Abstract
Little is known about the differences in patterns and drivers between species richness (SR) and functional diversity (FD) in aquatic plants at large scales, and the underlying assembly mechanisms are not well studied. We compared SR and FD patterns of aquatic plant assemblages in 29 subtropical lakes, and detected the underlying assembly rules. Environmental drivers of SR and FD were revealed by GLM and GAM models, and the relative importance of assembly rules was determined by a null model approach. SR and FD of aquatic plants presented different patterns and drivers in this region. SR was significantly correlated with geographic, hydrological and water quality variables. We found a lower functional richness but higher functional evenness and divergence in the highland lakes. There was no significant correlation between functional richness and environmental variables. Null model analyses showed that most values of standardized effect size were located between the confidence interval, indicating a dominance of randomness. Deterministic processes such as limiting similarity and habitat filtering were also important in individual lakes. Habitat filtering plays a stronger role shaping the hydrophyte assemblages especially with the increase of elevation, area and AWLF (amplitude of water level fluctuation). Our results demonstrated that FD, in contrast to SR, were more resistant to environmental variations, and hydrology played an important role in shaping both SR and FD patterns in lake ecosystems. Furthermore, we revealed complex assembly rules and emphasized the importance of both stochastic and deterministic mechanisms in structuring aquatic plant assemblages at the regional scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding patterns and drivers of biodiversity at various scales remains a fundamental issue in ecology and conservation practices (Hortal et al. 2015). Classical studies on biodiversity are largely based on species richness (SR) (i.e. taxonomic unit), treating all species almost equally with little consideration of phylogenetic and trait variations (Mouchet et al. 2010). Over the last two decades, functional diversity (FD) and phylogenetic diversity (PD) have been combined into analyses of community assemblages, revealing a multifaceted nature of biodiversity (Jarzyna and Jetz 2016; Pool et al. 2014). FD is defined as “the value and range of the functional traits of the organisms in a given ecosystem” (Tilman 2001). FD is linked to ecosystem functioning and ecological interactions, since the responses of species to environmental gradients and their ecological functions are determined by their functional traits (Hooper et al. 2005; Mori 2016; Mouillot et al. 2013; Petchey et al. 2004). Consequently, a FD approach is increasingly incorporated into addressing a variety of ecological and environmental questions, including the patterns and drivers of biodiversity, community assembly, diversity-ecosystem functioning relationship, ecological restoration and conservation, and impact of global change (e.g. Arthaud et al. 2012; Barbet-Massin and Jetz 2015; Mouchet et al. 2010; Paillex et al. 2013; Santos et al. 2016; Schleuter et al. 2012; Swenson et al. 2012).
FD can be generally decomposed into three components, i.e. functional richness, functional evenness and functional divergence (Mason et al. 2005; Villéger et al. 2008). The three facets are complementary, and each provides independent information of the distribution of species in functional space. Although a number of indices have been developed to quantify each facet of functional diversity (e.g. FRic, FEve and FDiv; Villéger et al. 2008), functional richness is the most concerned while functional evenness and divergence are rarely documented (Mason et al. 2013; Mouchet et al. 2010; Schleuter et al. 2010). To date, only a very small number of studies document a complete quantification of functional diversity in terms of functional richness, evenness and divergence (e.g. Ding et al. 2013, Karadimou et al. 2015, 2016). In addition, theoretical and empirical studies show that functional richness is highly correlated with SR, while functional evenness and divergence are more independent (Ding et al. 2013; Mouchet et al. 2010; Paillex et al. 2013; Schleuter et al. 2010). It indicates that FD might not always be consistent with SR in responses to environmental gradients. Therefore, understanding patterns and drivers of FD might provide additionally valuable insights for biodiversity conservation and ecological theory.
Recently, applying FD measures to detect assembly rules that control biodiversity patterns has received a lot of attention (e.g. Karadimou et al. 2015; Mouchet et al. 2010; Mouillot et al. 2007; Santos et al. 2016). Generally, two hypotheses driven by functional traits are commonly discussed: limiting similarity and habitat filtering. The limiting similarity principle (Macarthur and Levins 1967) assumes that coexisting species are functionally dissimilar and complementary, while the habitat filtering principle (Zobel 1997) emphasizes the role of environmental condition as a filter and thus coexisting species are more similar to one another. Among the three FD components, functional richness is found to be efficient in detecting the above assembly rules (Mouchet et al. 2010). Since functional richness increases with SR, a null model approach that randomize the community or trait matrix is introduced to remove the effects of SR (e.g. Ding et al. 2013; Mason et al. 2008; Swenson 2014; Swenson et al. 2012). Observed FD indices are compared with expected values generated by the null model, where a positive departure from random expectation indicates limiting similarity and a negative departure habitat filtering (Cornwell and Ackerly 2009; Mori et al. 2015). In summary, FD analysis becomes a useful tool in revealing assembly rules of biological community, establishing a linkage between community ecology and ecosystem functioning.
Empirical studies reveal that both deterministic and stochastic mechanisms are important in assembling biological communities (Chase and Myers 2011; Cornwell and Ackerly 2009; Geheber and Geheber 2016; Laliberté et al. 2014; Lasky et al. 2014). These assembly rules can occur simultaneously or sequentially along environmental gradients (Helmus et al. 2007; Karadimou et al. 2015; Mason et al. 2007), indicating that biological communities might be controlled by multiple mechanisms. Therefore, it’s important to understand which mechanism is stronger under a specific condition and how their influences vary along environmental gradients (Mouchet et al. 2010). Although community assembly of terrestrial plant is progressing rapidly within a FD framework, little is known about the relative influence of mechanisms in aquatic plants (but see, Fu et al. 2014; Ruhí et al. 2014), especially at large scales. A number of factors that affect distribution of aquatic plants have been identified, among them, hydrological variables and water quality are the most concerned (Bornette and Puijalon 2011). Since these factors can also exert influences of various degrees on functional traits (Baattrup-Pedersen et al. 2015; Fu et al. 2014), they might potentially drive the FD patterns and community assembly of aquatic plants.
In the present study, we described patterns and drivers of SR and FD, and detected underlying assembly rules of aquatic plants in subtropical lakes. Our study had three specific purposes. First, we compare biodiversity patterns of aquatic plants in two sub-regions: highland and lowland. Since SR declines with elevation (Rahbek 1995) and isolated habitats form dispersal barriers for species (Schleuter et al. 2012), we expect a lower functional richness and higher functional evenness and divergence in the more isolated highland lakes. Second, we examine the differences between SR and FD in response to environmental variables at the regional scale. On the one hand, since FD increases monotonically with SR (Mouchet et al. 2010; Villéger et al. 2008), their responses to environmental gradients might be similar. On the other hand, FD might be more resistant to environmental disturbances due to functional redundancy (Carmona et al. 2016), potentially blurring the observed patterns. Third, we examine the relative influence of limiting similarity and habitat filtering on aquatic plant community. Habitat filtering is assumed to structure communities at regional scales, while limiting similarity usually drives communities at local scales (Santos et al. 2016; Swenson et al. 2012). We then expect a stronger effects of habitat filtering in the subtropical region.
Methods
Species survey
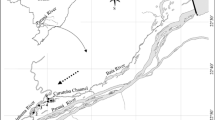
We studied aquatic plant diversity of 29 lakes in the subtropical area of China (Fig. 1). These lakes can be generally divided into two subsets, i.e. highland and lowland. Thirteen highland lakes are located in the Yunnan-Guizhou plateaus, making up about 90% of the total lake area in this region, while the other 16 lowland lakes were in the Yangtze River floodplain. The highland lakes are most tectonic and isolated, while the lowland lakes are fluviatile and connected or used to be connected with the Yangtze mainstem (Wang et al. 2016; Wang and Dou 1998).
Aquatic plants including hydrophytes (i.e. macrophytes) and hygrophytes (i.e. amphiphytes) (Zhang 2009) were investigated during 2007–2012. Surveys were mainly carried out in the lakeshore region. The upper limit of the lakeshore zone is defined as where terrestrial species or embankment is encountered, and the lower limit is where submerged hydrophytes disappear and determined by field surveys of this study and historical records. Therefore, the survey area is subject to change from site to site given the heterogeneity of lake bathymetry. Field surveys were carried out in autumns of 2007 and 2010, and summer of 2008 for highland lakes, with each lake being visited twice. For lowland lakes, three field surveys were taken for each lake in spring, summer and autumn in 2009–2012. Sampling transects (each 20 m width) perpendicular to the shoreline were set in lakeshore zone avoiding sites with obvious human activities. Three to nine transects were set for plateau lakes, and one to seven for floodplain lakes given the size of lake area and habitat heterogeneity. From the upper limit to the water edge of each transect, all species encountered (mainly hygrophytes and emergent hydrophytes) were identified and recorded. From the water edge to the lower limit, hydrophytes were sampled by scythes or grabs, and any species encountered were recorded. All lakeshore plants were identified to species level when possible according to Flora of China (www.efloras.org) (Flora of China Editorial Committee 1988–2013) and other taxonomic monographs (Institute of Botany 1994; Zhang 2009; Zhao and Liu 2009). Eight species including one woody species (Triadica sebifera), one charophyte (Chara sp.) and six fern species were excluded from analysis although they occurred in the sampling transects. The presence/absence data of 215 species, including 42 hydrophytes and 173 hygrophytes, were used to describe plant assemblages of the 29 lakes. Hygrophytes dominated in most lakes, accounting for 66.4% of the total in average.
Environmental data
We considered 15 environmental variables representing the geographic, hydrological, lake morphological and water quality condition of the lakes (Online Resource 1). All the variables are regarded as important to various degrees in shaping aquatic plant communities (Bornette and Puijalon 2011; Heino and Toivonen 2008). Geographic variables included longitude, latitude, elevation and area. Regarding hydrological characters, water-level fluctuation (WLF) is regarded as the chief hydrological variable structuring biological communities in lakes and wetlands (Cott et al. 2008; Wantzen et al. 2008). In this study, amplitude of WLF (AWLF) was considered and defined as the within-year variation in water level, i.e. the maximum minus the minimum. Precipitation had great impacts on water level, and was also treated as a hydrological variable in this study. In terms of lake morphology, the mean and maximum water depth together with the shoreline development index (SDI) were included. SDI is calculated as \({\text{SDI}} = L/(2\sqrt {\pi A} )\), where L is shoreline length and A is lake area (Aronow 1982). For water quality, six indices were included as Secchi depth (SD), pH, conductivity, and concentrations of total nitrogen (TN), total phosphorus (TP), and chlorophyll a (Chla). All environmental data were derived from literature, publications or government datasets. Geographic, hydrological and morphological data were mainly from Records of Chinese Lakes (Wang and Dou 1998). AWLF values of some lakes were calculated from daily water-level records of local gauge stations of the survey years. Water quality data of the field survey periods were derived from literature (Liu et al. 2011; Yu et al. 2010; Zhang 2013), and unpublished monitoring data of local governments and scientific institutes.
Trait data
We selected 13 functional traits representing vegetative, regenerative and ecological characteristics of aquatic plants (Table 1). These traits are generally used in functional diversity analysis and they represent different functional strategies of plants under certain environmental conditions (Adler et al. 2014; Karadimou et al. 2015; Weiher et al. 1999). The other traits such as hydraulic and foliar traits (SLA, N% and P%) were of interest (Adler et al. 2014; Wright et al. 2004), but they were not included in this study due to lack of data. Among the selected traits, seven are continuous variables and the remains are either categorical or binary. Functional trait values were collected mainly from the monograph Flora of China (www.efloras.org) (Flora of China Editorial Committee 1988–2013) and other literature (Institute of Botany 1994; Zhang 2009; Zhang 2013; Zhao and Liu 2009). Leaf width/length ratio was calculated based on the collected data.
Functional diversity
We used three independent indices, i.e. FRic, FEve and FDiv, developed by Villéger et al. (2008) to quantify FD of each assemblage. FRic quantifies the volume of functional space filled by the community, and FEve and FDiv measure evenness and divergence of species distribution in this volume, respectively (Mason et al. 2005; Villéger et al. 2008). To calculate the three indices, we followed methods provided by Swenson (2014). First, we calculated the functional distance for each pair of species using Gower’s distance which allows mixing quantitative and qualitative variables while giving them equal weight (Podani and Schmera 2006). Secondly, we performed a Principal Coordinate Analysis (PCoA) on this functional distance matrix (Villéger et al. 2008). The first five axes of the PCoA, accounting for 77.8% of total variation, were selected and treated as the new “traits” for computation. Finally, functional indices for each plant assemblage were calculated using the new “traits” and species presence/absence data.
Null model
We used a null model approach to detect the assembly rules that structure the plant assemblages in the studied lakes (Gotelli and Colwell 2001; Mouchet et al. 2010). According to Swenson (2014), we created 999 random assemblages by randomizing the trait data while maintaining the community. Randomization were carried out for highland and lowland lakes separately, considering that they might have different species pools due to geographic isolation. FRic values of generated assemblages were calculated and compared with observed values using two-tailed Wilcoxon Signed-Ranks Tests (Hollander and Wolfe 1999). We measured the standardized effect size (SES) for each assemblage according to Gotelli and Rohde (2002). The SES is calculated as SES = (Obs − Exp)/SDExp, where Obs corresponds to indices for the observed assemblage, Exp is the mean of index values for randomization and SDExp is the standard deviation of randomization. A significant difference at P < 0.05 is considered where the SES value falls outside the range − 1.96 to 1.96, assuming a normal distribution of deviations (Gotelli and Rohde 2002; Wittman et al. 2010). A greater SES value than 1.96 indicates limiting similarity, and a value lower than − 1.96 indicates habitat filtering (Ding et al. 2013; Mouchet et al. 2010). In addition, general linear models were used to test the differences between observed and expected FRic values across lakes, with SR as a covariate.
Statistical analyses
Patterns of SR and FD between the highland and lowland lakes were compared using Wilcoxon Rank Sum Tests (Hollander and Wolfe 1999). To determine environmental responses of SR and FD, generalized linear models (GLMs) and generalized additive models (GAMs) were constructed for each biodiversity index (Hastie and Tibshirani 1990). To avoid multicollinearity, bivariate correlations between environmental variables were detected using Spearman rank–order correlation analysis. Variables that highly correlated at P < 0.05 or with the coefficient |r| > 0.70, were removed and only one was kept considering their biological meanings (Alahuhta et al. 2011). Among the 15 variables, eight were selected to represent geographic, hydrological, morphological and water quality characteristics of studied lakes (Table 2). To determine which variables were statistical significant, we first constructed a global GLM model including all selected variables for each SR and FD index. Next, we used a full stepwise selection procedure by removing variables one by one from the global model to detect whether the model changed significantly using a Chi square test at P < 0.05. The variables that significantly correlated with a biodiversity index were used to construct the GAMs. A Poisson distribution was assumed and a logarithmic link function was used in the GAMs of SR, and a normal distribution with an identity link function was applied for FD models. A stepwise selection procedure was also applied to the GAMs, and the candidate models were compared using the Akaike information criterion (AIC) (Akaike 1974). Models with the lowest AIC values were selected as the best models (Burnham and Anderson 2002). If small difference (0–2) between two AIC values occurred, we choose the model with less variables to avoid overfitting or the model with higher explanatory power. Spatial autocorrelation was checked for residuals of each final model based on Moran I correlograms (Bivand et al. 2013), and none to very low spatial autocorrelation was detected. Similar procedure was applied to determine the responses of SES to environmental factors. All calculations and analyses were performed in R 3.3.2 (R Core Team 2017).
Results
Patterns and drivers of SR and FD
SR of aquatic plants was significantly different between the highland and lowland lakes. The total species and hygrophytes were more abundant in the lowland, while SR of hydrophyte was higher in the highland. Regarding FD, FRic was significant lower and FEve and FDiv were higher in the highland (Fig. 2).
The environmental analyses using GAMs showed that the deviances explained were > 56%, indicating that the models were well fitted with the data (Table 2). Aquatic plant diversity was closely correlated with geographic, hydrological and water quality variables, while lake morphological characters were less important (Table 2). The total SR was significantly correlated with area, AWLF, conductivity and TP (P < 0.01). It decreased with AWLF and conductivity, and increased with TP, while the species-area relationship was not monotonic (Fig. 3). Differences were also found between hydrophytes and hygrophytes. Hydrophytes only presented significant relationships with elevation and area, while hygrophytes showed patterns quite similar to the total SR. In contrast, FD seemed to be more resistant to environmental variables. No significant relationship was detected between the total FRic and environmental variables. FRic of hydrophytes varied with area and mean water depth, while that of hygrophytes with elevation and TP (Table 2). FEve had positive relationships with elevation and AWLF, and FDiv increased with elevation.
Responses of the total species richness to major environmental variables. The curved lines are splines fitted by the generalized additive model (Table 2), and the shaded areas indicate the ranges of 95% confidence intervals
Observed versus expected FRic
All observed values of total FRic were significantly different from the means of expected (P < 0.05). SES values were either positive or negative and most were located between the confidence intervals, and only in five lakes they showed significant departure from randomization (Fig. 4). The observed and expected values of FRic were strongly correlated with SR across lakes (P < 0.05). General linear model analyses showed that the slope of observed FRic was significantly shallower (F = 37.7, P < 0.001) than that of expected in hydrophytes, but no significant difference in slopes was found in the total as well as hygrophyte assemblages. SES values of hydrophytes were negatively related with elevation, area and AWLF (Online Resource 2).
Discussion
Patterns and drivers in SR versus FD
The present study revealed that lake area, AWLF, conductivity and TP were important in determining species diversity of aquatic plants in subtropical lakes at the regional scale. Although we found SR of aquatic plants was significantly correlated with lake area, the species-area curve was non-monotonic, and quite different from the classical power or exponential function (Lomolino 2000; Tjørve 2003; Williams et al. 2009). Previous studies have also failed to detect any significant relationship between aquatic plant species with lake area (e.g. Heegaard 2004; Hinden et al. 2005; Vestergaard and Sand-Jensen 2000). It seems that lake area per se can hardly explain species diversity pattern of aquatic plants. Both AWLF and conductivity had negative relationships with SR. Such results were different from previous studies in temperate lakes where SR peaked at the middle AWLF (ca. 1–2 m) (e.g. Geest et al. 2005; Hill et al. 1998; Riis and Hawes 2002). The difference might be attributed to the scale of AWLF involved, where AWLF was greater in the subtropical lakes (maximum 11.9 m) than temperate ones. Moreover, such a large AWLF might exert strong detrimental effects on both hygrophytes and hydrophytes, since it is usually associated with a deeper submergence and a quick decrease in water transparency in this region (Zhang 2013). Conductivity can be regarded as a measure of salinity, and the latter is proved to have strong detrimental effects on aquatic plants (Nielsen et al. 2003). There was a positive relationship between SR and TP, indicating that a certain level of nutrient increase might promote plant diversity especially hygrophytes in lakeshore. Regarding submerged macrophytes, however, overloading of TP can prohibit their development by reducing water transparency (Hough et al. 1989; Jin et al. 2005). In the present study, hydrophytes showed patterns and drivers quite different from those of hygrophytes. Although they were significantly correlated with elevation, their responses were in reverse ways (Fig. 2, Table 2), potentially blurring the relationship between elevation and total SR.
In contrast to SR, FD of aquatic plants presented different patterns and drivers. As we expected, FD was more resistant to environmental variables than SR, indicating that loss of species might not result in loss of FD due to functional redundancy (Carmona et al. 2016; Pool et al. 2014). No significant relationship was detected between FRic of the total and environmental variables, mainly due to the reason that hydrophyte FRic and hygrophyte FRic responded to different variables (Table 2). Both FEve and FDiv were significantly correlated elevation, and they were higher in highland lakes than in lowland lakes as we expected. Such results were consistent with the isolation hypothesis, which predicted a higher functional evenness and divergence but lower functional richness in isolated habitats (Field et al. 2009; Schleuter et al. 2012). In this study, the highland lakes are more isolated than the lowland floodplain lakes (Wang et al. 2016). Our results showed that AWLF was positively related with FEve of aquatic plants, suggesting that a certain level of hydrological disturbance can promote functional evenness. Such result was in agreement with studies on birds and terrestrial plants (e.g. Cardinale et al. 2000; Ding et al. 2013; Pakeman 2011). In lakes with a large AWLF, aquatic plant assemblages are usually dominated by a small number of species such as Carex and Phragmites australis (Wang et al. 2016). It’s probably that community in highly disturbed environments would be highly uneven due to dominance of a few species.
Underlying assembly rules
Our results showed that most SES values of total assemblages fell between the confidence intervals, indicating that stochastic processes might dominate in aquatic plants. This might partly explain why FRic was not related to any environmental variables (Table 2). Stochastic processes were also found to control macroinvertebrate assemblages in lakes (Heino and Tolonen 2017). It seemed that randomness could be common in aquatic assemblages. However, the dominance of randomness might be overestimated in the present study since randomness can also result from combined effects of deterministic processes such as limiting similarity and habitat filtering (Chesson 2000; Heino and Tolonen 2017; Tilman 2004).
Hydrophyte and hygrophyte assemblages seemed to be controlled by different mechanisms. Although only one significant departure was detected in hydrophytes, the observed values increased at a much lower pace with SR than expected, suggesting a stronger role of habitat filtering across lakes. By contrast, hygrophyte assemblages seemed to be controlled mainly by stochastic processes such as stochasticity and drift (Kraft and Ackerly 2014; Tilman 2004). The difference in assembly mechanisms might be related to their different life history strategies, where hygrophytes are more opportunistic with shorter life span (usually 3–5 months) than hydrophytes (Zhang 2009). As environmental gradients or disturbance intensity increase, biological communities are assumed to be driven by habitat filtering (Santos et al. 2016). SES values of hydrophytes showed significantly negative correlations with elevation, area and AWLF, indicating that habitat filtering would become stronger along these environmental gradients. Such results supported the hypothesis that habitat filtering would drive community at the regional scale (Laliberté et al. 2014; Santos et al. 2016).
Conclusions and implications
The present study revealed FD patterns and assembly rules of aquatic plants at a regional scale. SR of aquatic plants showed strong correlations with environmental variables, while FD was more resistant. Our analyses revealed complex assembly rules in structuring aquatic plants assemblages in this region. Aquatic plant assemblages seemed to be controlled mainly by stochastic processes. In individual lakes, deterministic mechanisms such as limiting similarity and habitat filtering were also important. Globally, freshwaters are seriously threatened and vulnerable to anthropogenic activities (Strayer and Dudgeon 2010; Vörösmarty et al. 2010). Our study provides important implications considering conservation and rehabilitation of aquatic plants. Since AWLF is important in determining both SR and FD of aquatic plants in lakes, conservation and rehabilitation should therefore take into consideration of water level management which is poorly implemented in the study region (Liu et al. 2017). As FD is related to ecosystem functioning (Mori 2016), water level management might also be a useful tool to promote the whole ecosystem health.
References
Adler PB, Salguero-Gómez R, Compagnoni A, Hsu JS, Ray-Mukherjee J, Mbeau-Ache C, Franco M (2014) Functional traits explain variation in plant life history strategies. Proc Natl Acad Sci 111:740–745
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Alahuhta J, Heino J, Luoto M (2011) Climate change and the future distributions of aquatic macrophytes across boreal catchments. J Biogeogr 38:383–393
Aronow S (1982) Shoreline development ratio. Beaches and coastal geology. Springer, New York, pp 754–755
Arthaud F, Vallod D, Robin J, Bornette G (2012) Eutrophication and drought disturbance shape functional diversity and life-history traits of aquatic plants in shallow lakes. Aquat Sci 74:471–481
Baattrup-Pedersen A, Göthe E, Larsen SE, O’Hare M, Birk S, Riis T, Friberg N (2015) Plant trait characteristics vary with size and eutrophication in European lowland streams. J Appl Ecol 52:1617–1628
Barbet-Massin M, Jetz W (2015) The effect of range changes on the functional turnover, structure and diversity of bird assemblages under future climate scenarios. Global Change Biol 21:2917–2928
Bivand RS, Pebesma E, Gómez-Rubio V (2013) Applied spatial data analysis with R. Springer, Berlin
Bornette G, Puijalon S (2011) Response of aquatic plants to abiotic factors: a review. Aquat Sci Res Across Bound 73:1–14
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cardinale BJ, Nelson K, Palmer MA (2000) Linking species diversity to the functioning of ecosystems: on the importance of environmental context. Oikos 91:175–183
Carmona CP, de Bello F, Mason NWH, Lepš J (2016) Traits without borders: integrating functional diversity across scales. Trends Ecol Evol 31:382–394
Chase JM, Myers JA (2011) Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc B 366:2351–2363
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Cornwell WK, Ackerly DD (2009) Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol Monogr 79:109–126
Cott PA, Sibley PK, Somers WM, Lilly MR, Gordon AM (2008) A review of water level fluctuations on aquatic biota with an emphasis on fishes in ice-covered lakes. JAWRA 44:343–359
Ding Z, Feeley KJ, Wang Y, Pakeman RJ, Ding P (2013) Patterns of bird functional diversity on land-bridge island fragments. J Anim Ecol 82:781–790
Field R, Hawkins BA, Cornell HV, Currie DJ, Diniz-Filho JAF, Guégan J-F, Kaufman DM, Kerr JT, Mittelbach GG, Oberdorff T, O’Brien EM, Turner JRG (2009) Spatial species-richness gradients across scales: a meta-analysis. J Biogeogr 36:132–147
Flora of China Editorial Committee (ed.) (1988–2013) Flora of China (Checklist & Addendum). Unpaginated. Science Press & Missouri Botanical Garden Press, St. Louis
Fu H, Zhong J, Yuan G, Xie P, Guo L, Zhang X, Xu J, Li Z, Li W, Zhang M, Cao T, Ni L (2014) Trait-based community assembly of aquatic macrophytes along a water depth gradient in a freshwater lake. Freshw Biol 59:2462–2471
Geest GJV, Wolters H, Roozen FCJM, Coops H, Roijackers RMM, Buijse AD, Scheffer M (2005) Water-level fluctuations affect macrophyte richness in floodplain lakes. Hydrobiologia 539:239–248
Geheber AD, Geheber PK (2016) The effect of spatial scale on relative influences of assembly processes in temperate stream fish assemblages. Ecology 97:2691–2704
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Gotelli NJ, Rohde K (2002) Co-occurrence of ectoparasites of marine fishes: a null model analysis. Ecol Lett 5:86–94
Hastie T, Tibshirani R (1990) Generalized additive models. Chapman & Hall, London
Heegaard E (2004) Trends in aquatic macrophyte species turnover in Northern Ireland—which factors determine the spatial distribution of local species turnover? Global Ecol Biogeogr 13:397–408
Heino J, Toivonen H (2008) Aquatic plant biodiversity at high latitudes: patterns of richness and rarity in Finnish freshwater macrophytes. Boreal Environ Res 13:1–14
Heino J, Tolonen KT (2017) Untangling the assembly of littoral macroinvertebrate communities through measures of functional and phylogenetic alpha diversity. Freshw Biol 62:1168–1179
Helmus MR, Savage K, Diebel MW, Maxted JT, Ives AR (2007) Separating the determinants of phylogenetic community structure. Ecol Lett 10:917–925
Hill NM, Keddy PA, Wisheu IC (1998) A hydrological model for predicting the effects of dams on the shoreline vegetation of lakes and reservoirs. Environ Manag 22:723–736
Hinden H, Oertli B, Menetrey N, Sager L, Lachavanne J-B (2005) Alpine pond biodiversity: what are the related environmental variables? Aquat Conserv 15:613–624
Hollander M, Wolfe DA (1999) Nonparametric statistical methods. Wiley, New York
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, La HS, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Hortal J, Bello Fd, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ (2015) Seven shortfalls that beset large-scale knowledge of biodiversity. Annu Rev Ecol Evol Syst 46:523–549
Hough RA, Fornwall M, Negele B, Thompson R, Putt D (1989) Plant community dynamics in a chain of lakes: principal factors in the decline of rooted macrophytes with eutrophication. Hydrobiologia 173:199–217
Institute of Botany CAoS (1994) The picture index of senior china plant. Science Press, Beijing
Jarzyna MA, Jetz W (2016) Detecting the multiple facets of biodiversity. Trends Ecol Evol 31:527–538
Jin X, Xu Q, Huang C (2005) Current status and future tendency of lake eutrophication in China. Sci China Ser C 48:948–954
Karadimou E, Tsiripidis I, Kallimanis AS, Raus T, Dimopoulos P (2015) Functional diversity reveals complex assembly processes on sea-born volcanic islands. J Veg Sci 26:501–512
Karadimou EK, Kallimanis AS, Tsiripidis I, Dimopoulos P (2016) Functional diversity exhibits a diverse relationship with area, even a decreasing one. Sci Rep 6:35420
Kraft NJB, Ackerly DD (2014) Assembly of plant communities. In: Monson RK (ed) Ecology and the environment. Springer, New York, pp 67–88
Laliberté E, Zemunik G, Turner BL (2014) Environmental filtering explains variation in plant diversity along resource gradients. Science 345:1602–1605
Lasky JR, Uriarte M, Boukili VK, Chazdon RL (2014) Trait-mediated assembly processes predict successional changes in community diversity of tropical forests. Proc Natl Acad Sci 111:5616–5621
Liu Q, Huo S, Zan F, Xi B (2011) Investigation and evaluation on the eutrophication of lakes in Anhui Province. J Anhui Agric Sci 39:4626–4629
Liu X, Yang Z, Yuan S, Wang H (2017) A novel methodology for the assessment of water level requirements in shallow lakes. Ecol Eng 102:31–38
Lomolino MV (2000) Ecology’s most general, yet protean pattern: the species-area relationship. J Biogeogr 27:17–26
Macarthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385
Mason NWH, Mouillot D, Lee WG, Wilson JB (2005) Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111:112–118
Mason NWH, Lanoiselée C, Mouillot D, Irz P, Argillier C (2007) Functional characters combined with null models reveal inconsistency in mechanisms of species turnover in lacustrine fish communities. Oecologia 153:441–452
Mason NWH, Lanoiselée C, Mouillot D, Wilson JB, Argillier C (2008) Does niche overlap control relative abundance in French lacustrine fish communities? A new method incorporating functional traits. J Anim Ecol 77:661–669
Mason NWH, de Bello F, Mouillot D, Pavoine S, Dray S (2013) A guide for using functional diversity indices to reveal changes in assembly processes along ecological gradients. J Veg Sci 24:794–806
Mori AS (2016) Resilience in the studies of biodiversity-ecosystem functioning. Trends Ecol Evol 31:87–89
Mori A, Fujii S, Kitagawa R, Koide D (2015) Null model approaches to evaluating the relative role of different assembly processes in shaping ecological communities. Oecologia 178:261–273
Mouchet MA, Villéger S, Mason NWH, Mouillot D (2010) Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol 24:867–876
Mouillot D, Dumay O, Tomasini JA (2007) Limiting similarity, niche filtering and functional diversity in coastal lagoon fish communities. Estuar Coast Shelf Sci 71:443–456
Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR (2013) A functional approach reveals community responses to disturbances. Trends Ecol Evol 28:167–177
Nielsen DL, Brock MA, Crosslé K, Harris K, Healey M, Jarosinski I (2003) The effects of salinity on aquatic plant germination and zooplankton hatching from two wetland sediments. Freshw Biol 48:2214–2223
Paillex A, Dolédec S, Castella E, Mérigoux S, Aldridge DC (2013) Functional diversity in a large river floodplain: anticipating the response of native and alien macroinvertebrates to the restoration of hydrological connectivity. J Appl Ecol 50:97–106
Pakeman RJ (2011) Functional diversity indices reveal the impacts of land use intensification on plant community assembly. J Ecol 99:1143–1151
Petchey OL, Downing AL, Mittelbach GG, Persson L, Steiner CF, Warren PH, Woodward G (2004) Species loss and the structure and functioning of multitrophic aquatic systems. Oikos 104:467–478
Podani J, Schmera D (2006) On dendrogram-based measures of functional diversity. Oikos 115:179–185
Pool TK, Grenouillet G, Villéger S (2014) Species contribute differently to the taxonomic, functional, and phylogenetic alpha and beta diversity of freshwater fish communities. Divers Distrib 20:1235–1244
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahbek C (1995) The elevational gradient of species richness: a uniform pattern? Ecography 18:200–205
Riis T, Hawes I (2002) Relationships between water level fluctuations and vegetation diversity in shallow water of New Zealand lakes. Aquat Bot 74:133–148
Ruhí A, Chappuis E, Escoriza D, Jover M, Sala J, Boix D, Gascón S, Gacia E (2014) Environmental filtering determines community patterns in temporary wetlands: a multi-taxon approach. Hydrobiologia 723:25–39
Santos AMC, Cianciaruso MV, De Marco P (2016) Global patterns of functional diversity and assemblage structure of island parasitoid faunas. Global Ecol Biogeogr 25:869–879
Schleuter D, Daufresne M, Massol F, Argillier C (2010) A user’s guide to functional diversity indices. Ecol Monogr 80:469–484
Schleuter D, Daufresne M, Veslot J, Mason NWH, Lanoiselée C, Brosse S, Beauchard O, Argillier C (2012) Geographic isolation and climate govern the functional diversity of native fish communities in European drainage basins. Global Ecol Biogeogr 21:1083–1095
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J N Am Benthol Soc 29:344–358
Swenson N (2014) Functional and phylogenetic ecology in R. Springer, New York
Swenson NG, Enquist BJ, Pither J, Kerkhoff AJ, Boyle B, Weiser MD, Elser JJ, Fagan WF, Forero-Montaña J, Fyllas N, Kraft NJB, Lake JK, Moles AT, Patiño S, Phillips OL, Price CA, Reich PB, Quesada CA, Stegen JC, Valencia R, Wright IJ, Wright SJ, Andelman S, Jørgensen PM, Lacher TE Jr, Monteagudo A, Núñez-Vargas MP, Vasquez-Martínez R, Nolting KM (2012) The biogeography and filtering of woody plant functional diversity in North and South America. Global Ecol Biogeogr 21:798–808
Tilman D (2001) Functional diversity. In: Levin SA (ed) Encyclopedia of biodiversity. Academic Press, San Diego, pp 109–120
Tilman D (2004) Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA 101:10854–10861
Tjørve E (2003) Shapes and functions of species–area curves: a review of possible models. J Biogeogr 30:827–835
Vestergaard O, Sand-Jensen K (2000) Aquatic macrophyte richness in Danish lakes in relation to alkalinity, transparency, and lake area. Can J Fish Aquat Sci 57:2022–2031
Villéger S, Mason NWH, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301
Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Liermann CR, Davies PM (2010) Global threats to human water security and river biodiversity. Nature 467:555–561
Wang S, Dou H (1998) Records of Chinese lakes. Science Press, Beijing
Wang H, Liu X, Wang H (2016) The Yangtze River floodplain: threats and rehabilitation. Am Fish Soc Symp 84:263–291
Wantzen KM, Rothhaupt K-O, Mörtl M, Cantonati M, László G, Fischer P (eds) (2008) Ecological effects of water-level fluctuations in lakes. Springer, New York
Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O (1999) Challenging Theophrastus: a common core list of plant traits for functional ecology. J Veg Sci 10:609–620
Williams MR, Lamont BB, Henstridge JD (2009) Species–area functions revisited. J Biogeogr 36:1994–2004
Wittman SE, Sanders NJ, Ellison AM, Jules ES, Ratchford JS, Gotelli NJ (2010) Species interactions and thermal constraints on ant community structure. Oikos 119:551–559
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Yu Y, Zhang M, Qian S, Li D, Kong F (2010) Current status and development of water quality of lakes in Yunnan-Guizhou Plateau. J Lake Sci 22:820–828
Zhang S (ed) (2009) Common wetland plants in China. Science Press, New York
Zhang X (2013) Water level fluctuation requirements of plants in the Yangtze floodplain lakes. University of Chinese Academy of Sciences, Huairou
Zhao J, Liu Y (eds) (2009) Aquatic plant. Huazhong University of Science & Technology Press, Wuhan
Zobel M (1997) The relative of species pools in determining plant species richness: an alternative explanation of species coexistence? Trends Ecol Evol 12:266–269
Acknowledgements
We thank Yaqiang Shen, Xiaoke Zhang, Qingzhang Ding and Saibo Yuan for fieldwork and data collection. We also thank two anonymous reviewers for valuable comments on this manuscript. The research was funded by National Natural Science Foundation of China (41371054), and National Basic Research Program of China (2015CB458900).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Hawksworth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, X., Wang, H. Contrasting patterns and drivers in taxonomic versus functional diversity, and community assembly of aquatic plants in subtropical lakes. Biodivers Conserv 27, 3103–3118 (2018). https://doi.org/10.1007/s10531-018-1590-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-018-1590-2