
Lichens are no exception to the trend of global decline (Conti and Cecchetti 2001; Fraser et al. 2014; Allen et al. 2019); The sensitivity of lichens to pollution (Pearson and Skye 1965; Nash 1976; Conti and Cecchetti 2001), climate change (Fraser et al. 2014; Lendemer and Allen 2014; Allen and Lendemer 2016) and other man-made habitat changes has been documented throughout more than a century and a half (Kettlewell 1955; Delendick 1994; Allen et al. 2019). As a result, researchers have documented major declines in many lichen species attributed to such anthropogenic influences (Nash and Gries 1991; Giordani 2007; Joly et al. 2009). However, lichens, and other fungi for that matter, have not received conservation research representation relative to that of flowering plants and vertebrates, despite their immense biodiversity (Allen and Lendemer 2015). At the time of writing, a total of 71,999 species of animals, 33,573 plants, and only 145 fungi (23 of which are lichens) have been evaluated under the IUCN red list criteria (IUCN 2019). Fungi are incredibly diverse (Blackwell 2011; Cuadros-Orellana et al. 2013), with more than 120,000 accepted species and 2.2–3.8 million estimated to exist (Hawksworth and Lücking 2017). Within fungi, lichens are a highly polyphyletic group that are also remarkably speciose, with almost 20,000 accepted species across 115 families (Lücking et al. 2017). Thus, the current representation of lichens and other fungi in conservation is especially disproportionate when one considers their overall high biodiversity, abundance throughout terrestrial biomes, and invaluable ecological functions. Most notably, lichens and other fungi serve critical roles in nutrient and mineral cycling; (Pike 1978; Read and Perez-Moreno 2003; Dighton 1995), and are important components of many food webs (Terry et al. 2000; McGonigle 2007; Jobard et al. 2010), while lichens specifically also often form major stabilizing components of soil crusts (Anderson et al. 1982; Eldridge and Leys 2003).
Considering the known threats and history of decline in lichens, this inequality in conservation reflects an organismal bias, rather than differences in actual need for conservation (Allen and Lendemer 2015; Allen et al. 2019). The lack of lichen conservation research in turn means that there are relatively few frameworks from which to structure effective lichen conservation strategies. To further complicate matters, the basic data essential for such strategies, such as complete knowledge of historical and extant occurrences, as well as demographic estimates, are incomplete or outdated for many taxa. Moreover, important population-level information such as dispersal patterns and capabilities are available for only a small subset of lichen species with relative confidence (Scheidegger et al. 2012; Alors et al. 2014; Allen et al. 2018; Dal Grande et al. 2017; Degtjarenko et al. 2018).
Conservation risk assessments under the IUCN can be performed for species with limited available data, but a complete understanding of the distribution, demographics and conservation threats for a species can influence the level of protection granted to it (Game et al. 2013). Given the limited resources available for species conservation, accurate ranking and assessment is key to effectively prioritize the most at-risk taxa.
While many lichen species are threatened or in decline, beach broccoli (C. submitis A. Evans; also called mid-Atlantic comb-over) stands out among them (Fig. 1a). It is a conspicuous macrolichen, almost entirely restricted to sand dunes and pine barrens in the Mid-Atlantic of eastern North America, where it often forms large colonies in natural habitats interwoven across a densely populated metropolitan landscape mosaic (Hinds and Hinds 2007). Considering the large size of both the lichen, and the colonies it forms, it may be difficult to consider that such a species could have suffered substantial declines in its population size and be threatened by diverse forces. Yet, at the same time, the natural landscape in its core range has become highly fragmented and degraded, such that many plants and animals are now recognized as threatened (Ehrenfeld 1983; Knisley and Hill 1992; Smith et al. 2017). Hence it is possible, given the lack of conservation attention for lichens, a decline of C. submitis has occurred and simply gone unnoticed.
The potential need for a conservation assessment of C. submitis was recognized in 2015. At that time, a preliminary assessment was completed based on the available data (Lendemer et al. 2015). The assessment noted the species likely faced threats from habitat loss and sea level rise. However, it also recognized the need for modern distribution and abundance data for the species, particularly determination of whether historical occurrences remained extant. Another complication briefly noted in the preliminary assessment was the taxonomy of the species; since the late 1950’s, some collections of Cladonia in east Asia, predominantly from Japan, have been identified as C. submitis, although the question has been raised as to whether these identifications are correct (Ahti 1961). If the collections from east Asia are C. submitis, the presence of a disjunct population may impact the results of an IUCN risk assessment. Thus, these collections must be compared against North American C. submitis to determine how to treat the east Asian records.
Here we present the results of a detailed survey of C. submitis populations across its core range (central and southern New Jersey Long Island, New York and Cape Cod, Massachusetts). We provide new estimates of range and population size, estimates of population decline over time, and place the conservation status of the species within the context of an IUCN risk assessment. In addition, we provide a discussion of morphology, secondary metabolites, distribution and habitat of C. submitis, comparing North American populations to a disjunct population from eastern Asia, whose taxonomic identity has been questioned for more than fifty years.
Methods
Assembly of occurrence data and examination of herbarium specimens
As part of the 2015 preliminary IUCN assessment for C. submitis, Lendemer et al. (2015) assembled a comprehensive database of known herbarium specimens for the species. For records whose identification was questionable (e.g., based on location data or lack of expert verification), the specimen was re-examined and its determination was evaluated using a combination of morphological examination under a dissecting microscope and thin layer chromatography (TLC) with solvent C following Culberson and Kristinsson (1970), as modified by Lendemer (2011a). This portion of the study was primarily based on specimens in the herbarium of The New York Botanical Garden (NY), supplemented by loans of material from CHRB and DUKE. In addition to the specimens of C. submitis from North America, a suite of specimens was borrowed from TNS in order to evaluate the taxonomic identity of the disjunct populations whose status was questioned previously (Ahti 1961).
When coordinates were not previously provided (e.g., for specimens collected before satellite-based navigation was implemented), GPS coordinates for these collections were obtained by either manually identifying sites on Google Earth based on collection locality descriptions, or georeferencing in the online program GeoLocate (Rios and Bart 2010). This database of occurrences revealed that the vast majority of known occurrences for C. submitis (82.7%) occur within the pine barren and sand dune habitats in New Jersey, New York, and Massachusetts. As such, we focused survey effort on this core range for the species and used the list of occurrences as a guide to survey historical sites, revisiting locations based on their coordinate information. Other sites in Connecticut and Rhode Island were also targeted due to their accessibility and proximity to other Massachusetts sites. We also identified other sites with suitable habitat that had not previously been surveyed, or where no records for the species existed. These sites were judged potentially suitable using Google Earth satellite imagery. Accessible areas that showed evidence of being exposed, sandy spaces behind dunes or in pine barrens were selected for survey. In some cases, local naturalists and employees of nearby parks were consulted. Additionally, opportunistic observations of the presence of other Cladonia lichens were made while traveling between field sites (i.e. Cladonia lichens seen from the car on small roads). These sites were subsequently also surveyed for the presence of C. submitis.
Survey overview
Between April and October of 2017, a total of 121 sites were surveyed for C. submitis. Each site surveyed consisted of a 0.5 ha area, which was visually assessed for the presence of Cladonia. A minimum of 10 min was spent at each site searching for Cladonia. Any Cladonia lichens found were identified to species using morphological and chemical characters (Evans 1943; Hinds and Hinds 2007). In most cases, C. submitis can be distinguished from other, morphologically similar Cladonia species by its thick, coarse podetia that branch infrequently and typically lie prostrate along the substrate. In some cases, however, particularly in highly dense aggregations of Cladonia species or for apparently young thalli, chemical analysis is also necessary. For chemical identification, C. submitis possesses the fatty acid pseudonorrangiformic acid, which is diagnostic for the species. However, there is no simple method to test for the presence of this fatty acid in the field. Another unique chemical fingerprint for C. submitis is its lack of fumarprotocetraric acid. Absent in C. submitis, this chemical is present in the majority of other morphologically similar species in the mid-Atlantic coast, such as C. subtenuis (Brodo et al. 2001). To test for the presence of fumarprotocetraric acid, a spot test was performed using Steiner’s solution (Thomson 1984), a stable solution of the spot test reagent Pd, which turns lichen material red in a reaction with the chemical (Fig. 1b). As the concentration of fumarprotocetraric acid tends to be highest on podetial tips for Cladonia, Steiner’s solution spot tests were performed on podetial tips. All surveys were conducted on warm, dry days, which reduces the likelihood of lichen secondary metabolites having been washed out from rain, which could lead to a false positive identification. At each site: (i) the presence or absence of C. submitis was recorded; (ii) the presence of other co-occurring conspicuous Cladonia species were documented; and (iii) if C. submitis was present, abundance was estimated (individual thalli tallied to 100, then > 100 if exceeding 100 counted individuals). For the purposes of this study, thalli that were distinct (i.e. with a clear border, not blending indiscriminately into nearby clumps) were considered to be individuals. A population at a site was determined ≫ 100 if the Cladonia community was dominated by C. submitis, and stretched across the majority or entirety of the 0.5 ha survey site.
Extent of occurrence and area of occupancy
Using the above data, Extent of Occurrence (EOO) and the Area of Occupancy (AOO, cell size 2 km) were calculated as per IUCN guidelines (IUCN 2018). It is hypothesized that lichen generations likely last upwards of 30 years (Nimis et al. 2002), but lichen lifespan and generation time is poorly researched and difficult to study (Bench et al. 2001). Lifespan may vary considerably among species. To account for the uncertainty of generation time for C. submitis, four “baseline” EOO and AOO values were calculated; (i) all occurrences, (ii) only occurrences ≤ 90 years old (30-year generation), (iii) only occurrences ≤ 60 years old (20-year generation), and (iv) only occurrences ≤ 30 years old (10-year generation). Older records where the species is presumed to still be extant were also included in the 20- and 10-year models. These values were also calculated for the presumed total extant population, using only occurrences that have been recorded or confirmed by a secondary visit in the last 20 years. The EOO and AOO for the presumed extant population were then compared against these baseline values to estimate losses over time. These data, along with new records of population sizes (local abundance), were analyzed to determine ranking in accordance with IUCN assessment regulations.
Specimens studied
Morphological and chemical studies were conducted on 64 collections of C. submitis, which were collected from New Jersey, Massachusetts and New York during the survey period. These data were compared against that of 70 georeferenced collections of east Asian Cladonia species currently identified as C. submitis. Diagnostic traits such as the relative coarseness of the podetia, the presence of non-terminal pycnidia-bearing tips, and presence of pseudonorrangiformic acid, and the absence of fumarprotocetraric acid were examined for each individual. Morphological characteristics were examined under a dissecting microscope, with a specimen marked as bearing a morphological trait if at least one podetium was observed to possess it. The secondary metabolites of all specimens was determined using TLC as outlined above (i.e., Solvent C following Lendemer (2011a)). In some cases, east Asian specimens had already received TLC as part of their initial identification as C. submitis. Habitat elevation was also gathered for every unique site from North American and east Asian herbarium records using either recorded label information or, when label information was not available, Google Maps. For all unique sites, elevation was then compared between the two populations using a One-Tailed Mann Whitney U test.
Results
Range of Cladonia submitis
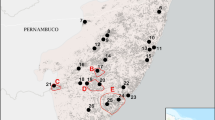
The core distribution of C. submitis, containing the majority of known collection sites, is concentrated in a strip of coastal habitats along the Atlantic Coast spanning from southern New Jersey and Cape Henlopen, Delaware to Long Island, New York and Cape Cod, Massachusetts (Fig. 2). These sites are predominantly dune, near-dune or pine barren habitat. The total known range of the species encompasses an area as far south as North Carolina, as far north as Maine, and as far west as West Virginia. Although the total land area covered by the range of the species is large, this is due to a small number of outlier sites located in the Piedmont and Appalachian Mountains where the species occurs as small numbers of individuals (Lendemer unpublished data). These sites uniformly consist of exposed montane rock outcrops in the Appalachian Mountains and exposed granite flat rocks in the Piedmont. While these outlier sites are geographically disjunct from the core of the range of C. submitis, they are known to host rare disjunct occurrences of typically Coastal Plain lichen species (Wyatt and Stoneburner 1982; Dirig 1994; Lendemer 2011b; LaGreca et al. 2018).
Field population assessment
Of the total 121 surveys conducted as part of this study, 36 were sites where C. submitis was found in the past, and 86 were new potential sites not previously surveyed. The species was found at 47 sites (38.5%), 11 of which were historical sites where it was known to occur previously (Fig. 3a). Of the 74 sites (61.2%) where C. submitis was not observed, 25 (33.3%) were historical sites where the species had been documented before. The species is considered to have been extirpated from those 25 sties. Fourteen (56.0%) of those 25 historical sites that no longer supported C. submitis had been entirely converted to residential or commercial land uses, and did not appear to support any Cladonia species. Hence those locations were likely subject to drastic land use changes since the original observations.
Where C. submitis was found, it frequently occurred in abundance. Of the 47 sites where the species was observed, 42 (89.4%) possessed populations that clearly exceeded 100 individuals. Moreover, 26 sites (55.3%) sustained C. submitis in numbers estimated to far exceed 100 individuals. Sites that supported the species were split between pine barrens (23 sites, 48.9%) and interdune habitat (24 sites, 51.1%, although 16 sites were contiguous along the length of Island Beach State Park; Fig. 3), Of the 23 sites in pine barrens, 5 (21.7%) were roadside non-forested open patches of sand that immediately bordered pine barren habitat.
Cladonia mat communities
The communal, mat-forming nature of C. submitis and other Cladonia, is also noteworthy (Supplementary Table S1). At the sites where C. submitis was found, the species was frequently a large component of the communal mat, in some cases being the most abundant species present. At least one other Cladonia species was found co-occurring with C. submitis at nearly every survey site where the species was found (46 of 47 sites, 97.9%). The most commonly co-occurring Cladonia species was C. subtenuis, which was found at 44 of 47 sites (93.6%). Other Cladonia species that were found to co-occur in abundance included C. atlantica (14 sites, 29.8%), C. dimorphoclada (13 sites, 27.7%), C. boryi (11 sites, 23.4%) and C. rangiferina (6 sites, 12.8%). Of the aforementioned species, two (C. dimorphoclada and C. boryi) exhibited differences in occurrence regionally; C. dimorphoclada was only observed at sites in New Jersey, and C. boryi was only observed in Cape Cod. At sites where C. submitis was absent, Cladonia communities were consistently dominated by C. subtenuis.
Extent of occurrence and area of occupancy
Taking into account all known occurrences of C. submitis, both extirpated and extant, the EOO and AOO would be 242,727.64 km2 and 472 km2, respectively. The total presumed extant population (i.e. all newly surveyed sites and sites not recently visited where C. submitis is still expected to persist; Fig. 2), however, had an EOO of 190,322.75 km2 and an AOO of 168 km2. Due to the lack of available data on lichen generation times, change in EOO and AOO was estimated using three different models of generation time (10 year, 20 year and 30 year). Subtracting the total presumed extant population model from that of each of the three baseline models of generation times revealed an EOO decline of up to 122,853.20 km2 (39.3% decline; 30-year model) and an AOO decline of up to 268 km2 (59.3% decline; 30-year model; Fig. 4).
Taxonomic assessment of east Asian specimens
In an examination of morphology between American and Asian C. submitis specimens, the podetia of the Japanese specimens were found to be less coarse and wrinkled than American specimens (Fig. 5), a morphological difference that had been recognized previously (Ahti 1961). Additionally, pycnidia-bearing branchlets that grow directly from the base of the podetium, a characteristic that appears to be unique to C. submitis, occur much less frequently in Japanese material than in North American specimens (Fig. 5). While these branchlets were found on at least one podetium from 37 of 64 (57.8%) North American samples, only five of 70 (7.2%) examined Japanese samples bore these branchlets. The branchlets also appeared to be less abundant on podetia of Asian material, but a dedicated study and larger sample size is needed to assess this potential variation with confidence. Secondary metabolite composition was found to be identical between Asian and North American C. submitis (i.e., lacking fumarprotocetraric acid and containing usnic and pseudonorrangiformic acid). The Asian material was also collected in habitats that differ considerably from the typical habitats of North American C. submitis. East Asian specimens were predominantly collected on rock and soil substrates from high-elevation locations, particularly along mountain ranges in Honshu and Hokkaido. These locations stand in stark contrast to the Mid-Atlantic region where C. submitis mainly grows in sandy habitats very close to sea level. The difference in elevation between Japanese and American specimens was significant (One-Tailed Mann–Whitney U Test, U = 256, p < < 0.05), with Japanese collections (n = 49 unique sites) occurring at far higher elevations than American collections (n = 142 unique sites).
IUCN assessment
Cladonia submitis meets the criteria for IUCN listing as Endangered under assessment categories A2c and B2b (i–iv). Under category A2c, the considerable estimated decline in AOO under a 30-year generation time model (> 50% decline) would classify C. submitis as Endangered. Similarly, the EOO losses observed in both the 30-year and 20-year generation time model (> 30% decline) would classify the species as Vulnerable. Cladonia submitis also meets the requirements for Endangered status under category B2b (i–iv) due to the calculated declines in EOO and AOO, as well as the extirpation from many sites where the species occurred historically, some due to land use changes. Taking the highest level of protective status supported by these data, C. submitis should be assessed as Endangered. Due to the large number of individuals observed at sites where C. submitis was found, the species does not meet the criteria for IUCN listing under categories C and D, which pertain to issues concerning small, restricted population sizes.
Discussion
Overview
Surveys of pine barren and sand dune sites in Massachusetts, New Jersey and New York revealed 47 populations of C. submitis, the majority of which were newly documented occurrences. More than 90% of the sites where C. submitis was found, supported > 100 thalli, and more than 50% of the sites supported ≫ 100 thalli. Hence, the species is often abundant where it occurs, suggesting that overall population size is unlikely to be a current concern for the species. It is important to note that the population size estimates provided here are physical counts only up to the first 100 individuals. After this threshold was reached during a hand-count of distinct thalli at a site, populations sizes were roughly estimated based on a visual assessment of abundance of C. submitis in the lichen mat. As a result of such a rough, by-sight estimation in the mats after that threshold was exceeded, the counts for almost 90% of sites are likely severe underestimates. As such, this method is not optimal for generating accurate estimates for sites where C. submitis is highly abundant. Because most of the sites exceeded 100 individuals and thus received a rough estimation, we cannot use the IUCN criterion C (Small population size and decline) to assess the population size of C. submitis. To do so with the data available would likely result in overestimating the risk the species, with regard to issues of population size. A future study employing more standard, regimented methods for estimating population size and abundance within mats, such as a transect approach (Buckland et al. 2007; Reisch et al. 2018), would generate more accurate estimates of population size many of the sites surveyed in this study. However, our counts presented here are still informative for the purposes of roughly judging the abundance of C. submitis where it occurs, establishing that the vast majority of mats where the species occurs support sizeable populations of at least 100 individuals.
While the total population size of C. submitis appears to be large, the species appears to have been extirpated from many sites where it occurred historically. Moreover, declines in EOO and AOO were considerable, with the species disappearing from many sites where it had once been observed. Multiple potential generation times were modeled when calculating EOO and AOO decline due to the general uncertainty of lichen generation times. However, some models are likely more appropriate for C. submitis than others. When habitat is exposed after a disturbance such as fire, it can take decades for establishing Cladonia species to develop into a sustainable mat, like those seen at nearly every site in this study (Maikawa and Kershaw 1976; Morneau and Payette 1989; Coxson and Marsh 2001). Further, the impact of fire on Cladonia in fire-adapted Coastal Plain systems in eastern North America, is complex and dependent on multiple factors (Ray et al. 2020). In some systems, the exposed habitat where a Cladonia mat develops can persist for more than a century, and lichen mats remain dominant at the site (Maikawa and Kershaw 1976). In fact, there is some evidence that Cladonia secondary metabolites may inhibit plant growth, warding off further succession by competing plants (Lawrey 1977; Hobbs 1985; Sedia and Ehrenfeld 2003). The long-term sustainability of lichen mats and the time it takes to suitably establish at a site support longer models of generation time, such as 20 or 30 years.
The declines observed in this study likely reflect habitat degradation as a result of land use change. In fact, more than half of revisited sites from which C. submitis seems to have disappeared have been significantly transformed through urbanization or other anthropogenic disturbances. The habitats in which the species occurs (primarily interdune, roadsides and sandy exposures within pine barrens) suggest that it is fairly tolerant of at least one type of disturbance, edge effects. Moreover, Cladonia species are often dependent on natural disturbances such as fire, which create exposures that the lichens can colonize (Menges and Kohfeldt 1995; Hawkes and Menges 1996; Coxson and Marsh 2001; Ray et al. 2020), although excessively frequent fires can instead be detrimental. In such systems, Cladonia mats become the late-stage state of succession for a patchy habitat type (Clément and Touffet 1990; Morneau and Payette 1989). While anthropogenic conversion of natural habitat does create edge effects, residential (e.g. housing construction, the implementation of grass lawns) and commercial land transformation (e.g. parking lots, construction of buildings of business) have very different outcomes compared to natural disturbances and often preclude reestablishment of natural communities and native species (Mckinney 2008; Aronson et al. 2014). As such, urbanization is not conducive to the growth of C. submitis, as has been demonstrated to be the case for many species (Van der Veken et al. 2004; Scheffers and Paszkowski 2012; Elmqvist et al. 2016).
Another form of habitat transformation that has impacted C. submitis, and other Cladonia, is dune destruction. For example, among the historical occurrences on sand dunes, the most notable is perhaps on Cape May Point, New Jersey. Raymond H. Torrey collected the species on the dunes or swales at this site in 1935, but prior to our survey, the site was not revisited to relocate the species since that observation. In that time, a significant nor’easter storm in 1962 destroyed a considerable portion of the sand dunes around Cape May Point, leaving few areas undamaged (Jordan 2003). Since then, the dunes have been reconstructed, but C. submitis appears to have been lost from the site, either destroyed with the dunes or disappeared as the dunes retreated towards residential property lines and suitable habitat shrank. Erosion and recession along Cape May beaches and dunes have been observed repeatedly over the last century (Jordan 2003), and continue to be cause for concern (Wu et al. 2002). In any case, habitat transformations, both natural and anthropogenic, have likely significantly impacted C. submitis, and stand as current threats to its survival. Whereas natural threats such as dune erosion are more challenging to address, anthropogenic threats could be mitigated by limiting alteration of the habitats where C. submitis is known to occur, and requiring surveys of similar habitat in the future before such alterations occur.
While this study investigated C. submitis across the majority of its range, observing declines and threats in predominantly coastal pine barren and sand dune habitat, the few scattered outlier populations further inland have not been investigated. These outlier populations inhabit exposed outcrops, mostly in montane localities, which stand apart from the sandy, low-elevation habitat the species typically occupies. Although the outlier populations comprise relatively small numbers of individuals, they may be genetically isolated to some degree, potentially representing small pockets of novel genetic diversity. Few Cladonia species have been studied using population genetics, but one study of C. mitis and C. arbuscula using commonly employed molecular markers demonstrated the capacity for high gene flow among Cladonia populations (Myllys et al. 2003). However, C. mitis and C. arbuscula are considerably more widespread than C. submitis, which is relatively geographically constrained to the mid-Atlantic coast of North America. Without further study, we cannot know whether the species will demonstrate the same capacity for dispersal and gene flow that C. mitis or C. arbuscula have shown. Hence, a population genetic examination of main and outlier populations merits future attention.
While this study focused on C. submitis, it is clear that habitat occupied by the species is also ideal for other Cladonia. Communal Cladonia mats have been well-documented previously (Lechowicz and Adams 1974; Crittenden 1991; Coxson and Marsh 2001) and are likely important to the establishment and survival of the species. In every case, C. submitis was never the only Cladonia species at a site, always forming dense lichen mats intermixed with several other species, most prominently C. subtenuis. As such, seeking out Cladonia mats can be a useful method of searching for additional occurrences of C. submitis. These mats are often easy to visually locate, especially those which occur along roadsides, appearing as pale green splotches against exposed sand or intermixed with darker green or brown plant matter. Protecting C. submitis habitat has the added benefit of protecting the habitat for an assortment of other Cladonia species that share its niche and hence likely face similar threats at the regional level. Moreover, this overlap in range and habitat and shared potential threats is an even greater motivator to conduct further conservation assessments on these and other co-occurring lichens.
Taxonomic status and identity of Asian populations
Cladonia submitis was first recognized as a species by Evans (1943) whom observed morphological and chemical variation in C. mitis among collections from locations on the mid-Atlantic coast. Ultimately C. submitis was split from C. mitis and differentiated from this and other species based on its combination of phenotypic characters, including its shorter, more robust podetia, its unique secondary metabolites and its restricted distribution. Four subspecies of C. submitis were also recognized by Evans (1943), though these have subsequently never been evaluated and treated in a taxonomic or phylogenetic study. The description of infraspecific taxa in Cladonia was a widespread phenomenon in the taxonomy of the genus during the early 20th century (Poelt 1994). This reflected attempts to classify the extensive, presumably environmentally induced, phenotypic variation often found in a single species (DePriest 1994; Osyczka and Rola 2013; Osyczka et al. 2014). While numerous such names were introduced, most are presumed to be synonyms of available names at the species level (Poelt 1994), and the majority lack any modern-day treatment.
While Evans (1943) believed C. submitis to be endemic to eastern North America, this has been challenged since the description of the species. Since 1958, collections of Cladonia from Japan and Sahkalin Island, Russia have been identified as C. submitis (Fig. 6). Some of these specimens were collected as early as 1919, though those collected prior to 1943 were initially determined to be other species, and subsequently reidentified as C. submitis. The Asian collections identified as C. submitis bear some morphological similarity to North American C. submitis, as well as share the same unusual secondary metabolites (Ahti 1961). The reports of C. submitis from Asia led the species to be included among the lichens with disjunct distributions between east Asia and eastern North America (Ahti 1961; Miyawaki 1994; Kurokawa 2006).
Despite the fact that C. submitis has been reported to occur in Asia, the conspecifity of the material with that from eastern North America has been questioned for more than half a century. Ahti (1961) examined many Japanese specimens and noted morphological differences that suggested they belonged to a distinct taxon. Our study supports the observations made by Ahti (1961), with the podetia of American specimens typically thicker and more coarse than Asian ones. While not discussed in Ahti (1961), the two populations also notably differed in the occurrence of non-terminal, pycnidia-bearing tips arising directly from the core of the podetium, with American specimens exhibiting the trait far more often. Finally, habitat appears to differ greatly between the two populations. East Asian collections grew at significantly higher elevations than their American counterparts, predominantly inhabiting montane rock outcrops rather than sandy coastal landscapes. While it is the case that C. submitis has been collected at a small number of higher elevation sites in Pennsylvania and West Virginia, and material has been collected at sea level at two sites in Japan, the elevational difference among collections is significant.
While Ahti (1961) described many of the same differences that we noted above, suggesting that the two populations were distinct, the taxonomic identity of the Asian material was left unresolved, and no further conclusions have subsequently been made. Thus, taxonomic comparisons alone are not sufficient to assess these populations. With the advent of molecular data, we now have the ability to more fully explore the relationship between North American and Asian material assigned to C. submitis once fresh material from the latter populations can be obtained. Such a study would benefit from employing phylogenetics and population genetics to determine whether the two populations are the same species, sister species, or more distantly related. It would also shed light on past connectivity and population history, potentially providing a new perspective on eastern Asian-eastern North American disjunctions.
However, molecular data for the Asian population are presently unavailable, and a comprehensive assessment of the population has not been carried out in modern times. Furthermore, the differences in morphology and ecology strongly suggest that the Asian material identified as C. submitis likely belongs to a distinct taxon that may, or may not be, sister North American C. submitis. Given the above, we treat the Asian material as having an uncertain taxonomic status and exclude it from the delimitation of C. submitis. While taxonomic study and conservation assessment of the Asian material previously assigned to C. submitis is urgently needed, it does not preclude conservation ranking and assessment of C. submitis in North America.
Conclusion
In summary, this study collectively provides a detailed assessment for C. submitis, and lays out an initial direction for conservation, both for this species, and for the other Cladonia that commonly co-occur with it. With its occurrence throughout fire-adapted habitats, often in close proximity to the coast, as well as its presence adjacent to some of the largest and most inter-connected metropolitan areas in the United States, this eastern North American endemic faces a number of threats to its long-term survival. As a result of these threats and observed declines, C. submitis meets the criteria to be ranked as Endangered under IUCN guidelines. The threats of fire and sea level rise are projected to become more severe over time (Keeley and Syphard 2016; Sweet et al. 2017). However, the threat of urbanization is the most immediate, though it is likely controllable through protecting C. submitis habitat and preventing future extirpations. With such few IUCN conservation assessments conducted on lichens, and with the many known threats they face, the need to examine their conservation need is higher now than ever before. Recognizing this need for scientific attention, the pool of lichen conservation research is steadily growing, both for Cladonia species (Lohmus and Lohmus 2009; Ravera et al. 2016) and many other species (Binder and Ellis 2008; Cameron et al. 2013; Allen et al. 2018, 2019). These studies have taken multiple different perspectives to examine their target species and assess them with IUCN and other conservation criteria, from employing general lichen inventories (Lohmus and Lohmus 2009) to in-depth literature and herbarium specimen review (Ravera et al. 2016) to predictive modeling (Binder and Ellis 2008; Cameron et al. 2013) to genomics (Allen et al. 2018). With our targeted field survey approach using herbarium data as a guide, this study now provides another useful framework for such future lichen assessments, and will hopefully inspire more workers to conduct systematic and detailed conservation assessments on at-risk lichen species.
References
Ahti T (1961) Taxonomic studies on reindeer lichens (Cladonia, subgenus Cladina). Ann Bot Soc Zool Bot Fenn 'Vanamo' 32(1):1–160
Allen JL, Lendemer JC (2015) Fungal conservation in the USA. Endanger Species Res 28(1):33–42
Allen JL, Lendemer JC (2016) Climate change impacts on endemic, high-elevation lichens in a biodiversity hotspot. Biodivers Conserv 25(3):555–568
Allen JL, McKenzie SK, Sleith RS, Alter SE (2018) First genome-wide analysis of an endangered lichen reveals isolation by distance and strong population structure. Am J Bot 105(9):1556–1567
Allen JL, McMullin RT, Tripp EA, Lendemer JC (2019) Lichen conservation in North America: a review of current practices and research in Canada and the United States. Biodivers Conserv 28(12):3103–3138
Alors D, Dal Grande F, Schmitt I, Kraichak E, Lumbsch HT, Crespo A, Divakar PK (2014) Characterization of fungus-specific microsatellite markers in the lichen-forming fungus Parmelina carporrhizans (Parmeliaceae). Appl Plant Sci 2(12):1400081
Anderson DC, Harper KT, Holmgren RC (1982) Factors influencing development of cryptogamic soil crusts in Utah deserts. Rangeland Ecol Manag 35(2):180–185
Aronson MF, La Sorte FA, Nilon CH, Katti M, Goddard MA, Lepczyk CA, Warren PS, Williams NSG, Cilliers S, Clarson B, Dobbs C, Dolan R, Hedblom M, Klotz S, Kooijmans JL, Kühn I, MacGregor-Fors I, Pyšek P, Siebert S, Sushinsky J, Werner P, Winter M (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc B 281(1780):20133330
Barnosky AD, Matzke N, Tomiya S, Wogan GO, Swartz B, Quental TB, Marshall C, McGuire JL, Lindsey EL, Maguire KC, Mersey B, Ferrer EA (2011) Has the earth’s sixth mass extinction already arrived? Nature 471(7336):51
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15(4):365–377
Bench G, Clark BM, Mangelson NF, Clair LS, Rees LB, Grant PG, Southon JR (2001) Accurate lifespan estimates cannot be obtained from 14 C profiles in the crustose lichen Rhizocarpon geographicum (L.) DC. Lichenologist 33(6):539–542
Binder MD, Ellis CJ (2008) Conservation of the rare British lichen Vulpicida pinastri: changing climate, habitat loss and strategies for mitigation. Lichenologist 40(1):63–79
Blackwell M (2011) The fungi: 1, 2, 3… 5.1 million species? Am J Bot 98(3):426–438
Brodo IM, Sharnoff SD, Sharnoff S. (2001). Lichens of North America, Yale University Press.
Buckland ST, Borchers DL, Johnston A, Henrys PA, Marques TA (2007) Line transect methods for plant surveys. Biometrics 63(4):989–998
Cameron R, Goudie I, Richardson D (2013) Habitat loss exceeds habitat regeneration for an IUCN flagship lichen epiphyte: Erioderma pedicellatum. Can J Forest Res 43(11):1075–1080
Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM (2015) Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci Adv 1(5):e1400253
Clavero M, García-Berthou E (2005) Invasive species are a leading cause of animal extinctions. Trends in Ecol Evol 20(3):110
Clément B, Touffet J (1990) Plant strategies and secondary succession on Brittany heathlands after severe fire. J Veg Sci 1(2):195–202
Conti ME, Cecchetti G (2001) Biological monitoring: lichens as bioindicators of air pollution assessment—a review. Environ Pollut 114(3):471–492
Coxson DS, Marsh J (2001) Lichen chronosequences (post-fire and post-harvest) in lodgepole pine (Pinus contorta) forests of northern-interior British Columbia. Can J Bot 79:1449–1464
Crittenden PD (1991) Ecological significance of necromass production in mat-forming lichens. Lichenologist 23(3):323–331
Cuadros-Orellana S, Leite LR, Smith A, Medeiros JD, Badotti F, Fonseca PL, Vas ABM, Oliveira G, Góes-Neto A (2013) Assessment of fungal diversity in the environment using metagenomics: a decade in review. Fungal Genet Biol. https://doi.org/10.4172/2165-8056.1000110
Culberson CF, Kristinsson HD (1970) A standardized method for the identification of lichen products. J Chromatogr A 46:85–93
Dal Grande F, Sharma R, Meiser A, Rolshausen G, Büdel B, Mishra B, Thines M, Otte J, Pfenniger M, Schmitt I (2017) Adaptive differentiation coincides with local bioclimatic conditions along an elevational cline in populations of a lichen-forming fungus. BMC Evol Biol 17(1):93
Degtjarenko P, Tõrra T, Mandel T, Marmor L, Saag A, Scheidegger C, Randlane T (2018) Unconstrained gene flow between populations of a widespread epiphytic lichen Usnea subfloridana (Parmeliaceae, Ascomycota) in Estonia. Fungal Biol. https://doi.org/10.1016/j.funbio.2018.03.013
Delendick TJ (1994) Notes on the lichens of eastern New York City: kings and queens counties, Long Island. New York Bull Torrey Bot Club 121(2):188–193
DePriest PT (1994) Variation in the Cladonia chlorophaea complex. II: ribosomal DNA variation in a southern Appalachian population. Bryologist 97(2):117–126
Dighton J (1995) Nutrient cycling in different terrestrial ecosystems in relation to fungi. Can J Bot 73(S1):1349–1360
Dirig R (1994) Lichens of pine barrens, pine plains, and "Ice Cave" habitats in the Shawangunk Mountains New York. Mycotaxon 52(2):523–558
Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS (2009) The sixth mass coextinction: are most endangered species parasites and mutualists? Proc R Soc Lond 276(1670):3037–3045
Ehrenfeld JG (1983) The effects of changes in land-use on swamps of the New Jersey Pine Barrens. Biol Conserv 25(4):353–375
Eldridge DJ, Leys JF (2003) Exploring some relationships between biological soil crusts, soil aggregation and wind erosion. J Arid Environ 53(4):457–466
Elmqvist T, Zipperer W, Güneralp B (2016) Urbanization, habitat loss, biodiversity decline: solution pathways to break the cycle. In: Seta K, Solecki WD, Griffith CA (eds) Routledge Handbook of Urbanization and Global Environmental Change. Routledge, London and New York, pp 139–151
Evans AW (1943) Microchemical studies on the genus Cladonia, subgenus Cladina. Rhodora 45(539):417–438
Farrier D, Whelan R, Mooney C (2007) Threatened species listing as a trigger for conservation action. Environ Sci Policy 10(3):219–229
Forest F, Crandall KA, Chase MW, Faith DP (2015) Phylogeny, extinction and conservation: embracing uncertainties in a time of urgency. Phil Trans R Soc B. https://doi.org/10.1098/rstb.2014.0002
Fraser RH, Lantz TC, Olthof I, Kokelj SV, Sims RA (2014) Warming-induced shrub expansion and lichen decline in the Western Canadian Arctic. Ecosystems 17(7):1151–1168
Game ET, Kareiva P, Possingham HP (2013) Six common mistakes in conservation priority setting. Conserv Biol 27:480–485
Giordani P (2007) Is the diversity of epiphytic lichens a reliable indicator of air pollution? A case study from Italy. Environ Pollut 146(2):317–323
Hawkes CV, Menges ES (1996) The relationship between open space and fire for species in a xeric Florida shrubland. Bull Torrey Bot Club 123(2):81–92
Hawksworth DL, Lücking R. (2017). Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectr, 5(4): FUNK-0052–2016.
Hinds JW, Hinds PL. (2007). The macrolichens of New England. The New York Botanical Garden Press.
Hobbs RJ (1985) The persistence of Cladonia patches in closed heathland stands. Lichenologist 17(1):103–109
International Union for the Conservation of Nature (IUCN). (2018). IUCN Redlist Categories and Criteria: version 3.1. Retrieved September 2018 from https://www.iucnredlist.org/resources/categories-and-criteria.
International Union for the Conservation of Nature (IUCN). (2019). IUCN Redlist Summary Statistics. Retrieved September 2019 from https://www.iucnredlist.org/resources/summary-statistics.
Jobard M, Rasconi S, Sime-Ngando T (2010) Diversity and functions of microscopic fungi: a missing component in pelagic food webs. Aquat Sci 72(3):255–268
Joly K, Jandt RR, Klein DR (2009) Decrease of lichens in arctic ecosystems: the role of wildfire, caribou, reindeer, competition and climate in north-western Alaska. Polar Res 28(3):433–442
Jordan J (2003) Cape May Point: The illustrated history: 1875 to the present. Schiffer Books, Atglen, PA
Keeley J, Syphard A (2016) Climate change and future fire regimes: examples from California. Geosciences 6(3):37
Kettlewell HBD (1955) Selection experiments on industrial melanism in the Lepidoptera. Heredity 9(3):323–342
Knisley CB, Hill JM (1992) Effects of habitat change from ecological succession and human impact on tiger beetles. VA J Sci 43(1):133–142
Kurokawa S (2006) Phytogeographical elements of the lichen flora of Japan. J Hattori Bot Lab 100:721–738
LaGreca S, Spencer Goyette S, Medeiros ID (2018) The lichens of lizard lick. North Carolina Evansia 35(2):53–57
Lawrey JD (1977) Inhibition of moss spore germination by acetone extracts of terricolous Cladonia species. Bull Torrey Bot Club 104(1):49–52
Lechowicz MJ, Adams MS (1974) Ecology of Cladonia lichens. I. Preliminary assessment of the ecology of terricolous lichen–moss communities in Ontario and Wisconsin. Can J Bot 52(1):55–64
Lendemer JC (2011a) A review of the morphologically similar species Fuscidea pusilla and Ropalospora viridis in eastern North America. Opusc Philolichenum 9:11–20
Lendemer JC (2011b) Contributions to the lichen flora of Pennsylvania—Rare and important lichen habitats and lichen communities: part 1, the northeastern counties. Bartonia 65:20–28
Lendemer JC, Allen JL (2014) Lichen biodiversity under threat from sea-level rise in the Atlantic Coastal Plain. Bioscience 64(10):923–931
Lendemer JC, Allen JL, McMullin RT. (2015). Cladonia submitis Global Red List Assessment Proposal. Retrieved Jan 15 2017 from https://iucn.ekoo.se/iucn/species_view/365718/.
Lohmus P, Lohmus A (2009) The importance of representative inventories for lichen conservation assessments: the case of Cladonia norvegica and C. parasitica. Lichenologist 41(1):61–67
Lotze HK, Coll M, Magera AM, Ward-Paige C, Airoldi L (2011) Recovery of marine animal populations and ecosystems. Trends Ecol Evol 26(11):595–605
Lücking R, Hodkinson BP, Leavitt SD (2017) The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 119:361–416
Maikawa E, Kershaw KA (1976) Studies on lichen-dominated systems. XIX. The postfire recovery sequence of black spruce–lichen woodland in the Abitau Lake Region, NWT. Can J Bot 54(23):2679–2687
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11(2):161–176
McGonigle TP (2007) 12 Effects of Animals Grazing on Fungi. In: Kubicek CP (ed) Environmental and Microbial Relationships. Springer, Berlin, Heidelberg, pp 201–212
Menges ES, Kohfeldt N (1995) Life history strategies of Florida scrub plants in relation to fire. Bull Torrey Bot Club 122(4):282–297
Miyawaki H (1994) Lecanora imshaugii, a lichen of eastern North America and eastern Asia. Bryologist 97(4):409–411
Morneau C, Payette S (1989) Postfire lichen–spruce woodland recovery at the limit of the boreal forest in northern Quebec. Can J Bot 67(9):2770–2782
Myllys L, Stenroos S, Thell A, Ahti T (2003) Phylogeny of bipolar Cladonia arbuscula and Cladonia mitis (Lecanorales, Euascomycetes). Mol Phylogenet Evol 27(1):58–69
Nash TH (1976) Lichens as indicators of air pollution. Naturwissenschaften 63(8):364–367
Nash TH, Gries C (1991) Lichens as indicators of air pollution. In: Gries C, Lipfert FW, Lippmann M, Nash TH (eds) Air Pollution. Springer, Berlin, Heidelberg, pp 1–29
Nimis PL, Scheidegger C, Wolseley PA (2002) Monitoring with Lichens—Monitoring Lichens. Springer, Dordrecht
Osyczka P, Rola K (2013) Phenotypic plasticity of primary thallus in selected Cladonia species (lichenized Ascomycota: Cladoniaceae). Biologia 68(3):365–372
Osyczka P, Rola K, Lenart-Boroń A, Boroń P (2014) High intraspecific genetic and morphological variation in the pioneer lichen Cladonia rei colonising slag dumps. Open Life Sci 9(5):579–591
Pearson L, Skye E (1965) Air pollution affects pattern of photosynthesis in Parmelia sulcata, a corticolous lichen. Science 148(3677):1600–1602
Pike LH (1978) The importance of epiphytic lichens in mineral cycling. Bryologist 81(2):247–257
Poelt J (1994) Different species types in lichenized ascomycetes. In: Hawkesworth DL (ed) Ascomycete systematics: Problems and perspectives in the nineties. Plenum, New York, pp 273–278
Ravera S, Isocrono D, Nascimbene J, Giordani P, Benesperi R, Tretiach M, Montagnani C (2016) Assessment of the conservation status of the mat-forming lichens Cladonia subgenus Cladina in Italy. Plant Biosyst 150(5):1010–1022
Ray DG, Cahalan G, Lendemer JC (2020) Factors influencing the persistence of reindeer lichens (Cladonia subgenus Cladina) within frequent-fire environments of the Mid-Atlantic Coastal Plain, USA. Fire Ecol. https://doi.org/10.1186/s42408-019-0063-7
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems–a journey towards relevance? New Phytol 157(3):475–492
Reisch C, Schmid C, Hartig F (2018) A comparison of methods for estimating plant population size. Biodivers Conserv 27(8):2021–2028
Rios NE, Bart HL. (2010). GEOLocate (Version 3.22) [computer software]. Belle Chasse, LA: Tulane University Museum of Natural History.
Rodrigues AS, Pilgrim JD, Lamoreux JF, Hoffmann M, Brooks TM (2006) The value of the IUCN red list for conservation. Trends Ecol Evol 21(2):71–76
Scheffers BR, Paszkowski CA (2012) The effects of urbanization on North American amphibian species: identifying new directions for urban conservation. Urban Ecosyst 15(1):133–147
Scheidegger C, Bilovitz PO, Werth S, Widmer I, Mayrhofer H (2012) Hitchhiking with forests: population genetics of the epiphytic lichen Lobaria pulmonaria in primeval and managed forests in Southeastern Europe. Ecol Evol 2:2223–2240
Sedia EG, Ehrenfeld JG (2003) Lichens and mosses promote alternate stable plant communities in the New Jersey Pinelands. Oikos 100(3):447–458
Smith DR, Brockmann HJ, Beekey MA, King TL, Millard MJ, Zaldívar-Rae J (2017) Conservation status of the American horseshoe crab, (Limulus polyphemus): a regional assessment. Rev Fish Biol Fisher 27(1):135–175
Sweet WV, Kopp RE, Weaver CP, Obeysekera J, Horton RM, Thieler ER, Zervas C (2017) Global and regional sea level rise scenarios for the United States. National Oceanic and Atmospheric Administration, United States Department of Commerce
Terry EL, McLellan BN, Watts GS (2000) Winter habitat ecology of mountain caribou in relation to forest management. J Appl Ecol 37(4):589–602
Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, Ferreira de Sigueira M, Grainger A, Hannah L, Hughes L, Huntley B, van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Townsend Paterson A, Philips OL, Williams SE (2004) Extinction risk from climate change. Nature 427(6970):145
Thomson JW. (1984). American Arctic Lichens: The Microlichens, University of Wisconsin Press, (Vol. 2).
Van der Veken S, Verheyen K, Hermy M (2004) Plant species loss in an urban area (Turnhout, Belgium) from 1880 to 1999 and its environmental determinants. Flora 199(6):516–523
Wake DB, Vredenburg VT (2008) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci 105:11466–11473
Wu SY, Yarnal B, Fisher A (2002) Vulnerability of coastal communities to sea-level rise: a case study of Cape May County, New Jersey, USA. Climate Res 22(3):255–270
Wyatt R, Stoneburner A (1982) Range extensions for some cryptogams from granite outcrops in Alabama. Bryologist 85:405–409
Acknowledgements
A great thanks is extended to the Rutgers Pinelands Field Station for providing housing and the use of their facility over the course of this study. Additionally, thank you to Boy Scout troop 48 of Egg Harbor Township and to Tom Walker for your assistance in the field. Dr. Jessica Allen was invaluable resources in structuring, preparing and executing this study. Mike Baxter was instrumental in gathering high-quality scanning electron microscopy of specimens examined. Keith Seager, Rick Radis and David Snyder were all critical in understanding what happened to the lichens on Cape May dunes. This manuscript is part of a dissertation by the first author and was supported by NSF Dimensions of Biodiversity award #1542629 and #154263, as well as the student research grant by the Philadelphia Botanical Club and the Culberson and Hale award by the American Bryological and Lichenological Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Pradeep Kumar Divakar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hoffman, J.R., Ohmura, Y. & Lendemer, J.C. Combing for beach broccoli: surveys of the endemic macrolichen Cladonia submitis determines endangered status under IUCN guidelines. Biodivers Conserv 29, 2439–2456 (2020). https://doi.org/10.1007/s10531-020-01983-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-01983-x