Abstract
In many tropical areas, monospecific tree plantations are replacing natural forest. The ecology of these plantations is quite different from that of natural forests, including the diversity and community structure of vascular and cryptogamic epiphytes. Few studies have looked at the ecology of guilds of epiphytes in plantations versus natural forest. Here, we investigated epiphytic, lirellate species of the family Graphidaceae, the largest family of tropical lichen fungi, which are widely distributed and abundant in tropical regions. We compared species richness and community structure in a monospecific plantation of the introduced tree species Eucalyptus globulus versus native oak forest dominated by Quercus humboldtii. Overall species richness was substantially higher in the natural oak forest (41 vs. 14 species, with eight shared between both stands, for a total of 47), whereas species abundance was significantly higher in the gum plantation. While species richness per tree (alpha diversity) was comparable between both stands, average species turnover between trees within each stand (beta diversity) was significantly higher in the natural oak forest, resulting in substantially higher overall species richness (gamma diversity). We conclude that the monospecific gum plantation exhibits both de-diversification (lower overall species richness) and homogenization (more similar communities between trees) of these epiphytic lichen guilds. This is not an effect of phorophyte diversity since in both stands, only a single tree species each was considered. Among the lichens identified, we detected six new to the Neotropics and 29 new records for Colombia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oak forests reach their natural southernmost limit in the Colombian Andes, where they form an important component of native subandine forest ecosystems, characterized by the dominance of Quercus humboldtii Bonpl. (Lozano and Torres 1974; Galindo et al. 2003; León et al. 2009). These forests are found in all three mountain ranges of the country, between 750 and 3,450 m altitude (Agudelo 2009; Muñoz and Camacho 2010). Fossil records suggests that these oak forests go back to the late Cretaceous (Lozano and Torres 1974). The combination of regional mesoclimate and microclimate generated by the canopy of these forests supports a high diversity of epiphytes, among them bryophytes, lichens, ferns, bromeliads, and orchids (Muñoz and Camacho 2010), which contribute substantially to their floristic richness (Lozano and Torres 1974). Quercus humboldtii, commonly known as Andean or Colombian oak, can reach up to 40 m height and a DBH of up to 45 cm (Lozano and Torres 1974; Agudelo 2009). Its fruits are eaten and dispersed by small mammals (Lozano and Torres 1974).
In many areas, these native oak forests are replaced by introduced Eucalyptus tree monocultures (FAO 1981; Kapelle 2006), mostly E. globulus Labill., but encompassing a large number of species originating from Australia. These adapt well to the conditions found in these areas, especially in the highlands of Cundinamarca, Boyacá, and Nariño, mostly between 1,900 and 3,000 m altitude (Hillis and Brown 1984; Muñoz et al. 2010). Eucalyptus globulus, also known as Tasmanian blue gum, was introduced approximately 200 years ago by Australian monks (Hofstede et al. 1998). Today, it is the economically most important tree species in the northern Andes, due to its rapid growth (Hillis and Brown 1984; Andrade 2008). It can reach up to 55 m height, under certain conditions in its native Australia (Tasmania) up to 100 m, and a massive DBH of up to 150 cm (Muñoz et al. 2010). Like many Myrtaceae, Eucalyptus trees have a flaking bark, making the colonization by epiphytes, especially lichens, difficult, and this bark structure appears to be contributing to the de-diversification of montane forests in the Andes and in other ecosystems (FAO 1981; Sanz et al. 2000; Fonseca et al. 2009).
Tropical montane forest ecosystems offer near-optimal growth conditions for epiphytes, among them lichens. Lichens are comparatively well-documented in Colombian montane forests, although most works focus on macrolichens (Aguirre-C 1982, 1985, 2008; Sipman and Aguirre 1982; Moncada 1998; Rivera and Córdoba 1998; Aguirre-C and Linares 2000; Linares and Pinzón 2001, 2006; Chaparro and Aguirre 2002; Sipman et al. 2008; Moncada et al. 2013a, b, c, 2014; Lücking et al. 2014). In contrast, few studies exist on crustose microlichens, focusing on the largest family of tropical crustose microlichens, Graphidaceae (Rincón-Espitia 2011; Soto-Medina et al. 2011; Soto-Medina and Bolaños 2012; Simijaca 2011; Peláez et al. 2014). Simijaca (2011) is the only study to report epiphytic lichens specifically from oak forest (Q. humboldtii). No data are available on epiphytic lichen diversity in Eucalyptus plantations, and studies on these introduced ecosystems focus entirely on their economic productivity (Andrade 2008; Muñoz et al. 2010). Little is known about how these introduced tree monocultures affect ecosystem diversity and function, in particular with respect to epiphyte communities, although studies from southern Brazil and Spain suggest that epiphytic lichen communities become depauperate on these trees (Fonseca et al. 2009; Käffer et al. 2009; Calviño-Cancela et al. 2012, 2013).
Here we use lirellate species of the family Graphidaceae to analyze the impact of Eucalyptus tree monocultures on epiphyte diversity and community structure, based on studies showing that general patterns of lichen diversity and community structure are correlated with that of other epiphytes at the stand level, even if there is variation at the microhabitat level (Wolf 1994; Hofstede et al. 2001; Kelly et al. 2004; Affeld et al. 2008; Mežka et al. 2008; Lõhmus and Lõhmus 2010). Graphidaceae is a dominant element of tropical lichen communities, with a total number of 2,500 currently accepted species (Wirth and Hale 1963; Staiger 2002; Staiger et al. 2006; Rivas Plata et al. 2013; Lücking et al. 2014). It contains both lirellate taxa formerly classified in Graphidaceae s.str., and taxa with rounded ascomata, formerly separated in the family Thelotremataceae (Rivas Plata et al. 2013). The latter are mostly characteristic of undisturbed forest ecosystems, whereas lirellate taxa colonize both pristine and disturbed vegetation (Rivas Plata et al. 2008). Furthermore, taxonomic treatments for their identification are now readily available (Rivas Plata et al. 2013), compared to other microlichens or tropical macrolichens whose taxonomy has recently been challenged substantially (Moncada et al. 2013a, b, c; Lücking et al. 2014). Lirellate Graphidaceae are therefore well suited to compare lichen community structure between forest stands of different disturbance levels.
Materials and methods
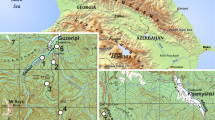
The study was carried out at Chicaque Natural Park, a private reserve situated on the western slope of the Eastern Cordillera in the Colombian Andes, in Cundinamarca and the municipality of San Antonio del Tequendama, between 74°18′25″ and 74°19′25″ western longitude and 4°36′21″ and 4°37′43″ northern latitude. The park is characterized by extensive forest areas with frequent cloud cover (Ojeda 2001; Armenteras et al. 2007), generating a vegetation ranging from montane cloud forest betwen 2,000 and 2,400 m (Rivera and Córdoba 1998) and subandine forest between 2,400 and 2,720 m, according to the classifications proposed by Cuatrecasas (1989) and Rangel (1997). The oak forest of the reserve is dominated by Q. humboldtii and is considered one of the last, well-preserved forests of its type near Colombia’s capital, Bogotá. Bordering the reserve are various extensive plantations of E. globulus, providing a sharp contrast with the native oak forest inside the park.
We delimited one rectangular plot (100 × 10 m) each within an area of native oak forest inside and an area of gum plantation outside the reserve. Within each plot, we randomly chose 28 trees with a DBH of more than 30 cm. On each selected tree, we placed a vertical line in northern direction from 50 to 150 cm height (McCune et al. 1997) and recorded all lichen thalli touching or crossing the line and representing lirellate species of the family Graphidaceae.
The collected samples were curated in the herbarium Emilio Mahecha, cryptogamic section, of the Universidad Distrital Francisco José de Caldas (UDBC). Morphological and anatomical characters were studied and documented using a LEICA stereoscope connected to a CANON Powershot SX 10015 digital camera and, by means of thin hand sections, with a ZEISS compound microscope. Spot tests were performed on sections using solutions of KOH (10 %) and PD (Lücking et al. 2009). For the identification of the species we used keys provided by Staiger (2002), Sipman (2006), Cáceres (2007), Lücking and Rivas Plata (2008), and Lücking et al. (2009).
We determined beta diversity between each pair of phorophytes and subsamples using Sørensen’s (1948) coefficient of similarity as distance measure (Villarreal et al. 2006). The distance matrix was ordinated by means of non-metric multidimensional scaling (NMS) and also subjected to cluster analysis, employing flexible beta set to −0.25 as clustering algorithm. For NMS, we used a strategy starting with six axes, with reduction in dimensionality, using 1,000 runs with real data, a maximum of 500 iterations per run, a step length of 0.20, and 0.00001 as stability criterion. We then employed a randomization test with 500 runs with randomized data, to evaluate the recommended level of dimensionality of the NMS. The distance matrix was also subjected to a non-parametric Kruskal–Wallis ANOVA to compare pairwise distance values between the two plots. We also applied indicator species analysis based on a randomization approach, using the two plots as grouping variable. The analyses were performed in PC-ORD 6.0 (McCune and Mefford 1999; McCune and Grace 2002) and Statistica 6.0™.
Results
We sampled a total of 294 specimens of lirellate Graphidaceae, 128 in the oak forest and 166 in the gum plantation, representing 47 species. Of the 56 trees sampled, one in each plot had no lirellate Graphidaceae, leaving 27 each for the statistical analysis. Most of the samples in the oak forest (75 %) corresponded to the genus Graphis, with 32 species, followed by Phaeographis (25 %), with ten species (Table 1). In the gum plantation, the majority of the samples (50 %) belonged to Phaeographis, with six species, followed by Graphis (43 %), also with six species, and Leiorreuma (7 %), with a single species (Table 1). Thus, while lirellate Graphidaceae were more abundant in the gum plantation, overall species richness was substantially higher in the oak forest (41 versus 14 species). Among the 47 species recorded, six were new records for the Neotropics and 29 new for Colombia (Table 1).
NMS ordination resulted in a two-dimensional plot as best solution (Table 2). The stress of 25.883 for the first two axes was comparatively high, suggesting that additional community structure is contained on the third axis (combined stress 17.423). However, the primary intention of this analysis was to compare beta diversity patterns between the two plots, which remain the same in all three axis combinations (2–1, 3–1, 3–1); therefore only the two principal axes are displayed here. The diagram showed a rather dense grouping of the Eucalyptus trees, whereas the oak trees appeared scattered around the lower periphery of the Eucalyptus group (Fig. 1). However, two Eucalyptus trees fell within the oak trees, whereas three oak trees clustered with the Eucalyptus trees. This pattern was confirmed by the cluster analysis, which resulted in two groups largely corresponding to the two forest plots (Fig. 2).
Statistical comparison of pairwise Sørensen distance values within each plot showed significantly lower values for the Eucalyptus plot (average 0.46 ± 0.17, median 0.47, range 0–0.86) than for the Quercus plot (average 0.92 ± 0.17, median 1.00, range 0.20–1.00; Mann–Whitney U test: Z adjusted = 21.17, p = 0.0000). Thus, trees within the natural Quercus forest have nearly complete species turnover on average, whereas trees within the Eucalyptus plantation have over 50 % similarity on average. Richness (number of species per tree) was slightly but not significantly lower for the Eucalyptus plot (average 2.74 ± 1.29, median 2, range 1–6) than for the Quercus plot (average 3.07 ± 1.57, median 3, range 1–6; Mann–Whitney U test: Z adjusted = 0.74, p = 0.4623). On the other hand, abundance (number of thalli per tree) was significantly higher for the Eucalyptus plot (average 6.33 ± 2.97, median 6, range 1–12) than for the Quercus plot (average 4.37 ± 2.11, median 5, range 1–8; Mann–Whitney U test: Z adjusted = −2.27, p = 0.0231). As a consequence, overal higher species richness (gamma diversity) for the oak forest (41) as compared to the gum plantation (14) was chiefly determined by the higher species turnover (beta diversity) and not by higher species richness per tree (alpha diversity), with gamma diversity exceeding average alpha diversity by the factor 13.36. This effect was much reduced in the gum plantation, with a factor of 5.11 only.
Indicator species analysis did not detect any species with significant association with oak forest, possibly due to the overall low frequency and abundance of species in that plot (Table 3). Only Graphis rhizocola was found with marginal significance to associate with natural oak forest. In contrast, three species were found to significantly associate with the gum plantation: Phaeographis dendritica, P. inusta, and G. elongata (Table 3).
Discussion
Effects of disturbed and secondary vegetation on epiphyte diversity and community structure have been demonstrated in various studies, generally showing a decrease in species richness, but sometimes also an increase in certain types of secondary forests due to a mixture of species characteristic of climax and pioneer vegetation (Hickey 1994; Sillett et al. 1995). These effects are mostly due to apparent adaptations of epiphytes to particular niches within native forest (Kelly 1985; ter Steege and Cornelissen 1989; van Leerdam et al. 1990; Zimmerman and Olmsted 1992; Wolf 1993, 1994; Hickey 1994; Hietz and Hietz-Seifert 1995; Andersson and Gradstein 2005). However, effects of monospecific tree plantations of epiphyte diversity and community structure have rarely been studied, and especially not much in tropical regions Sawyer (1993; Hietz and Hietz-Seifert 1995; Merwin et al. 2003; Hietz 2005; Brockerhoff et al. 2008; Käffer et al. 2009; Calviño-Cancela et al. 2012, 2013).
The results of our analysis showed a substantial effect on species richness and community structure in lirellate Graphidaceae lichens in Eucalyptus plantations compared to natural Quercus forest. While species richness per tree sample was not significantly affected, overall species richness or gamma diversity in the gum plantation decreased by 65 %. This de-diversification was mainly caused by a strong effect of homogenization, with beta diversity between tree samples reduced by approximately 50 % in the gum plantation compared to the oak forest and hence the lichen communities in the gum plantation becoming significantly more similar between individual trees. De-diversification and homogenization are thereby two partially independent effects, since homogenization does not necessarily mean de-diversification, if individual communities per tree retain high species richness. Likewise, de-diversification can occur without homogenization, if individual communities become species-poor but differ between individual trees. Thus, while lichens in terms of abundance did rather well in the Eucalyptus plot, in fact significantly better than in the Quercus plot, the lichen communities on individual trees were dominated by a few, abundant species, which basically repeated themselves on each tree. This effect is similar to what can be observed under the influence of air pollution, where lichen diversity dramatically decreases but a few species become abundant and even dominant, such as Lecanora conizaeoides in Europe (Cook et al. 1990; Wirth 1993; Bates et al. 2001; LaGreca and Stutzman 2006; Massara et al. 2009; Purvis et al. 2010; Lisowska 2011). The much fewer species found under anthropogenic conditions are then often a subset of the species found under natural conditions, with shifted abundance values. In the present case, seven out of 14 species where unique to the Eucalyptus plantation, but were all found in single samples only, so this suggested association with Eucalyptus has no statistical support. Although not found on the oak trees, these species are expected to occur on other trees in the natural oak forest, with similar bark characteristics as Eucalyptus, such as other Myrtaceae. On the other hand, the three dominant species in that plot, in particular P. dendritica, were also present in the natural oak forest, but became abundant only in the gum plantation. The high level of beta diversity in the oak forest detected here makes the characterization of an “oak forest community” based on the two-dimensional NMS diagram impossible, since it causes a comparatively high stress value for the first two axes. Hence, distinctive oak forest community can not be discerned; rather, the oak tree samples represent fragments of a hypothetical community with very low levels of species co-occurrences. The usefulness of the NMS ordination in this case lies in the powerful visualization of the very distinctive beta diversity patterns between the oak forest and the gum plantation.
The flaking bark of the Eucalyptus trees did not affect overall lichen abundance values, which were higher in the gum plantation than in the oak forest. We have two possible explanations for this: (1) the lichens found in the gum plantation grow faster or mature earlier than those in the oak forest, adjusting to the more dynamic substrate, or (2) the rhythm of bark shedding is not fast enough to prevent certain lichen communities from establishing. Cáceres et al. (2007) found similar result when comparing naturally occurring trees within a lowland rain forest preserve in northeastern Brazil: while lichen diversity was negatively correlated with bark shedding and species composition changed markedly, lichen abundance was not affected. These findings indicate that bark shedding has a filtering effect, allowing for fewer species, namely pioneer taxa that have low levels of competitivity under other circumstances, to colonize such substrates and increase their abundance values. The studies by Fonseca et al. (2009) and Käffer et al. (2009) in southern Brazil and by Calviño-Cancela et al. (2012, 2013) in Spain, comparing natural forest with tree plantations, also supports this point. The same effect is known under the influence of air pollution, with the aforementioned example of L. conizaeoides (Cook et al. 1990; Wirth 1993; Bates et al. 2001; LaGreca and Stutzman 2006; Massara et al. 2009; Purvis et al. 2010; Lisowska 2011).
Similar effects of reduced alpha and beta diversity in managed tree plots, with increased abundance of a few, otherwise rare species, were found for both vascular and non-vascular epiphytes in other studies. Thus, Hietz and Hietz-Seifert (1995) reported de-diversification and homogenization of vascular epiphytes in alder plantations (Alnus acuminata) versus native cloud forest in Mexico. Merwin et al. (2003) found monospecific tree cultured to be dominated by a few species of bromeliads, compared to a much higher diversity in natural forest. Studies in subtropical and extratropical areas in southern Brazil and northeastern Spain found substantial reduction of species richness and homogenization of community structure for Eucalyptus (and pine) plantations compared to natural forest (Fonseca et al. 2009; Käffer et al. 2009; Calviño-Cancela et al. 2012, 2013). Work on tropical herpetofauna and invertebrates discovered similar effects, including for Eucalyptus plantations in the Brazilian Amazon (Barlow et al. 2007, 2008; Gardner et al. 2007, 2008; Hung et al. 2008). These findings suggest a general pattern of changes in alpha and beta diversity in monospecific tree cultures, and our study demonstrates the same effect for Eucalyptus plantations in tropical montane areas. On the other hand, when monospecific tree plantations are formed by native tree species, this effect appears much less pronounced. For instance, monospecific shade tree stands in coffee plantations, mainly made up by trees of the native genus Inga, maintain a comparatively rich and well structured epiphyte community (Hietz 2005). Fonseca et al. (2009) and Käffer et al. (2009) demonstrated much higher lichen diversity for native Araucaria plantations compared to introduced Eucalyptus and Pinus monocultures in southern Brazil.
Many of the aforementioned studies suggest that the diversity of microhabitats generated by the diversity of tree species in natural forest is largely responsible for higher epiphyte diversity compared to monospecific tree plots, possibly due to subtle phorophyte preferences related to bark structure and bark pH (Cáceres et al. 2008). However, our results suggest that also the factor of forest management come into play. By selecting a single tree species in the natural forest, Q. humboldtii, we eliminated the factor of phorophyte diversity; hence, if phorophyte preferences alone would explain effects on lichen communities in tree plantations, we would expect the natural oak trees to display similar levels of low beta diversity as the Eucalyptus trees in the gum plantation. Yet, we found significant differences in terms of community structure, specifically beta diversity. Therefore, in addition to a reduction in tree species in monocultures, there must be other factors affecting epiphyte diversity. Among these are stand demography, with trees in monocultures having the same age whereas a natural forest, even if dominated by a single species, contains trees of a wide age range. Another factor is spatial structure, with trees in monocultures being evenly spaced whereas in a natural forest they follow a stochastic pattern determined by the interaction of dispersal and competition (Connell 1970; Janzen 1970; Clark and Clark 1984; Wright 2002; Nathan and Casagrandi 2004; Mangan et al. 2010). These factors affect canopy development and hence light regimes (Menalled et al. 1998), as well as the dispersal of epiphytes between trees. We therefore hypothesize that the lichen community structure in the natural oak forest is largely driven by the demography and spatial dispersion of the sampled oak trees and that a managed oak stand, with equally spaced trees of the same age, would have a community structure more similar to the gum plantation, albeit likely composed of different species.
In conclusion, our work supports findings from other studies that monospecific, standardized tree plots, especially those composed of alien tree species, affect diversity patterns of epiphytic communities and particularly lead to less diverse and more homogeneous assemblies. The present study appears to be the first to quantify this effect for Eucalyptus plantations replacing natural oak forest in tropical montane regions; our data also suggest that this effect is quite substantial. Analyzing these effects for other epiphytes and forest organisms should therefore be made a priority, considering that E. globulus is one of the most extensively planted alien tree species in the Andes (Hillis and Brown 1984).
References
Affeld K, Sullivan J, Worner SP, Didham RK (2008) Can spatial variation in epiphyte diversity and community structure be predicted from sampling vascular epiphytes alone? J Biogeogr 35:2274–2288
Agudelo MI (2009) Biomasa aérea y contenido de carbono en bosques de Quercus humboldtti y Colombobalanus excelsa, corredor de conservación de robles Guantiva-La Rusia-Iguaque (Santander-Boyacá). Administración del medio ambiente y de los recursos naturales. Universidad Autónoma de Occidente, Cali
Aguirre-C J (1982) Contribución al conocimiento de los líquenes de Colombia II. Observaciones acerca de Hypotrachyna y Parmotrema (Líquenes). Bol Dept Biol 1(4):29–44
Aguirre-C J (1985) Algas, líquenes, hongos, hepáticas y musgos. In: Flora de la Real Expedición Botánica del Nuevo Reino de Granada (1783–1816) Promovida y Dirigida por José C. Vol. II. Mutis, Madrid
Aguirre-C J (2008) Los líquenes de la región del Sumapaz (composición florística, distribución y ecología). In: Cramer J (ed) La Cordillera Oriental Colombiana Transecto Sumapaz (Studies on Tropical Andean Ecosystems). Stuttgart, pp 211–234
Aguirre-C J, Linares E (2000) Briófitos y Líquenes de la Sábana de Bogotá. Departamento Administrativo del Medio Ambiente, DAMA, Bogotá
Andersson MS, Gradstein SR (2005) Impact of management intensity on non-vascular epiphyte diversity in cacao plantations in western Ecuador. Biodivers Conserv 14:1101–1120
Andrade L (2008) Determinar la viabilidad financiera del cultivo de 100 hectáreas de Eucalipto en el departamento del Atlántico. Graduate Thesis, Fundación Universidad del Norte, Barranquilla, Colombia
Armenteras D, Cadena C, Moreno R (2007) Evaluación del Estado de los Bosques de Niebla y de la Meta 2010 en Colombia. Instituto Humboldt, Bogotá
Barlow J, Gardner TA, Araujo IS, Avila-Pires TC, Bonaldo AB, Costa JE, Esposito MC, Ferreira LV, Hawes J, Hernandez MM, Hoogmoed MS, Leite RN, Lo-Man-Hung NF, Malcolm JR, Martins MB, Mestre LAM, Miranda-Santos R, Nunes-Gutjahr AL, Overal WL, Parry L, Peters SL, Ribeiro-Junior MA, Da Silva MNF, Motta CD, Peres CA (2007) Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc Natl Acad Sci USA 104:18555–18560
Barlow J, Araujo IS, Overal WL, Gardner TA, Mendes FD, Lake IR, Peres CA (2008) Diversity and composition of fruit-feeding butterflies in tropical eucalyptus plantations. Biodivers Conserv 17:1089–1104
Bates JW, Bell JNB, Massara AC (2001) Loss of Lecanora conizaeoides and other fluctuations of epiphytes on oak in S.E. England over 21 years with declining SO2 concentrations. Atmos Environ 35:2557–2568
Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv 17:925–951
Cáceres MES (2007) Corticolous crustose and microfoliose lichens of northeastern Brazil. Libri Bot 22:1–168
Cáceres MES, Lücking R, Rambold G (2007) Phorophyte specificity and environmental parameters versus stochasticity as determinants for species composition of corticolous crustose lichen communities in the Atlantic rainforest of northeastern Brazil. Mycol Prog 6:117–136
Cáceres MES, Lücking R, Rambold G (2008) Efficiency of sampling methods for accurate estimation of species richness of corticolous microlichens in the Atlantic rainforest of northeastern Brazil. Biodivers Conserv 17:1285–1301
Calviño-Cancela M, Rubido-Bará M, Van Etten EJ (2012) Do eucalypt plantations provide habitat for native forest biodiversity? For Ecol Manage 270:153–162
Calviño-Cancela M, López de Silanes ME, Rubido-Bará M, Uribarri J (2013) The potential role of tree plantations in providing habitat for lichen epiphytes. For Ecol Manage 291:386–395
Chaparro M, Aguirre J (2002) Hongos Liquenizados. Editorial El Malpensante, Bogotá
Clark DA, Clark DB (1984) Dynamics of a tropical rain forest tree: evaluation of the Janzen–Connell model. Am Nat 124:769–788
Connell JH (1970) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: Den Boer PJ, Gradwell GR (eds) Dynamics of populations. Pudoc, Wageningen, pp 298–310
Cook LM, Rigby KD, Seaward MRD (1990) Melanic moths and changes in epiphytic vegetation in north-west England and north Wales. Biol J Linn Soc 39:343–354
Cuatrecasas J (1989) Aspectos de la vegetación natural en Colombia. Pérez-Arbeláezia 2:155–283
FAO (1981) El eucalipto en la repoblación forestal. Colección FAO 11. Montes, Rome
Fonseca CR, Grande G, Baldissera R, Becker CG, Boelter CR, Brescovit AD, Campos LM, Fleck T, Fonseca VS, Hartz SM, Joner F, Käffer MI, Leal-Zanchet AM, Marcelli MP, Mesquita AS, Mondin CA, Paz CP, Petry MV, Piovensan EN, Putzke J, Stranz A, Vergara M, Vieira EM (2009) Towards an ecologically-sustainable forestry in the Atlantic Forest. Biol Cons 142:1209–1219
Galindo T, Betancur J, Cadena M (2003) Estructura y composicion floristica de cuatro bosques andinos del Santuario de Flora y Fauna Guanenta-Alto Rio Fonce, Cordillera Oriental colombiana. Caldasia 25:313–335
Gardner TA, Ribeiro-Junior MA, Barlow J, Cristina T, Avila-Pires S, Hoogmoed MS, Peres CA (2007) The value of primary, secondary, and plantation forests for a neotropical herpetofauna. Cons Biol 21:775–787
Gardner TA, Hernandez MIM, Barlow J, Peres CA (2008) Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. J Appl Ecol 45:883–893
Hickey JE (1994) A floristic comparison of vascular species in Tasmanian oldgrowth mixed forest with regeneration resulting from logging and wildfire. Aust J Bot 42:383–404
Hietz P (2005) Conservation of vascular epiphyte diversity in Mexican coffee plantations. Cons Biol 19:391–399
Hietz P, Hietz-Seifert U (1995) Structure and ecology of epiphyte communities of a cloud forest in central Vera Cruz, Mexico. J Veg Sci 6:719–728
Hillis WE, Brown AG (1984) Eucalypts for wood production. CSIRO/Academic Press, London
Hofstede R, Lips J, Jongsma W, Sevink Y (1998) Geografía. Ecología y Forestación de la Sierra Alta del Ecuador, Abya Yala, Quito
Hofstede RGM, Dickinson KJM, Mark AF (2001) Distribution, abundance and biomass of epiphyte-lianoid communities in a New Zealand lowland Nothofagus-podocarp temperate rain forest: tropical comparisons. J Biogeogr 28:1033–1049
Hung N, Gardner TA, Ribeiro MA, Barlow J, Bonaldo AB (2008) The value of primary, secondary, and plantation forests for neotropical epigeic arachnids. J Arachnol 36:394–401
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:940
Käffer MI, Ganade G, Marcelli MP (2009) Lichen diversity and composition in Araucaria forests and tree monocultures in southern Brazil. Biodiv Conserv 18:3543–3561
Kapelle M (ed) (2006) Ecology and conservation of neotropical montane Oak Forests. Springer, Berlin
Kelly DL (1985) Epiphytes and climbers of a Jamaican rain forest: vertical distribution, life forms and life histories. J Biogeogr 12:223–241
Kelly DL, O’Donovan G, Feehan J, Muphy S, Drangeid SO, Marcano-Berti L (2004) The epiphyte communities of a montane rain forest in the Andes of Venezuela: patterns in the distribution of the flora. J Trop Ecol 20:643–666
LaGreca S, Stutzman BW (2006) Distribution and ecology of Lecanora conizaeoides (Lecanoraceae) in eastern Massachusetts. Bryologist 109:335–347
León J, Vélez G, Yepes A (2009) Estructura y composición florística de tres robledales en la región norte de la cordillera central de Colombia. Rev Biol Trop 57:1165–1182
Linares E, Pinzón M (2001) Catálogo comentado de los líquenes y briófitos de la región subxerofítica de La Herrera (Mosquera, Cundinamarca). Caldasia 23:237–246
Linares E, Pinzón M (2006) Diversidad de líquenes y briófitos en la región subxerofítica de La Herrera, Mosquera. Cundinamarca I. Riqueza y estructura. Caldasia 28:243–257
Lisowska M (2011) Lichen recolonisation in an urban-industrial area of southern Poland as a result of air quality improvement. Environ Monit Assess 179:177–190
Lõhmus A, Lõhmus P (2010) Epiphyte communities on the trunks of retention trees stabilise in 5 years after timber harvesting, but remain threatened due to tree loss. Biol Cons 143:891–898
Lozano C, Torres J (1974) Aspectos generales sobre la distribución sistemática fitosociológica y clasificación ecológica de los bosques de robles (Quercus) en Colombia. Ecol Trop 1:45–79
Lücking R, Rivas Plata E (2008) Clave y guía ilustrada para géneros de Graphidaceae. Glalia 1(1):1–47
Lücking R, Archer AW, Aptroot A (2009) A world-wide key to the genus Graphis (Ostropales: Graphidaceae). Lichenologist 41:363–452
Lücking R, Dal-Forno M, Sikaroodi M, Gillevet PM, Bungartz F, Moncada B, Yánez A, Chaves JL, Coca LF, Lawrey JD (2014) A single macrolichen constitutes hundreds of unrecognized species. Proc Natl Acad Sci USA 111:11091–11096
Mangan SA, Schnitzer SA, Herre EA, Mack KML, Valencia MC, Sanchez EI, Bever JD (2010) Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466:752–755
Massara AC, Bates JW, Bell JNB (2009) Exploring causes of the decline of the lichen Lecanora conizaeoides in Britain: effects of experimental N and S applications. Lichenologist 41:673–681
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software, Gleneden Beach, Oregon
McCune B, Mefford MJ (1999) PC-ORD. Multivariate analysis of ecological data, Version 4.0. MjM Software, Gleneden Beach
McCune B, Amsberry KA, Camacho FJ, Clery S, Cole C, Emerson C, Felder G, French P, Greene D, Harris R, Hutten M, Larson B, Lesko M, Majors S, Markwell T, Parker GG, Pendergrass K, Peterson EB, Peterson ET, Platt J, Proctor J, Rambo T, Rosso A, Shaw D, Turner R, Widmer M (1997) Vertical profile of epiphytes in a Pacific Northwest old-growth forest. Northwest Sci 71:145–152
Menalled FD, Kelty MJ, Ewel JJ (1998) Canopy development in tropical tree plantations: a comparison of species mixtures and monocultures. Forest Ecol Manag 104:249–263
Merwin MC, Rentmeester SA, Nadkarni NM (2003) The influence of host tree species on the distribution of epiphytic bromeliads in experimental monospecific plantations, La Selva, Costa Rica. Biotropica 35:37–47
Mežka A, Brūmelis G, Piterāns A (2008) The distribution of epiphytic bryophyte and lichen species in relation to phorophyte characters in Latvian natural old-growth broad leaved forests. Folia Cryptogam Eston 44:89–99
Moncada B (1998) Contribución al Estudio de la flora liquénica del Parque Natural Chicaque, San Antonio del Tequendama-Cundinamarca, Santa Fé de Bogotá. Graduate Thesis, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia
Moncada B, Coca LF, Lücking R (2013a) Neotropical members of Sticta (lichenized Ascomycota: Lobariaceae) forming photosymbiodemes, with the description of seven new species. Bryologist 116:169–200
Moncada B, Lücking R, Coca LF (2013b) Six new apotheciate species of Sticta (lichenized Ascomy-cota: Lobariaceae) from the Colombian Andes. Lichenologist 45:635–656
Moncada B, Lücking R, Betancourt-Macuase L (2013c) Phylogeny of the Lobariaceae (lichenized Ascomycota: Peltigerales), with a reappraisal of the genus Lobariella. Lichenologist 45:203–263
Moncada B, Lücking R, Suárez A (2014) Molecular phylogeny of the genus Sticta (lichenized Ascomycota: Lobariaceae) in Colombia. Fung Div 64:205–231
Muñoz A, Camacho L (2010) Conservación y uso sostenible de los bosques de roble en el corredor de conservación Guantiva-La Rusia-Iguaque, departamentos de Santander y Boyacá, Colombia. Rev Colombia Forestal 13:5–25
Muñoz M, Barbosa V, Vargas J, Pinzón M, Rodríguez M, Triana W (2010) Diagnóstico del Huerto Semillero de Eucalyptus globulus, Parque La Florida, Facultad del Medio Ambiente y Recursos Naturales, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia
Nathan R, Casagrandi R (2004) A simple mechanistic model of seed dispersal, predation and plant establishment: Janzen-Connell and beyond. J Ecol 92:733–746
Ojeda D (2001). Ecosistemas en Leyva (El medio Ambiente en Colombia). Instituto de Hidrología, Meteorología y Estudios Ambientales. Ideam
Peláez RN, Moncada B, Lücking R (2014) High diversity of Ocellularia (Ascomycota: Graphidaceae) in the Colombian Llanos, including two species new to science. Phytotaxa 189:245–254
Purvis OW, Tittley I, Chimonides PDJ, Bamer R, Hayes PA, James PW, Rumsey FJ, Read H (2010) Long-term biomonitoring of lichen and bryophyte biodiversity at Burnham Beeches SAC and global environmental change. Syst Biodivers 8:193–208
Rangel O (1997). Diversidad Biótica II. Tipos de vegetación en Colombia. Universidad Nacional de Colombia, Instituto de Ciencias Naturales, Bogotá, Colombia
Rincón-Espitia A (2011) Composición de la flora de líquenes corticícolas en el Caribe colombiano. Master Thesis, Universidad Nacional de Colombia, Bogotá
Rivas Plata E, Lücking R, Lumbsch HT (2008) When family matters: an analysis of Thelotremataceae (Lichenized Ascomycota: Ostropales) as bioindicators of ecological continuity in tropical forests. Biodiv Cons 17:1319–1351
Rivas Plata E, Parnmen S, Staiger B, Mangold A, Frisch A, Weerakoon G, Hernández JE, Cáceres MES, Kalb K, Sipman HJM, Common RS, Nelsen MP, Lücking R, Lumbsch HT (2013) A molecular phylogeny of Graphidaceae (Ascomycota, Lecanoromycetes, Ostropales) including 428 species. MycoKeys 6:55–94
Rivera D, Córdoba C (1998) Guía Ecológica Parque Natural Chicaque. Jardín Botánico José Celestino Mutis, Bogotá
Sanz M, Calatayud V, Sanchez G (2000) Macrolíquenes epífitos en las parcelas de nivel II de la Red de Seguimiento de la Salud de los Bosques en España. Ecología 14:117–128
Sillett SC, Gradstein SR, Griffin D III (1995) Bryophyte diversity of Ficus tree crowns from cloud forest and pasture in Costa Rica. Bryologist 98:251–260
Simijaca D (2011) Líquenes epífitos de Quercus humboldtii en el parque natural municipal robledales de Tipacoque (Boyacá–Colombia). Graduate Thesis, Facultad de Ciencia Básicas, Universidad Pedagógica y Tecnológica de Colombia. Tunja, Boyacá
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biol Skrif Kong Dansk Vidensk 5:1–34
Sipman HJM (2006) Provisional determination keys for the Graphidales of Costa Rica. http://www.bgbm.org/BGBM/STAFF/Wiss/Sipman/Zschackia/Diorygma/intro.htm
Sipman HJM, Aguirre J (1982) Contribución al conocimiento de los líquenes de Colombia I. Clave genérica para los líquenes foliosos y fruticosos de los páramos colombianos. Caldasia 13:603–634
Sipman HJM, Hekking W, Aguirre J (2008) Checklist of Lichenized and Lichenicolous Fungi from Colombia. Instituto de Ciencias Naturales, Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá D.C
Soto-Medina EA, Bolaños A (2012) Diversidad de líquenes cortícolas en el bosque subandino de la finca zíngara (Cali, Valle del Cauca). Rev Cienc Univalle 14:35–44
Soto-Medina EA, Lücking R, Bolaños A (2011) Especificidad de forófito y preferencias microambientales de los líquenes cortícolas en cinco forófitos del bosque premontano de finca zíngara, Cali, Colombia. Rev Biol Trop 60:843–856
Staiger B (2002) Die Flechtenfamilie Graphidaceae. Studien in Richtung einer natürlicheren Gliederung. Biblioth Lichenol 85:1–526
Staiger B, Kalb K, Grube M (2006) Phylogeny and phenotypic variation in the lichen family Graphidaceae (Ostropomycetidae, Ascomycota). Mycol Res 110:765–772
Ter Steege H, Cornelissen JHC (1989) Distribution ecology of vascular epiphytes in lowland rain forest of Guayana. Biotropica 21:331–339
Van Leerdam A, Zagt RJ, Veneklaas EJ (1990) The distribution of epiphyte growth-forms in the canopy of a Colombian cloud-forest. Vegetatio 87:59–71
Villarreal H, Álvarez M, Córdoba S, Escobar F, Fagua G, Gast F, Mendoza H, Ospina M, Umaña AM (2006). Métodos para el análisis de datos: una aplicación para resultados provenientes de caracterizaciones de biodiversidad. Manual de Métodos Para el Desarrollo de Inventarios de Biodiversidad. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Bogotá, Colombia, pp 185–226
Wirth V (1993) Trendwende bei der Ausbreitung der anthropogen geförderten Flechte Lecanora conizaeoides? Phytocoenologia 23:625–636
Wirth M, Hale ME (1963) The lichen family Graphidaceae in México. Contrib United States Natl Herb 36:63–119
Wolf JHD (1993) Diversity patterns and biomass of epiphytic bryophytes and lichens along an altitudinal gradient in the northern Andes. Ann Mo Bot Gard 80:928–960
Wolf JHD (1994) Factors controlling the distribution of vascular and non-vascular epiphytes in the northern Andes. Vegetatio 112:15–28
Wright JS (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14
Zimmerman JK, Olmsted IC (1992) Host tree utilization by vascular epiphytes in a seasonally inundated forest (Tintal) Mexico. Biotropica 24:402–407
Acknowledgments
This study was partially supported through the following projects funded by the National Science Foundation: Neotropical Epiphytic Microlichens—An Innovative Inventory of a Highly Diverse yet Little Known Group of Symbiotic Organisms (DEB 715660 to The Field Museum; PI R. Lücking), and ATM—Assembling a taxonomic monograph: The lichen family Graphidaceae (DEB-1025861 to The Field Museum; PI T. Lumbsch, CoPI R. Lücking). The first and second author thank the Universidad Distrital Francisco José de Caldas, especially the Proyecto Curricular de Licenciatura en Biología, and the Herbario Forestal Gilberto Emilio Mahecha Vega (UDBC), Sección Criptógamas, for the logistic support. David Escobar, subdirector of the Parque Natural Chicaque, is thanked for authorizing the study within the limits of the natural reserve. Alejandra Suárez and the Grupo Colombiano de Liquenología (GCOL) assisted in various aspects of the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Karen E. Hodges.
Rights and permissions
About this article
Cite this article
Rios, A.I.A., Moncada, B. & Lücking, R. Epiphyte homogenization and de-diversification on alien Eucalyptus versus native Quercus forest in the Colombian Andes: a case study using lirellate Graphidaceae lichens. Biodivers Conserv 24, 1239–1252 (2015). https://doi.org/10.1007/s10531-014-0855-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0855-7