Abstract
As human impacts and demands for ocean space increase (fisheries, aquaculture, marine reserves, renewable energy), identification of marine habitats hosting sensitive biological assemblages has become a priority. Epifaunal invertebrates, especially the structure-forming species, are an increasing conservation concern as many traditional (bottom-contact fishing) and novel (marine renewable energy) ocean uses have the potential to displace or otherwise impact these slow-growing organisms. The differences in mega-invertebrate species assemblages between high-relief rocks and low-relief sediments are well documented and likely hold for most marine environments. In anticipation of potential development of marine renewable energy faculties off Oregon and Washington (USA), a survey of the benthic invertebrate assemblages and habitats was conducted on the continental shelf of the Pacific Northwest, using video footage collected by ROV, to more finely characterize these assemblage–habitat associations. Four main associations were found: pure mud/sand dominated by sea whips and burrowing brittle stars; mixed mud–rock (which may be further divided based on size of mixed-in rocks) characterized by various taxa at small densities; consolidated rocks characterized by high diversity and density of sessile or motile mega-invertebrates; and rubble rocks showing less diversity and density than the consolidated rocks, possibly due to the disturbance generated by movement of the unconsolidated rocks. The results of this study will help classify and map the seafloor in a way that represents benthic habitats reflective of biological species assemblage distributions, rather than solely geological features, and support conservation and management planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the oceans provide a variety of valuable goods and services, societies sometimes fail to consider the damage that resource exploitation may cause to marine ecosystems over time (Jackson et al. 2001). Examples of anthropogenic impacts and over-exploitations of these ecosystems are numerous, and hard continental shelves and rocky reefs are among those most impacted (Lotze et al. 2006; Halpern et al. 2008). Fisheries using bottom gear such as trawls and dredges are by far the most damaging for the seafloor, acting like forest clear-cutting (Watling and Norse 1998). Due to technological improvements during the last decades, bottom-fishing gears are now used from polar to tropical waters on every type of seafloor; few places on the world’s continental shelves remaining non-affected (Watling and Norse 1998; Halpern et al. 2008). Other human uses of the oceans like aquaculture, mining or tourism activities threaten continental shelf ecosystems (Rossi 2013) and their effects, both direct and indirect, can be synergistic (Jackson et al. 2001; Kaplan et al. 2013). Human use changes such as marine protected areas (MPAs) and marine renewable energy developments (MREs), like wave energy or offshore wind farms, both may have some benefits for ecosystems by closing some areas to fisheries (Sheehan et al. 2013). However, potential negative effects of MREs arise from introducing hard structure to sedimentary seafloor habitats as well as changing current and sediment flow patterns. The intensity and extent of such effects on seafloor assemblages by MRE installations are as yet poorly characterized, mostly hypothesized from studies of artificial reefs and oil platforms (see reviews in Boehlert and Gill 2010; Henkel et al. 2013, 2014). However, some hard-bottom (Keenan et al. 2011) and structure colonization studies (Leonhard and Pedersen 2006; Wilhelmsson and Malm 2008; Langhamer et al. 2009) have been conducted in relation to MRE installations in Europe (see also review by Leeney et al. 2014).
One of the major threats of seafloor exploitation to continental shelf ecosystems is a reduction of habitat complexity and heterogeneity by damage to or smothering of slow-growing structure-building organisms like sponges or gorgonians, which may create biogenic habitat (Watling and Norse 1998; Kaiser et al. 2006; Sheehan et al. 2013) as well as damage to or sedimentation of rocky outcrop or reefs themselves. Habitat heterogeneity can be a major driver of variability in the abundance and diversity of marine species (Benedetti-Cecchi and Cinelli 1995; García-Charton et al. 2004), supporting global species diversity by increasing niche availability and community complexity and facilitating the formation of distinct species assemblages (Cerame-Vivas and Gray 1966; García-Charton et al. 2004; McClain and Barry 2010).
The Pacific Northwest (PNW) continental shelf, especially in its northern part (i.e. off Oregon and Washington), is mostly characterized by mud and gravel habitats, but rocky outcrops and reefs occur in several areas (Romsos et al. 2007), supporting structure–building invertebrates that increase the habitat complexity of the seafloor (Strom 2006). This region has a long history of intense fisheries with a variety of fleets using bottom gears dedicated to benthic and/or demersal species: groundfishes, demersal rockfishes, crabs and shrimps. Moreover, it is becoming a focus for offshore wave and wind energy installations on the continental shelf and slope, with an estimate of about 1000 TWh of just wave energy resource available per year for the PNW continental shelf (EPRI 2011). However, despite the abundance (and some documentation of) of invertebrate bycatch, little is known about mega-invertebrate assemblages on this part of the continental shelf. Hixon and Tissot (2007) and Hannah et al. (2010, 2013) compared trawled versus untrawled mud assemblages at two locations on the Oregon continental shelf, and Tissot et al. (2007) described the invertebrate and fish assemblages at a single outer continental shelf reef off Oregon. Only Strom (2006) has summarized the distribution of structure-forming invertebrates at multiple sites along the continental margin off Oregon. On the southern part of the eastern Pacific continental shelf (i.e. southern California), different invertebrate assemblages have been distinguished based on the physical structure of the habitats: habitats composed of high-relief rocks were associated with sessile and structure-forming mega-invertebrates including sponges and gorgonians, while low-relief habitats composed of fine sediments were associated with motile mega-invertebrates including sea stars, crustaceans, bivalves, and sea cucumbers (Allen and Moore 1996; Allen et al. 1997; Stull et al. 1999; Tissot et al. 2006). Large structure-forming mega-invertebrates such as sponges, corals, crinoids and basket stars have been suggested to provide shelter and additional resources for fish and other invertebrates by increasing the availability of microhabitats through their large surface area (Tissot et al. 2006).
The differences in mega-invertebrate species assemblages between high-relief rocks and low-relief unconsolidated sediment as described above likely hold for most marine environments. However, the diversity of assemblage–habitat associations on the seafloor is more complicated than this dual opposition and management decisions regarding protection or development of seafloor habitats require a more detailed understanding of associated affected species. Thus the objectives of this study were to distinguish finer differences in habitats based on substrata (and depth if significant in the study range) and to characterize the diversity and composition of mega-invertebrate assemblages in those habitats. The following substratum differentiations were investigated. How mega-invertebrate assemblages found on pure sediment differ from assemblages found on mud mixed with unconsolidated rocks (hereafter called mixed mud–rock), which in turn differ from assemblages living in rocky habitats. Within rocky habitats, if the slope of the rocks (i.e. flat rocks vs. ridge rocks) and the cover of the rocks (i.e. a large consolidated outcrop with a cover of unconsolidated smaller rocks, hereafter called rubble rocks; rocks with a veneer of sediment; or bare rocks) affect the diversity and density of associated epifauna. To test these hypotheses, underwater video footage from three different sampling sites along the Washington (Grays Bank) and Oregon (Siltcoos Reef and Bandon–Arago outcrop) coast were analyzed, to identify and enumerate the sessile and motile mega-invertebrates from the images, and characterize the substrata encountered. These three sites were selected for this study because they are located in areas of potential interest for the development of different MRE projects and have been mapped with high-resolution multi-beam sonar.
Materials and methods
Study sites
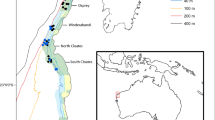
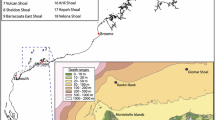
In late August 2011 and September 2012, we used the remotely operated vehicle (ROV), Hammerhead, a modified Deep-Ocean Engineering Phantom ROV customized and implemented by Marine Applied Research & Exploration (http://www.maregroup.org/the-hammerhead-rov.html), to survey habitats and mega-invertebrates at three sites on the Pacific Northwest continental shelf (Fig. 1): Grays Bank (GB, 14 stations, off Grays Harbor, Washington) and Siltcoos Reef (SC, 10 stations, off Charleston, Oregon) in 2011 and Bandon–Arago (BA, 12 stations, off Bandon, Oregon) in 2012. Each site was composed of several stations, themselves composed of three transects, each approximately 250 m long each separated by 250 m (Fig. 2). The ROV was kept at a regular speed (~0.5 m s−1) and a regular height from the bottom (~1 m) to provide images of good quality to identify and enumerate the mega-invertebrates. This sampling plan was designed to maximize the number of bottom types surveyed at each study site. The ROV Hammerhead was equipped with two color HD video cameras attached at the front of the ROV: one facing downward and perpendicular to the sea surface, and the other facing outward, angled roughly 30° from the dorsal surface of the ROV. The ROV Hammerhead was equipped with sizing lasers for each camera, a CTD that measured depth (meters), temperature (Celsius), and salinity (PSU) continuously, and was integrated with a navigation system that measured latitude and longitude every second.
Video analyses
Each video was watched a minimum of three times: one for substratum identification, one for sessile mega-invertebrate identification and enumeration, one for motile mega-invertebrate identification and enumeration. While two observers were used for classifying substrata, a single observer identified all organisms to reduce potential observer-related differences in organism detection or classification. Only benthic epifauna and some endofauna taxa showing recognizable body parts above the sediment were recorded. Both the outward and downward facing cameras were used to identify substratum patches and invertebrates. Since one camera faced downward at a fixed angle from the vehicle, all footage viewed by the downward-facing camera was considered “on-transect” and this view was used to count the invertebrates. Generally, video analysis followed guidelines established by Tissot (2008). Each invertebrate entry was accompanied with a time code that was used to determine in which substratum patch a particular invertebrate was found.
Substratum
Substratum patches were identified based on the grain size class estimated from the video footage and, for consolidated rocks, relief angle, with the start and end times of each substratum patch recorded. Each substratum patch was coded with two letters; the first letter indicated the primary substratum (comprising 50–80 % of the duration of the patch) and the second letter indicated the secondary substratum (comprising 20–50 % of the duration of the patch): R for ridge rock (angle >30°), F for flat rock (angle <30°), B for boulder (>25.5 cm), C for cobble (6.5–25.5 cm), P for pebble (2–6.5 cm), G for gravel (4 mm–2 cm), and M for mud (not distinguished from sand), refined from Stein et al. (1992). If a substratum patch was comprised of two substrata in equal proportions, the patch was coded with the first letter indicating the substratum with larger grain size. If a patch comprised over 80 % of a single substratum, the patch was coded with the same two letters (e.g. MM).
Sessile mega-invertebrates
Only sessile invertebrates taller than 5 cm were identified and enumerated, as recommended by Riedl (1971) and Tissot et al. (2006) because smaller individuals were difficult to see and identify on the images. Sponges and gorgonians, difficult to identify on video, were characterized based on their morphology and sometimes color (e.g., branching sponge, shelf sponge, branching red gorgonian). Encrusting ascidians and bryozoans, impossible to distinguish on video from encrusting sponges, were all gathered under the name shelf sponge, while possible branching bryozoans were counted as branching sponges. These two names thus describe a life form more than a systematic group and patches (shelf sponges) or tufts (branching sponges) were counted as individuals.
Motile mega-invertebrates
Motile invertebrates taller than 5 cm were identified to the lowest possible taxonomic level and enumerated. Some taxa were only identified to the family or genus level, since many species in these families/genera have overlapping morphological features and are difficult to distinguish without specimens to analyze. When the abundance of motile invertebrates was high, one to three additional viewings were needed to identify and enumerate all the individuals. In the BA footage, small orange brittle stars were too numerous to be counted all along each transect and were only enumerated every 30 s.
Substratum patch area and species density
The ROV Hammerhead was equipped with a navigator beam that was used to calculate the transect width and the approximate distance traveled every second. The area covered per second was calculated based on the transect width and the distance the ROV traveled from the previous second. Thus, the area of each different patch was calculated by adding all area entries from one second after the start time of the patch to the end time of the patch. The density (individuals/m2) of each taxon for each patch was calculated by dividing the count for that taxon by the total area of that patch covered by the ROV.
Statistical analyses
The sample units considered here were the different patch types in a whole site: data from all the same substratum patches were pooled at the site level. Only patch types observed longer than 1 min in total for a site were kept in the analyses. A matrix of Bray–Curtis similarities between patch types was calculated on log-transformed density data. Nonmetric multidimensional scaling (nMDS), analyses of similarities (ANOSIM), SIMPER, and DIVERSE were performed using PRIMER 6th Edition (Clarke and Gorley 2006). The nMDS analysis plotted sample units (patch types) on a two-dimensional ordination plane based on taxa composition similarities and dissimilarities. Groups of patch types (hereafter ‘habitat types’) were discerned from the nMDS plot and an ANOSIM was performed to test the strengths of similarities within and differences between these habitat type groups, using permutation and randomization methods on the resemblance matrix. SIMPER (Similarity of Percentage) was used to determine which taxa and their densities contributed to defining each group and the percent contribution of each defining taxon. DIVERSE was used to calculate the diversity indices (average number of taxa S, average density N, Pielou’s evenness J’) on the untransformed abundances for each habitat group, and a series of ANOVAs and Tukey HSD tests was performed in the open-source software R (R Development Core Team 2013) to test whether or not the indices were significantly different among habitat type groups. To test for a possible bathymetric structuring of the organisms, a second set of nMDS was performed at the transect level on the density of taxa within a patch, coded by the habitat type defined at the first round of analyses, using depth bin (sections 10 m deep) as a factor. For this second set of nMDS, the sample units were the patch types within a transect, that is all the patches of a same substratum type pooled at the transect level because the depth range varied within a site but not so much along a transect. An ANOSIM was also performed on the seven depth bins.
Results
Site characteristics
The three sites showed slightly different physical characteristics (Table 1). BA and GB were shallower than SC. The temperature was the coldest at the northern stations (GB) and up to one degree Celsius warmer in 2012 at BA as compared to SC in 2011. No bathymetric or latitudinal variation in salinity was noticed among the three sites.
A total of 28 different substrata (two-letter code combinations) were identified in the transects: 16 at SC, 20 at BA as well as GB. Eight substrata were discarded at GB, seven at SC, and two at BA because of durations shorter than a minute, resulting in a grand total of 22 different substrata (Fig. 3) that were analyzed and are discussed further. Substrata found in large proportion across all sites were flat rock–mud (average = 23 %), mud–mud (average = 20 %), ridge rock–ridge rock (average = 19 %) and ridge rock–mud (average = 18 %). A total of 85 taxa representing eight phyla were found across all three sites (Table 2, Online Resource 1). The phyla Echinodermata, Porifera and Cnidaria together comprised over 91 % of all the invertebrates encountered in the survey (Table 2). Porifera and Echinodermata were the most abundant at BA whereas Cnidaria were the most abundant at GB and Echinodermata at SC (Fig. 4).
Assemblage composition
Six habitats (groups of patches hosting similar invertebrate taxa) were identified from the nMDS ordination (Fig. 5). The habitat groups were mostly organized by substratum characteristics (e.g. pure mud, mixed mud–rock, rock) and subsequently by sites. Unconsolidated sediment patches from the sites split into three groups: group MM-GBSC consisted of pure mud patches from GB and SC; group Mx-GBSC was made of mixed mud–rock patches from GB and SC; and group Mx-BA gathered pure and mixed mud–rock patches from BA only. Rock-based patches clustered into two main groups: cR made of consolidated rocks, both bare and covered with a veneer of mud (BM, FM, RM, RR), from the three sites; and group rR made of rubble rocks (e.g. BC, FB, RG) from the three sites. No distinction was observed between ridge rocks and flat rocks meaning that the slope does not seem to matter. Group PG (pebble–gravel), was a patch type found only at BA in a single transect and will not be discussed further. The ANOSIM performed on the five remaining groups (MM-GBSC, Mx-GBSC, Mx-BA, cR and rR) demonstrated significant overall differences in the compositions of assemblages between the habitats (Global R statistic = 0.700, p < 0.01). In the pairwise test, comparisons were considered reliable when more than ten permutations were possible. Nine of the ten possible pairwise comparisons showed significant differences between groups (Table 3). The only non-significant pairwise comparison was MM-GBSC vs. Mx-GBSC (p = 0.067). This was not surprising because of the low number of permutations possible for this pairwise comparison. The SIMPER analysis showed large dissimilarities for each pairwise comparison, ranging from 70.81 to 99.47 % of difference in the taxonomic composition of the groups (Table 4). Differences also were found among habitats based on the univariate analyses of number of taxa S, density N and evenness J’ (Fig. 6).
Nonmetric multidimensional scaling (nMDS) ordination of the substratum types based on invertebrate assemblages. cR consolidated rocks, MM-GBSC pure mud at Grays Bank and Siltcoos Reef, Mx-BA mixed mud–rock at Bandon–Arago, Mx-GBSC mixed mud–rock at Grays Bank and Siltcoos Reef, PG pebble–gravel, rR rubble rocks
Pure mud at GB and SC (33 % similar) showed a medium number of taxa and a high density of individuals with a significantly lower Pielou’s evenness than all other habitats. Pure mud habitat at these sites was characterized by high density of burrowing brittle stars and Subselliflorae (sea whips) (Table 5). Mixed mud–rock habitats at GB and SC were characterized by medium to high density of anemones and low density of sponges with the lowest within group similarity (16 %; Table 5); they also showed lower number of taxa and density of individuals than the same habitats at BA. Mixed mud–rock habitats at BA (which included pure mud at this site; patches 46 % similar) showed a medium number of taxa, a low density of individuals and were characterized by many of the same taxa as the consolidated rocks (minus the anemones and squat lobsters) but in much lower densities (Table 5). What made the two mixed mud–rock groups 93.18 % dissimilar was the higher density of several echinoderm species (brittle stars, sea stars and sea cucumbers), sponges, branching gorgonians and tunicates at BA than GB and SC, and a higher density of sea anemones at GB and SC than BA (Online Resource 2).
Consolidated rocks showed 37 % within-group similarity, supported the highest number of taxa and density of individuals (Fig. 6), and were characterized by high density of sponges, branching gorgonians, giant plumose anemones, echinoderms (brittle stars, sea cucumbers and sea stars) and squat lobsters (Table 5). In contrast, rubble rocks supported significantly fewer taxa (three-fold) and much smaller densities of individuals (88-fold) and were characterized by low density of sponges and sea cucumbers with nearly 36 % within-group similarity (Table 5). What made the consolidated rock group 90.47 % different than the rubble rock group was higher density and diversity of sponges, gorgonians, echinoderms (brittle stars, basket stars, sea stars, sea cucumbers), anemones, squat lobsters and tunicates on the consolidated rock (Online Resource 2).
There appeared to be some distinction of groups by depth; however separation on the ordination plane was dominated by habitat (Fig. 7) and the ANOSIM performed on the seven depth bins did not demonstrate significant overall differences in the compositions of assemblages between depth (Global R statistic = 0.193, p < 0.01). Based on taxa densities pure mud transects at GB and SC clustered together in the top right section of the graph with further separation by depth bin; mixed mud–rock transects at GB and SC (50–79 m) clustered in the bottom right. Mixed mud–rock at BA (50–69 m) and consolidated rocks (50–119 m) from the three sites mixed together on the left side of the two-dimensional plot with rubble rocks (50–119 m) in the lower left. Clearer distinctions among the three habitat groups appeared on the three-dimensional plot (results not shown).
Discussion
This study aimed to distinguish finer resolution in benthic habitats that support distinct epifaunal invertebrate assemblages on temperate continental shelves. Specifically, groups of benthic mega-invertebrate epifauna were described from three rocky reefs and the surrounding soft sediments off the Oregon and Washington coast and associated with the substrata on which they were observed. In addition to building an understanding of the diversity, density, and taxa various habitats support, this study provides data on benthic mega-invertebrate abundances and distributions on the Pacific Northwest continental shelf at a specific time point, which may be compared to future similar surveys for assessments of the effects of global warming, fisheries management and MRE on the distribution of such taxa.
Hundreds of thousands of sessile and motile individuals were identified and enumerated, as well as the characteristics of the substratum. However, several identifications were not able to reach the species level without actual specimens to check and dissect for diagnostic morphological characters. For example, the different species within the sea star genera Henricia and Solaster are impossible to differentiate without a check of the aboral plates, the adambulacral spines and the pedicellariae (Lambert 2000; C. Mah, pers. comm.); similarly, species identification via images is nearly impossible for organisms like sponges, which are usually identified on the structure of their spicules. All branching and encrusting organisms (trickier to enumerate and discriminate) were gathered as functional groups under the names “branching sponge” and “shelf sponges” respectively, even if these groups included more than just sponge taxa (e.g. bryozoans or colonial ascidians). Since different species use different ecological niches and suitable habitats, a full understanding of which taxa might be most susceptible to small habitat changes would require sampling these organisms, particularly the sessile invertebrates, and identifying them to species.
Despite these taxonomic limitations, the review of the video footage and the statistical analyses performed on taxa densities allowed the discrimination of different assemblages on particular substrata based on their taxonomic composition. Like previous studies (Allen and Moore 1996; Allen et al. 1997; Stull et al. 1999; Tissot et al. 2006), differences were observed between habitats composed of higher-relief rocks (greater densities of sessile and structure-forming mega-invertebrates and greater diversity) versus low-relief habitats composed of fine sediments (more motile mega-invertebrates). However, finer distinction was also characterized within both low-relief (between pure mud and mixed mud–rock) and higher-relief (among rock types) habitats as described in the following sections. Although the goal was to describe habitats that were generalizable across sites, some differences among sites were observed. However, this did not seem to be driven by latitudinal or depth differences, which might be suspected to affect species distributions. SC was more similar to GB, which is ~500 km north, than to BA, which is only 50 km south (Fig. 1), and GB and BA had overlapping depth ranges, while Siltcoos was deeper. Thus, observed differences likely stem from differences in the geologic history of the sites such that the assemblage–habitat associations are not unique to a site per se but rather are based on characteristics of the substratum. The major habitat types discerned across this ROV survey are described here below.
Pure mud
Not surprisingly, the assemblages found along patches of pure mud (not distinguished from sand) were very different from the assemblages found in other types of habitats. The diversity and evenness of taxa living on the mud or partially burrowed in it were quite low while the abundance of some of these taxa numbered in the hundreds. The pure mud community was thus largely dominated by a very few taxa, like Subselliflorae sea whips and burrowing brittle stars with occasional sea anemones and sponges. This dominance of sea whips on mud communities previously has been noted along the Oregon coast (Hixon and Tissot 2007; Hannah et al. 2010, 2013), as well as on the southern California shelf (Tissot et al. 2006; de Marignac et al. 2008), the Gulf of Alaska and the Bering Sea (Malecha and Stone 2009). This type of mega-invertebrate can live in dense populations and provides structure and habitat heterogeneity for other invertebrates in this otherwise non-complex environment (Tissot et al. 2006; Malecha and Stone 2009). However, Subselliflorae are adapted to life in very homogeneous and stable habitats and are more vulnerable to habitat alteration (e.g. from bottom-fishing gears) than benthic communities found in less consolidated coarse sediments like the mixed mud–rock (Collie et al. 2000; Malecha and Stone 2009). Nonetheless, despite the high number of shrimp-trawl records in the vicinity of SC (R. Hannah, pers. comm.), the observed high abundance of Subselliflorae indicates that the populations observed on the video transects might be in areas around the reef not really accessible to bottom-trawling and could act as source populations to refill the impacted ones nearby. Burrowing brittle stars were also identified in de Marignac et al. (2008) as dominant taxa along what they called the ‘recovering transects’ in central California.
In contrast to SC and GB, the pure mud patches at BA were not differentiated in their benthic assemblages from the mixed mud–rock patches at the same site and were comprised of very few to no Subselliflorae and burrowing brittle stars. BA is a large and old rock outcrop on the mid Oregon shelf (Romsos et al. 2007) and the pure mud and mixed mud–rock patches were found within the reef itself (Fig. 2). In contrast, SC and GB are smaller rock outcrops and pure mud was mostly found around the reefs. The ‘pure mud’ at BA might rather be a thin layer of mud on the bedrock, not really stable and not suitable enough for the species characteristic of pure mud communities to settle in.
Mixed mud–rock
Mixed mud–rock habitats were made of mud (or sand) more or less assorted with coarser sediments like gravel, pebble, cobble or even boulder. These unconsolidated rocks act as physical supports for sessile organisms. The taxa inhabiting the mixed mud–rock at BA were sessile organisms like sponges (both shelf and branching) and gorgonians, known as structure-forming mega-invertebrates. They add complexity and heterogeneity to this habitat and supply support, shelter, or food to motile invertebrates like sea stars, sea cucumbers and nudibranchs. However, some of the most abundant motile taxa in this habitat were partially burrowing organisms such as the sea cucumbers Cucumaria spp. or the small orange brittle stars that live with the body hidden in tiny cracks in the mud or between small rocks and the arms extending out. At SC and GB, in addition to the structure-forming sessile organisms (gorgonians and sponges), the taxa inhabiting the mixed mud–rock habitats were mostly sea anemones and a few motile species like sea stars.
Mixed mud–rock has not been described as a major benthic habitat type on the PNW continental shelf in previous studies. On other temperate continental shelves like the Bay of Biscay or the English Channel, mixed mud–rock habitat is described and is further divided into different categories, depending on the size and abundance of the unconsolidated rocks involved, with different assemblages (Brind’Amour et al. 2014). Within this study, the differences between mixed mud–rock at BA versus the other two sites similarly may be related to the difference of the size and abundance of the unconsolidated rocks. At SC and GB, the mud was mixed with gravel and occasionally pebbles (small rocks). At BA the mixed mud also included cobbles and boulders. It is thus not certain whether the differences observed between the two mixed mud–rock assemblages here described are locally-induced differences from a general mixed mud–rock habitat, or two distinct habitats differentiated by the characteristics of the mixed-in rocks which support different assemblages. More occurrences of each substratum across sites might have helped highlight differences in benthic assemblages related to the size of the unconsolidated rocks mixed in the mud. Given these findings, ‘mixed mud–rock’ should be mapped as a distinct habitat characterized by low densities of a diversity of taxa, particularly sponges, gorgonians, anemones, and burrowing echinoderms. Since Brind’Amour et al. (2014) have shown that this habitat can be divided in several categories, future studies should be designed to obtain thorough coverage of transition areas between consolidated rock and mud habitats to discern whether the different sizes of the interstitial rocks in the transition zone support distinct mega-invertebrate assemblages.
Consolidated rocks
Most of the species diversity and individual densities were associated with consolidated rocks, which include boulders, flat rocks and ridge rocks with a veneer of mud as well as bare ridge rocks. Across all sites, this habitat had the highest abundance of sessile and structure-forming invertebrates such as sponges, gorgonians, giant plumose anemones, sometimes in very dense aggregations, and other sea anemones. The motile mega-invertebrates were very diverse, with an average of forty taxa, including a variety of crabs, echinoderms (basket stars, brittle stars, feather stars, sea cucumbers and sea stars), nudibranchs, octopuses, scallops and squat lobsters. This diversity can be attributed to the physical complexity of higher-relief substrata where there may be greater variation in depth, temperature, current direction and velocity, nutrient transport, and the substrata may be composed of different elements (Taylor and Wilson 2003). Furthermore, the large diversity of structure-forming sessile and motile invertebrates (e.g. basket stars and feather stars) further increases the habitat complexity and heterogeneity and provides a variety of shelters, refuges, spawning grounds and ecological niches for both invertebrates and fishes (Cerame-Vivas and Gray 1966; Benedetti-Cecchi and Cinelli 1995; Tissot et al. 2006).
Rubble rocks
On the other hand, although some of the major species were the same, the substrata composed of rubble rocks (flat or ridge rocks with a cover of unconsolidated rocks) showed very different assemblages. Despite these substrata being rock-based, they did not support the greater densities of sessile and structure-forming mega-invertebrates and greater diversity generally attributed to high-relief rocks (Allen and Moore 1996; Allen et al. 1997; Stull et al. 1999; Tissot et al. 2006). This habitat had the lowest diversity (an average of only ten different species) and densities. This difference might be due to the weak stability of the unconsolidated rocks on a high-relief substratum, probably engendered by hydrodynamic movements due to the strong currents found on the Oregon continental shelf (Kurapov et al. 2003; Osborne et al. 2014). This instability of the substratum may result in frequent disturbance not suitable for the establishment of dense populations of structure-forming organisms able to attract a variety of motile invertebrates. The role of natural disturbance in structuring marine communities has been well described in the intertidal (Dayton 1971; Lubchenco and Menge 1978; Sousa 1979, 1984; Paine and Levin 1981) and shallow subtidal, especially for algae (Airoldi et al. 1996; Airoldi 1998; Scheibling et al. 2008). Disturbance due to the movement of rubble rocks might similarly affect the recruitment and persistence of mega-invertebrates in this habitat. Mapping efforts have not yet distinguished this habitat from consolidated rocks and will be challenging to differentiate from complex, yet still consolidated rocks using sonar. However, it should be classified as a separate habitat since it certainly supports a different species assemblage and lower abundances than consolidated rocks without associated rubble.
Rocky reefs in the PNW continental shelf were highly targeted by fishing activities due to the high diversity of associated rockfish species. Repeated contacts of bottom-trawls on the reefs have damaged or even eradicated slow-growing structure-forming sessile invertebrates and the motile species they attract (Watling and Norse 1998; Kaiser et al. 2006; Sheehan et al. 2013). Nevertheless, because of the decline in rockfish stocks along the PNW coast at the end of the twentieth century (see review in NRC 2002), the Pacific Fishery Management Council established in the early 2000’s new regulations leading to a drastic decrease of the fishing pressure on part of the rocky reefs particularly on the outer continental shelf (Hannah 2003; Bellman et al. 2005; Bellman and Heppell 2007). Since that time, some studies have focused on the recovery of rockfish populations on reefs (Bellman et al. 2005; Bellman and Heppell 2007) or invertebrate populations on mud substrata (de Marignac et al. 2008; Hannah et al. 2010, 2013) after fishing closures, but much remains to be done on the recovery of structure-forming invertebrate species on rocky reefs. The three reefs in our study are not included in the Essential Fish Habitat conservation areas (NMFS 2013) and are thus still open to bottom-trawling, as evidenced by fishing gear debris seen on the video footage at GB and SC. Although the fishing pressure is not too high on these three inner shelf reefs (R. Hannah, W. Wakefield, pers. comm.), it is not the case for all the non-protected rocky reefs on the PNW continental shelf, and a comprehensive description of the benthic assemblages is needed to understand the effect of bottom-contact ocean-use activities (e.g. fishing, renewable energy development) and integrate this benthic component into the conservation and management plans. The present results could encourage the design of a video survey on rocky reefs now protected from fishing activities to compare the mega-invertebrate assemblages of reefs now recovering from bottom-gear disturbance to those of reefs clearly still impacted by bottom-fishing activities.
Conclusions
Before management decisions can be made about the ocean (for example where to close to fishing practices, where to allow renewable energy installations) it is useful to know what is being protected from potential impacts. While biological communities are shaped by a variety of bottom–up and top–down processes, and species interactions, a major driver structuring benthic mega-invertebrate communities is substratum. Thus, more precise habitat mapping is necessary. This study identified at least four habitats for mega-invertebrate assemblages: (1) pure mud (not distinguished from sand on the video footage) dominated by sea whips and burrowing brittle stars; (2) mixed mud–rock (which may be further divided based on size of mixed-in rocks) characterized by medium diversity of species in low density; (3) consolidated rocks (big rocks with or without a veneer of sediment) characterized by high diversity and density of sessile and motile taxa; and (4) rubble rocks (big rocks with a cover of unconsolidated rocks) showing less diversity and density than the consolidated rocks, probably due to the disturbance generated by the unconsolidated rocks. These four habitats were consistent across the sites, even if some differences were observed between the mixed mud–rock habitats at BA versus GB and SC, probably due to the different geologic history of the reefs. It may be possible to map mixed mud–rock separately from other unconsolidated sediment with existing data. Future survey methods should attempt to distinguish rubble-rock from consolidated rock.
References
Airoldi L (1998) Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf. Ecology 79(8):2759–2770
Airoldi L, Fabiano M, Cinelli F (1996) Sediment deposition and movement over a turf assemblage in a shallow rocky coastal area of the Ligurian Sea. Mar Ecol Prog Ser 133:241–251
Allen MJ, Moore SL (1996) Recurrent groups of megabenthic invertebrates on the mainland shelf of southern California in 1994. In: Allen MJ, Francisco C, Hallock D (eds) Southern California coastal water research project annual report 1994–1995. Southern California Coastal Water Research Project, Westminster, pp 129–135
Allen MJ, Diener D, Mubarak J, Weisberg SB, Moore SL (1997) Megabenthic invertebrate assemblages of the mainland shelf of southern California in 1994. In: Weisberg SB, Hallock D (eds) Southern California coastal water research project annual report 1997–1998. Southern California Coastal Water Research Project, Westminster, pp 113–124
Bellman MA, Heppell SA (2007) Trawl effort distribution off the U.S. Pacific coast: regulatory shifts and seafloor habitat conservation. In: Heifetz J, Dicosimo J, Gharrett AJ, Love MS, O’Connell VM, Stanley RD (eds) Biology, assessment, and management of North Pacific rockfishes. Alaska Sea Grant College Program, Fairbanks, pp 275–294
Bellman MA, Heppell SA, Goldfinger C (2005) Evaluation of a US west coast groundfish habitat conservation regulation via analysis of spatial and temporal patterns of trawl fishing effort. Can J Fish Aquat Sci 62:2886–2900
Benedetti-Cecchi L, Cinelli F (1995) Habitat heterogeneity, sea urchin grazing and the distribution of algae in littoral rock pools on the west coast of Italy (western Mediterranean). Mar Ecol Prog Ser 126:203–212
Boehlert GW, Gill AB (2010) Environmental and ecological effects of ocean renewable energy development, a current synthesis. Oceanography 23(2):68–81
Brind’Amour A, Laffargue P, Morin J, Vaz S, Foveau A, Le Bris H (2014) Morphospecies and taxonomic sufficiency of benthic megafauna in scientific bottom trawl surveys. Cont Shelf Res 72:1–9
Cerame-Vivas MJ, Gray IE (1966) The distributional pattern of benthic invertebrates of the continental shelf off North Carolina. Ecology 47:260–270
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth
Collie JS, Hall SJ, Kaiser MJ, Poiner IR (2000) A quantitative analysis of fishing impacts on shelf-sea benthos. J Anim Ecol 69:785–798
Dayton PK (1971) Competition, disturbance, and community organization—provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr 41(4):351–389
de Marignac J, Hyland J, Lindholm J, De Vogelaere A, Balthis WL, Kline D (2008) A comparison of seafloor habitats and associated benthic fauna in areas open and closed to bottom trawling along the central California continental shelf. Marine Sanctuaries Conservation Series ONMS-09-02. U.S. Department of Commerce, NOAA, Office of National Marine Sanctuaries, Silver Spring, pp 1–48
Electric Power Research Institute (EPRI) (2011) Mapping and assessment of the United States ocean wave energy resource. EPRI Technical Report, Palo Alto, CA, 1024367, pp 1–176
García-Charton J, Pérez-Ruzafa A, Sánchez-Jerez P, Bayle-Sempere J, Reñones O, Moreno D (2004) Multi-scale spatial heterogeneity, habitat structure, and the effect of marine reserves on Western Mediterranean rocky reef fish assemblages. Mar Biol 144:161–182
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Hannah RW (2003) Spatial changes in trawl fishing effort in response to footrope diameter restriction in the U.S. West Coast bottom trawl fishery. North Am J Fish Manag 23:693–702
Hannah RW, Jones SA, Miller W, Knight JS (2010) Effects of trawling for ocean shrimp (Pandalus jordani) on macroinvertebrate abundance and diversity at four sites near Nehalem Bank, Oregon. Fish Bull 108:30–38
Hannah RW, Jones SA, Kupillas S, Miller W (2013) A comparison of 2007 and 2013 macroinvertebrate surveys of mud habitats at Nehalem Bank, Oregon: changes in areas with continued trawling and those closed to trawling in 2006. Oregon Department of Fish and Wildlife Information Reports 2014–2003, pp 1–30
Henkel SK, Conway FDL, Boehlert GW (2013) Environmental and human dimensions of ocean renewable energy development. Proc IEEE 101(4):991–998
Henkel SK, Suryan RM, Lagerquist B (2014) Marine renewable energy and environmental interactions: baseline assessments of seabirds, marine mammals, and benthic communities on the Oregon shelf. In: Shields MA, Payne AIL (eds) Marine renewable energy technology and environmental interactions. Springer, Dordrecht, pp 93–110
Hixon MA, Tissot BN (2007) Comparison of trawled vs untrawled mud seafloor assemblages of fishes and macroinvertebrates at Coquille Bank, Oregon. J Exp Mar Biol Ecol 344:23–34
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, PandolÞ JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Kaiser MJ, Clarke KR, Hinz H, Austen MCV, Somerfield PJ, Karakassis I (2006) Global analysis of response and recovery of benthic biota to fishing. Mar Ecol Prog Ser 311:1–14
Kaplan IC, Gray IA, Levin PS (2013) Cumulative impacts of fisheries in the California Current. Fish Fish 14:515–527
Keenan G, Sparling C, Williams H, Fortune F (2011) SeaGen Environmental Monitoring Programme—final report for marine current turbines. Royal Haskoning Enhancing Society, Edinburgh, pp 1–81
Kurapov AL, Egbert GD, Allen JS, Miller RN, Erofeeva SY, Kosro PM (2003) The M2 internal tide off Oregon: inferences from data assimilation. J Phys Oceanogr 33:1733–1757
Lambert P (2000) Sea stars of British Columbia, southeast Alaska and Puget Sound. Royal British Columbia Museum Handbook, UBC Press, Vancouver, pp 1–186
Langhamer O, Wilhelmsson D, Engström J (2009) Artificial reef effect and fouling impacts on offshore wave power foundations and buoys—a pilot study. Est Coast Shelf Sci 82(3):426–432
Leeney RH, Greaves D, Conley D, O’Hagan AM (2014) Environmental impact assessments for wave energy developments—learning from existing activities and informing future research priorities. Ocean Coast Manag 99:14–22
Leonhard SB, Pedersen J (2006) Benthic communities at horns rev before, during and after construction of horns rev offshore wind farm. Final Report—Annual Report 2005. Copenhagen: Vattenfall, pp 1–134
Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson JBC (2006) Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312:1806–1809
Lubchenco J, Menge BA (1978) Community-development and persistence in a low rocky inter-tidal zone. Ecol Monogr 48(1):67–94
Malecha PW, Stone RP (2009) Response of the sea whip Halipteris willemoesi to simulated trawl disturbance and its vulnerability to subsequent predation. Mar Ecol Prog Ser 388:197–206
McClain CR, Barry JP (2010) Habitat heterogeneity, disturbance, and productivity work in concert to regulate biodiversity in deep submarine canyons. Ecology 91:964–976
National Marine Fisheries Service (NMFS) (2013) Groundfish essential fish habitat synthesis: a report to the Pacific Fishery Management Council. NOAA NMFS Northwest Fisheries Science Center, Seattle, pp 1–107
National Research Council (NRC) (2002) Effects of trawling and dredging on seafloor habitat. National Academy Press, Washington, DC
Osborne JJ, Kurapov AL, Egbert GD, Kosro PM (2014) Intensified diurnal tides along the Oregon coast. J Phys Oceanogr 44:1689–1703
Paine RT, Levin SA (1981) Inter-tidal landscapes—disturbance and the dynamics of pattern. Ecol Monogr 51(2):145–178
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Riedl R (1971) Water movement: animals. In: Kinne O (ed) Marine ecology: a comprehensive, integrated treatise on life in oceans and coastal waters. Wiley-Inter Science, London, pp 1123–1156
Romsos CG, Goldfinger C, Robison R, Milstein RL, Chaytor JD, Wakefield WW (2007) Development of a regional seafloor surficial geologic habitat map for the continental margins of Oregon and Washington, USA. In: Todd BJ, Greene HG (eds) Mapping the seafloor for habitat characterization. Geological Association of Canada, Special Paper 47: 209–234
Rossi S (2013) The destruction of the ‘animal forests’ in the oceans: towards an oversimplification of the benthic ecosystems. Ocean Coast Manag 84:77–85
Scheibling RE, Kelly NE, Raymond BG (2008) Physical disturbance and community organization on a subtidal cobble bed. J Exp Mar Biol Ecol 368:94–100
Sheehan EV, Stevens TF, Gall SC, Cousens SL, Attrill MJ (2013) Recovery of a temperate reef assemblage in a marine protected area following the exclusion of towed demersal fishing. PLoS One 8(12):e83883
Sousa WP (1979) Disturbance in marine inter-tidal boulder fiels—the non-equilibrium maintenance of species-diversity. Ecology 60(6):1125–1239
Sousa WP (1984) The role of disturbance in natural communities. Annu Rev Ecol Syst 15:353–391
Stein DL, Tissot BN, Hixon MA, Barss W (1992) Fish habitat associations on a deep reef at the edge of the Oregon continental shelf. Fish Bull 90:540–551
Strom N (2006) Structure-forming benthic invertebrates: habitat distributions on the continental margins of Oregon and Washington. MS thesis, Oregon State University, Corvallis, OR
Stull JK, Allen MJ, Moore SL, Tang CL (1999) Relative abundance and health of megabenthic invertebrate species on the southern California shelf in 1994. In: Weisberg SB, Elmore D (eds) Southern California coastal water research project annual report 1999–2000. Southern California Coastal Water Research Project, Westminster, pp 189–209
Taylor PD, Wilson MA (2003) Palaeoecology and evolution of marine hard substrate communities. Earth-Sci Rev 62:1–103
Tissot BN (2008) Video analysis, experimental design, and database management of submersible-based habitat studies. In: Reynolds JR, Greene HG (eds) Marine habitat mapping technology for Alaska, Alaska Sea Grant College Program, University of Alaska Fairbanks, pp 157–167
Tissot BN, Yoklavich MM, Love MS, York K, Amend M (2006) Benthic invertebrates that form habitat on deep banks off southern California, with special reference to deep sea coral. Fish Bull 104:167–181
Tissot BN, Hixon MA, Stein DL (2007) Habitat-based submersible assessment of macro-invertebrate and groundfish assemblages at Heceta Bank, Oregon, from 1988 to 1990. J Exp Mar Biol Ecol 352:50–64
Watling L, Norse EA (1998) Disturbance of the seabed by mobile fishing gear: a comparison to forest clearcutting. Conserv Biol 12:1180–1197
Wilhelmsson D, Malm T (2008) Fouling assemblages on offshore wind power plants and adjacent substrata. Estuar Coast Shelf Sci 79:459–466
Acknowledgments
The authors would like to thank K. Politano and T.S. Lee for their participation in the ROV survey cruises and video review. The authors acknowledge funding from the Bureau of Ocean Energy Management via a Cooperative Agreement to SKH, which supported both TSL and LGH, and Hatfield Marine Science Center’s Mamie Markham Research Award to TSL. We acknowledge C. Goldfinger (OSU College of Earth, Ocean, and Atmospheric Sciences) for providing high-resolution maps for survey areas. We thank Marine Applied Research and Exploration (MARE) and the crew of the R/V Pacific Storm for the completion of ROV surveys in 2011 and the Derek M. Baylis for vessel support in 2012. We also thank B. Tissot (Washington State University Vancouver) for assistance in study design and for providing patience, enthusiasm, and thorough guidance during the substratum type and invertebrate classification training. We finally thank W. Austin (Khoyatan Marine Laboratory) and C. Mah and D. Pawson (Smithsonian Institution) for their help on identifying some tricky sponges, sea stars and sea cucumbers on the video footage, as well as W. Wakefield (NOAA Fisheries, Northwest Fisheries Science Center) and R. Hannah (Oregon Department of Fisheries and Wildlife) for their help to understand the fishing rules along the Pacific Northwest coast. We are grateful to the three anonymous reviewers whose comments greatly helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Hawksworth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hemery, L.G., Henkel, S.K. Patterns of benthic mega-invertebrate habitat associations in the Pacific Northwest continental shelf waters. Biodivers Conserv 24, 1691–1710 (2015). https://doi.org/10.1007/s10531-015-0887-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-015-0887-7