Abstract
Our knowledge of the spatial distribution of bryophyte diversity still suffers from low sampling efforts. Here we try to determine the spatial diversity patterns of liverworts and mosses and their environmental drivers more accurately by correcting for this sampling bias. We compiled bryoflora from 49 localities in eastern China, including data on sampling effort. Both sampling bias uncorrected (raw) species richness and bias corrected (estimated) species richness, as derived from species-sampling curves, were used as response variables. Model selection based on Akaike’s information criterion was used to evaluate the impact of collection bias on the selection of environmental and spatial variables in the regression models. Variation partitioning was used to assess the independent and joint effects of environmental, spatial and sampling variables on raw and estimated species richness. Liverwort richness increased significantly with decreasing latitude, while moss richness showed no latitudinal pattern, whether for raw or estimated species richness. However, estimated species richness showed stronger correlation with environmental variables than raw species richness. Importantly, selected environmental variables in the raw species richness models changed after correcting for collection bias. Despite their ability to produce copious amounts of spores, our sampling bias corrected models indicated that bryophyte richness showed strong spatial structuring, indicating dispersal limitation. Environmentally, liverwort richness was primarily controlled by water availability, while the richness of mosses was mainly determined by available energy. Our results highlight that biological features, dispersal ability and environmental sorting may account for the discrepancies between species richness of liverworts and mosses. Given the considerable impact that controlling for sampling effort had on analysis outcome, we like to stress the importance of controlling for sampling bias when studying spatial richness patterns in bryophytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most knowledge on spatial patterns and underlying mechanisms of distribution and diversity have so far been based on studies of macro-organisms, which tend to exhibit latitudinal gradients in species richness and are predominately driven by contemporary environment (Willig et al. 2003; Field et al. 2009; Beck et al. 2012). Whether microscopic organisms (such as prokaryotes, protists, algae, and bryophytes) possess similar spatial patterns of species diversity as those observed for macro-organisms and whether these patterns are controlled by similar environmental factors are hotly debated (Fenchel and Finlay 2004; Foissner 2006; Fontaneto and Brodie 2011). Early biogeographers thought geography to be irrelevant for the distribution of microscopic organisms due to their well developed dispersal capabilities, often summed up as ‘everything is everywhere, but the environment selects’, the so-called “everything is everywhere” hypothesis (O’Malley 2007). This hypothesis states that the biogeography of microscopic organisms is not affected by dispersal barriers or historical events, but is only influenced by local contemporary habitat conditions. This hypothesis was proposed to describe spatial diversity patterns of any organism smaller than 2 mm, although this hypothesis is now controversial since many microscopic organisms have restricted distributions (Finlay 2002; Foissner 2006; Fontaneto and Brodie 2011). Because bryophytes produce copious spores that disperse readily, predictions made for free-living microbes under the “everything is everywhere” hypothesis should also hold for these organisms (Foissner 2006; Frahm 2008; Medina et al. 2011).
Bryophytes, comprising liverworts, hornworts and mosses, exhibit high levels of diversity, and play important ecological roles in terms of water balance, erosion control, nitrogen budget, and providing habitat for other organisms (Glime 2007). However, species richness of bryophytes has rarely been tested in terms of spatial patterns and their correlations with environmental variables at geographic scales (Medina et al. 2011; Beck et al. 2012). The currently available analyses have yielded controversial results which are widely open for debate (Medina et al. 2011). For liverworts, von Konrat et al. (2008) used data from 400 geo-political units to produce a global map showing that species density per 10,000 km2 was highest in the tropics, indicating the presence of a latitudinal species richness gradient. However, this study suffers from incomplete data for many regions limiting its robustness. For mosses, several recent studies did not support the idea of a latitudinal species richness gradient (Shaw et al. 2005; Mutke and Geffert 2010; Geffert et al. 2013). Geffert et al. (2013) argue that more field work and data collection in the tropical regions are needed to draw final conclusions on the presence of a latitudinal gradient for moss species richness. With the exception of Möls et al. (2013), who found a positive correlation between moss richness and both precipitation and coastline length (which forms a proxy for high humidity), few studies have related species richness of bryophytes or their subgroups to environmental factors at biogeographical scales.
Sampling effort, in terms of specimens collected or area surveyed, can heavily influence species richness estimates and thus the analyses of geographic patterns of species diversity and their environmental drivers (Gotelli and Colwell 2010; Yang et al. 2013; Zhang et al. 2014). This is particularly true for bryophytes, since many regions have not been adequately surveyed due to their small size, simplicity in structure and subsequent difficulties in field identification (Aranda et al. 2010; Mutke and Geffert 2010; Medina et al. 2013). To be able to compare richness patterns between bryophyte studies and relate these patterns to environmental variables, the effect of sampling effort has to be taken into account. However, it has long been recognized that most bryophyte biogeographical studies suffer from incomplete sampling (Aranda et al. 2010; Mutke and Geffert 2010; Medina et al. 2013). Studies that did include measures of sampling completeness generally only considered the effect of sampling area, but not specimen number (e.g., von Konrat et al. 2008; Mutke and Geffert 2010), even though the latter is a more direct measure of sampling effort (Willott 2001).
China is covered by a continuous transition of vegetation from boreal to tropical forests in its eastern part. The complicated topography and diverse climate of China have resulted in a diverse bryoflora (1050, 26, and 1945 species of liverworts, hornworts and mosses, respectively; Jia and He 2013). Here we compiled a data set of bryophyte richness and collection intensity for 49 localities in eastern China, and to ask whether: (i) the spatial species richness patterns of the two subgroups of bryophytes (i.e., liverworts and mosses) differ, (ii) liverwort and moss richness differ in environmental and spatial correlations, and (iii) sampling effort affects the results of spatial analyses on bryophyte biogeography.
Materials and methods
Data sources
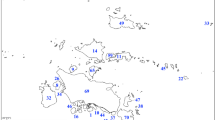
We compiled data on species richness of bryophytes (liverworts, hornworts, and mosses, respectively) from 49 localities (nature reserves or national parks, Fig. 1) in eastern China from the peer-reviewed scientific literature and dissertations published between 1986 and 2013 (Appendix in supplementary material). Weipu (http://bjvip.csdl.ac.cn/) and Wanfang (http://www.wanfangdata.com.cn/) were used to survey literature on regional bryoflora. All the localities had information on coordinates, topography (minimum and maximum elevation), sampling area and specimen numbers. The included localities followed a combined strategy of floristic habitat sampling based on vegetation types and altitudinal bands, and completely random plot sampling within each floristic habitat. Specimen collection within all included locations was conducted during at least 1 year to avoid missing seasonal species. Since there are only 26 hornworts in China, and because hornworts exhibit similar functional vegetative traits and ecological features as liverworts, we merged the two groups (see Patiño et al. 2013). Of the 49 localities, 12 had separate information on the specimen number of liverworts and mosses. For these 12 locations, which were representative for the whole environmental space encompassed by all 49 locations (Fig. S1), we determined if the specimen numbers of liverworts and mosses could be predicted from the number of bryophyte specimens with correlation analysis. Both liverwort and moss specimen numbers were strongly correlated with total bryophyte specimen numbers (Fig. S2). Using the obtained correlation models we subsequently predicted the specimen numbers of liverworts and mosses for the 37 localities which lack this information.
Response variables
The relationship between raw species richness (i.e., the species numbers reported for each location without correction for sample effort) and specimen number across localities followed a significant power-function (Fig. 2a–c), meaning that raw species richness can be extrapolated up, or down, to a given number of specimens using the power model of the species-sampling curve, analog to the method of Kier et al. (2005) seen in Eq. (1):
where S e and S are the estimated species richness (i.e., species numbers corrected for sample effort) and raw species richness in each locality, respectively. N e is the base number of specimens, i.e., the number of specimens used to calculate estimated species richness for each of the 49 locations. This base number was set at 3,000 for liverworts, 6,000 for mosses and 9,000 for bryophytes. These numbers were chosen based on the maximum number of specimens available for bryophytes (8,500), and the approximate ratio between specimen number of liverworts and mosses (ca. 1:2). N is the observed specimen number of each locality, and α is the slope in regression of S against N in ln–ln space (Fig. 2a–c).
The effect of specimen number on the latitudinal gradient of species richness for bryophytes (a, d), liverworts (b, e) and mosses (c, f). In the upper band, lnb denotes intercept, and α denotes slope in regression of S against N in ln–ln space. Only significant regressions were shown. * p < 0.05, ** p < 0.01, *** p < 0.001
Environmental variables
Based on previous studies on distribution and diversity of bryophytes at geographical scales (Grau et al. 2007; Möls et al. 2013), biological characteristics of bryophytes (Glime 2007), and studies on ferns with microscopic dispersing stages in China (Chen et al. 2011), we selected mean annual temperature (AMT), mean temperature of the coldest quarter (TCQ), annual precipitation (AP), and precipitation of the driest quarter (PDQ) as macroclimatic variables, while elevational range (maximum elevation minus minimum elevation, ELEra) was added to reflect environmental heterogeneity. Data for temperature and precipitation between 1950 and 2000 were obtained from WorldClim (http://www.worldclim.org/; Hijmans et al. 2005) with a spatial resolution of ~1 km for the 49 localities according to their geographical midpoints.
Sampling effort
Both sampling area (AREA) and specimen number (N) of each locality can be regarded as measures of sampling effort. Because species richness was significantly correlated with specimen number for bryophytes, liverworts, and mosses (Fig. 2a–c), while AREA and species richness were poorly correlated (R 2 < 0.05; p > 0.2), we included only specimen number in subsequent analyses of sampling effort. We controlled for sampling effort in two ways. First, we used estimated species richness of liverworts, mosses, and bryophytes, based on Eq. (1) for the base number of specimens as response variables. Second, we treated specimen number as a potential predictor in regression models (Dunn et al. 2009; Gotelli and Colwell 2010). The latter method has been regarded as the best way to control the effect of sampling effort in the absence of identities of individuals (Gotelli and Colwell 2010).
Spatial variables
To determine to what extent bryophyte diversity patterns were driven by spatial variables, we computed principal coordinates of neighbor matrices (PCNMs) from the geographical distance matrix based on the geographical coordinates (latitude and longitude) of the 49 locations (Borcard and Legendre 2002; Dray et al. 2006). Each PCNM variable represents an independent spatial filter. The higher the eigenvalue of a given filter, the broader the spatial gradient it represents (Dray et al. 2006; Rangel et al. 2006). Twelve filters were generated and ranked in declining order according to eigenvalue (Table S1). We then used corrected Akaike’s information criterion (AICc) to rank all the possible combinations of models for raw and estimated species richness in which the 12 spatial filters were the predictors to be selected. The model with the minimum AICc was considered as the best one. This procedure selected Filter 2 and 7 for raw species richness of bryophytes and mosses, and Filter 1, 2, 4, and 7 for raw liverwort richness (Table S1). To explore the effect of sampling effort on the selection of spatial filters, we used specimen number as fixed variable in all models to select spatial filters for raw species richness. This procedure selected Filter 1, 4, 6, 7 and 10 for raw bryophyte richness, Filter 1, 3, 4, 6, and 10 for raw liverwort richness, and Filter 1, 4, 6, and 10 for raw moss richness. The spatial filters selected for estimated species richness were identical to those selected for ‘raw species richness with specimen number as fixed variable’ (Table S1).
Data analyses
First we ln-transformed all response and environmental variables to reduce their skewed distribution. The relationships between the environmental variables were examined by Pearson’s correlation analyses (Table S2). Univariate regression analyses were used to examine the relationships between response variables and putative predictors. Adjusted R 2 was used to estimate the explanatory power of regression models. Statistical significance of the correlations and regressions were based on corrected degrees of freedom, calculated using Dutilleul’s (1993) modified t test. We used Moran’s I at different distances to test the spatial autocorrelation of species richness.
Because environmental variables were significantly correlated with each other (Table S2), we used AICc to select the best environment models for raw and estimated species richness, and included the selected environmental variables in subsequent analyses. To assess the influence of sampling effort and spatial autocorrelation on selection of environmental variables, specimen number, spatial filter, or both were used as fixed variables in model selection. The selected environmental variables in the best model and corresponding specimen number and spatial filters were used to run multiple regressions (Table 1). For simplicity, we only reported the models including all the variables representing environment, space and sampling effort for raw species richness, and the models including environmental and spatial variables for estimated species richness. The remaining models can be found in Table S3.
We estimated independent effects of spatial, environmental and sampling variables on response variables by variation partitioning (VP; Peres-Neto et al. 2006). We used VP because this technique calculates R 2, which is either confounded (joint effect) or not confounded (independent effect) by other variables and thus can quantify the independent effect of each explanatory variable (Quinn and Keough 2002). We conducted a series of VP with different combinations of response variables and independent variables, and portioned the variance in response variables into the effects of two or three groups of independent variables. To show how the sampling effort affected the results of VP, we partitioned the spatial variance of response variables into the pure effect of space and environment and their joint effect. For raw species richness, we used the spatial filters and the environmental variables selected without considering sampling effort. Then, each component of VP for raw species richness was compared with that of VP for estimated species richness, which incorporated the variables of sampling effort. VP on three groups of variables (environment, space and sampling effort) was conducted twice for raw species richness, i.e., with and without considering sampling effort.
To test the ‘everything is everywhere’ hypothesis, we compared species richness–ELEra relationships between two regions: the (sub)tropical region (latitude <30°) and the temperate region (latitude >30°) (Telford et al. 2006). All the data analyses were performed separately for bryophytes, liverworts, and mosses, by using Spatial Analysis in Macroecology (SAM) software (Rangel et al. 2010).
Results
General species richness–environment relationships
Raw species richness of each locality ranged from 37 to 517 for bryophytes, from 1 to 215 for liverworts, and from 36 to 364 for mosses. The specimen numbers of bryophytes collected at a particular site ranged from 38 to 8,500, and averaged 1,206 ± 1,570. Significant linear relationships were observed between ln-transformed specimen number and ln-transformed raw species richness for bryophytes, liverworts and mosses, respectively (Fig. 2a–c). Estimated species richness was positively correlated with raw species richness, while the regression slopes were obviously lower than the 1:1, indicating that raw species richness was underestimated in localities with lower sampling effort (Fig. S3).
Both raw and estimated species richness of liverworts were negatively correlated with latitude, while moss richness had no significant latitudinal gradient, whether for raw or estimated species richness (Fig. 2d–f). Moran’s I correlogram indicated that estimated species richness showed a stronger spatial structure (higher absolute values of Moran’s I) than raw species richness (Fig. S4).
Generally, liverwort richness showed more significant correlations with climatic variables, than moss richness. Estimated richness was better explained by climatic variables than raw richness, especially for liverworts (Fig. 3; Table S4). ELEVra and AREA explained only a small part of the variance in estimated species richness. When tested separately, climatic variables generally showed higher explanatory power than ELEVra and AREA (Table S4). Species richness–ELEra relationships differed among regions (Fig. S5).
Multi-regressions models for richness of bryophytes
When sampling effort was considered, the selected environmental and spatial variables for raw species richness were the same as those for estimated species richness for each taxonomic group. For both raw and estimated species richness, AMT and TCQ were selected for bryophytes and mosses, and AP and PDQ for liverworts. Species richness of bryophytes and mosses showed significantly positive correlation with TCQ (p < 0.05), and negative correlation with AMT (p < 0.05). Liverwort richness, however, was positively correlated with AP, and negatively with PDQ. Spatial filters at finer scales (e.g., Filter 10) generally showed significantly positive effects on species richness. The explanatory power of global models was higher for raw species richness than for estimated species richness of bryophytes (90.1 vs. 55.1 %), mosses (86.8 vs. 40.2 %), and liverworts (83.3 vs. 65.9 %; Table 1). Sampling effort and spatial variables had an important influence on the selection of environmental variables. For instance, the selected environmental variables were ELEra and AP for raw species richness of bryophytes, liverworts, and mosses without specimen number and spatial filters in model selection (Table S3).
Variation partitioning (VP)
When the variance in species richness was partitioned into two groups of variables (space and environment), the pure effect of space was much lower than that of environment for raw species richness, while for estimated species richness, the pure effect of space was dominant (Fig. S6). When the variance in raw species richness was partitioned into three groups of variables (space, environment and sampling effort), the strength of pure effects of space and environment depended on the combination of spatial and environmental variables. The pure effects of specimen number were large and comparable for the two VP analyses (Fig. S7).
Discussion
Geographic patterns of bryophyte diversity
Using a database containing bryofloristic data from 49 localities in eastern China, we demonstrate, after accounting for the influence of sampling effort and spatial autocorrelation, that bryophytes in general and liverworts in particular show a significant negative latitudinal species richness gradient. Moss richness, however, does not change along the latitudinal gradient. Apparently, the latitudinal gradient in bryophyte richness is mainly determined by liverworts, not mosses. Although several previous studies have tested latitudinal gradients in species richness of mosses (Shaw et al. 2005; Geffert et al. 2013; Möls et al. 2013) and liverworts (van Konrat et al. 2008) at the global scale, the effects of sampling effort had not been fully considered, as recognized by some of the authors themselves (e.g., Mutke and Geffert 2010). Some of these studies used the species–area relationship of Arrhenius (1921) to estimate species richness per standardized area (e.g., Geffert et al. 2013). But as indicated by our analysis and other studies on bryophytes (Mutke and Geffert 2010) and ants (Dunn et al. 2009), area, as opposed to number of individuals sampled, is a poor predictor of species richness at large scales. Therefore, accounting for specimen numbers in our analyses makes the results on latitudinal gradients in species richness of bryophytes, liverworts, and mosses more reliable.
Several factors may account for the discrepancies between latitudinal gradients in species richness of liverworts and mosses. First, the liverworts analyzed here included a high proportion of epiphyllous species, which are abundant in humid tropical and subtropical forests, and gradually diminish in abundance towards temperate and boreal forests (Jiang et al. 2013). Epiphyllous liverworts account for about 20 % of all liverwort species in China (Zhu and So 2001; Jia and He 2013). Most of these belong to Lejeuneaceae and Radulaceae, which dominate in the bryoflora of South China (Hu 1990; Pócs 1996), and show higher endemism than other subgroups of bryophytes (Pócs 1996). Second, mosses may have stronger dispersal ability than liverworts, because mosses produce more spores per sporophyte and have a higher proportion of species with sporophytes compared with liverworts (During and Van Tooren 1987). Most mosses have erect or creeping stems and tiny leaves, but hornworts and some liverworts have only a flat thallus and no leaves. Third, some major subgroups of mosses have extremely varied distribution patterns. For example, Hookeriales, Hypnodendrales, and Hedwigiales are rich in tropical regions, while Splachnales and Sphagnopsida occur mainly at high-latitude regions (Geffert et al. 2013). The contrasting species richness patterns of the different orders counteract each other and may lead to the absence of a latitudinal gradient in species richness for mosses.
Environmental correlates of bryophyte richness
Our results show that, at regional scales, liverwort richness is primarily controlled by water availability (PRE and PDQ), while moss richness is determined more by energy availability (AMT and TCQ). This finding is contrary to Möls et al. (2013), who found that moss richness was significantly correlated with water availability. However, our finding that liverwort richness is more strongly influenced by water availability than moss richness fits well with current understanding of bryophyte ecophysiology which states that liverworts as a group tend to favor more mesic habitats than mosses (Qiu et al. 1998; Oliver et al. 2000; Proctor et al. 2007). Bryophytes receive little water from the soil and have poor control over water loss; this is particularly true for liverworts, which are one of the most primitive groups of land plants (Qiu et al. 1998; van Konrat et al. 2008). Assuming that the driving force behind the evolution of terrestrial plants involved an improved ability to cope with water stress, mosses should be better adapted to conserving water than liverworts (Oliver et al. 2000). Contrary to the popular belief that mosses must grow in wet places, a number of species are xerophytic, having adapted to hot, dry desert conditions (Glime 2007). In such habitats, some mosses are able to absorb water from dew and night air, permitting brief periods of photosynthesis during the early morning hours (Alpert 1979).
It is surprising that, in eastern China, moss richness showed much weaker correlations with macroclimatic variables than liverwort richness. This may indicate that mosses exhibit a broader range of physiological optima and ecological amplitudes than liverworts, possibly as a consequence of more divergent evolution and contrasting distribution patterns of different orders in mosses (Rice et al. 2001; Geffert et al. 2013). The distribution of mosses may arise simply because of extreme habitat sorting of different orders (e.g., Whittaker 1972). Another possible explanation for the weak correlations between species richness and the environment in mosses may be attributable to the stronger tolerance of mosses to cold and drought than liverworts. For instance, most mosses (18 species tested) were resistant to temperatures up to −20 °C, with seven of these species being resistant to −27 °C, while liverworts are less cold resistant, even for Arctic species (Glime 2007). In addition, moss species disproportionately possess vegetative desiccation tolerance (globally 158 species of mosses out of 1,250–1,300 species in total versus 52 species of liverworts and hornworts out of 5,100–5,150 species; Wood 2007). Our results highlight the need for more studies on the evolution and ecophysiology of liverworts and mosses as independent species groups.
Do bryophytes conform to the ‘everything is everywhere’ hypothesis?
During recent decades, there has been an increasing interest in the biogeography of microscopic organisms, with focus on both geographic patterns of diversity and underlying mechanisms generating those patterns (Fenchel and Finlay 2004; Foissner 2006; Fontaneto and Brodie 2011). Bryophytes reproduce via spores, which are generally small in size (usually between 10 and 20 µm in diameter; Frahm 2008). In addition, the number of spores per capsule is very large for most species (104–106; Vanderpoorten and Goffinet 2009). Therefore, wind dispersal may be the most common dispersal mechanism (Porley and Hodgetts 2005), and these species may therefore disperse well and over long distances. Acknowledging that our analyses on spatial patterns and environmental correlates of species richness of liverworts and mosses are not explicit tests of the “everything is everywhere” hypothesis, the results can provide some useful clues on this issue. If the idea that “everything is everywhere” is true for bryophytes, there should be no dispersal limits for bryophytes and the variation of species richness of bryophytes, liverworts, and mosses should be completely explained by the environmental variables. However, our results of VP analyses showed that the pure effects of environmental variables were much lower than those of spatial variables. Although we cannot fully describe every aspect of environmental conditions for bryophytes, such as pH, vegetation types and soil, which may control bryophyte distribution at local scales (e.g., Vanderpoorten and Engels 2003), our results do suggest that the distribution of liverworts and mosses do not follow the ‘everything is everywhere’ hypothesis.
Telford et al. (2006) argued that the ‘everything is everywhere’ hypothesis could be tested by comparing regional species richness–environment relationships. The species richness–environment relationship should be similar for separate regions if dispersal is not limited. After we split the whole dataset into two regions (sub)tropical (latitude <30°) versus temperate (latitude >30°), we found that the species richness–ELEra relationships differed markedly among regions, for both liverworts and mosses. Despite the enormous quantities of spores and high passive dispersal potential, their dispersal seems limited, consistent with the result of previous studies (During and Van Tooren 1987; Foissner 2006; Virtanen 2014). In fact, even for small spores (<20 µml), most will fall within several meters of the source (During and Van Tooren 1987).
The influence of sampling effort on macroecological inference
Our analyses on macroecology of bryophytes showed the overriding effects of sampling bias on bryophyte richness estimations. Ignoring this sampling bias can result in serious misinterpretation of macroecological patterns of bryophyte richness and its correlations with environmental and spatial variables. Several methods have been used to compensate for the geographical bias in sampling effort, such as rarefaction curves based on individuals or samples (Gotelli and Colwell 2010), which need information on the total number of records, observed species richness, and species identity of individuals. Unfortunately, most studies on local fauna or flora only report the number of specimens collected and the number of species identified, but do not include lists of species and the identity of the corresponding specimen(s). Our study shows that the species-sampling curve across sites, which can be extrapolated up or down to a given specimen number, is a straightforward and sound method to account for the effect of sampling effort on species richness. First, estimated species richness showed more evident spatial patterns, indicated by their higher absolute values of Moran’s I at all classes of distances and was more strongly correlated with latitude than raw species richness. Second, the R 2 values of univariate regression on climatic variables strongly and consistently increased when estimated, instead of raw, species richness was used as the response variable. This indicates that the effect of climate predictors was severely underestimated when measures of raw species richness were used as response variable. Importantly, the strongest single predictor of species richness switched from elevational heterogeneity to temperature in coldest quarter (TCQ) for bryophytes and mosses when using estimated richness as a response variable. Third, using estimated species richness as a response variable can aid in selecting the statistically proper spatial and environmental predictors. Spatial filters at broader and finer scales were more frequently selected as spatial variables for estimated species richness. For bryophytes and mosses, the selected variables switched from ELEra and AP for raw species richness to AMT and TCQ for estimated species richness. For liverworts, the selected variables switched from ELEra and AP to AP and PDQ for estimated species richness. The above changes in variable selection also took place when raw species richness was response variable, and specimen number was included as a fixed variable.
Our results show that sampling effort strongly affects biogeographical inference on spatial patterns of species richness and underlying mechanisms for bryophytes. The results also highlight the difficulty to test species richness–environment relationships when sampling effort is ignored or not properly accounted for if the sampling is incomplete. This is also true for macroecological studies on other organisms (e.g., Chen et al. 2014; Zhang et al. 2014). Therefore, we strongly suggest incorporating sampling effort by using it as a potential predictor or, alternatively, estimating species richness based on species-sampling curves across sites.
References
Alpert P (1979) Desiccation of desert mosses following a summer rainstorm. Bryologist 82:65–71
Aranda SC, Gabriel R, Borges PA, Lobo JM (2010) Assessing the completeness of bryophytes inventories: an oceanic island as a case study (Terceira, Azorean archipelago). Biodivers Conserv 19:2469–2484
Arrhenius O (1921) Species and area. J Ecol 9:95–99
Beck J, Ballesteros-Mejia L, Buchmann CM, Dengler J, Fritz SA et al (2012) What’s on the horizon for macroecology? Ecography 35:673–683
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Model 153:51–68
Chen SB, Jiang GM, Ouyang ZY, Xu WH, Xiao Y (2011) Relative importance of water, energy, and heterogeneity in determining regional pteridophyte and seed plant richness in China. J Syst Evol 49:95–107
Chen S, Mao L, Zhang J, Zhou K, Gao J (2014) Environmental determinants of geographic butterfly richness pattern in eastern China. Biodivers Conserv 23:1453–1467
Dray S, Legendre P, Peres-Neto PR (2006) Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Model 196:483–493
Dunn RR, Agosti D, Andersen AN, Arnan X, Bruhl CA et al (2009) Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol Lett 12:324–333
During HJ, Van Tooren BF (1987) Recent developments in bryophyte population ecology. Trends Ecol Evol 2:89–93
Dutilleul P (1993) Modifying the t test for assessing the correlation between two spatial processes. Biometrics 49:305–314
Fenchel T, Finlay BJ (2004) The ubiquity of small species: patterns of local and global diversity. Bioscience 54:777–784
Field R, Hawkins BA, Cornell HV, Currie DJ, Diniz-Filho JAF et al (2009) Spatial species-richness gradients across scales: a meta-analysis. J Biogeogr 36:132–147
Finlay BJ (2002) Global dispersal of free living microbial eukaryote species. Science 296:1061–1063
Foissner W (2006) Biogeography and dispersal of micro-organisms: a review emphasizing protists. Acta Protozool 45:111–136
Fontaneto D, Brodie J (2011) Why biogeography of microorganisms? In: Fontaneto D (ed) Biogeography of microscopic organisms: is everything small everywhere. Cambridge University Press, Cambridge, pp 3–10
Frahm JP (2008) Diversity, dispersal and biogeography of bryophytes (mosses). Biodivers Conserv 17:277–284
Geffert JL, Frahm JP, Barthlott W, Mutke J (2013) Global moss diversity: spatial and taxonomic patterns of species richness. J Bryol 35:1–11
Glime JM (2007) Bryophyte ecology, Vol. 1. Physiological ecology. EBook sponsored by Michigan Technological University and the International Association of Bryologists. www.bryoecol.mtu.edu. Accessed Feb 2014
Gotelli NJ, Colwell RK (2010) Estimating species richness. In: Magurran AE, McGill BJ (eds) Biological diversity: frontiers in measurement and assessment. Oxford University Press, Oxford, pp 39–54
Grau O, Grytnes JA, Birks HJB (2007) A comparison of altitudinal species richness patterns of bryophytes with other plant groups in Nepal, Central Himalaya. J Biogeogr 34:1907–1915
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hu R (1990) Distribution of bryophytes in China. Trop Bryol 2:133–137
Jia Y, He S (2013) Species catalogue of China (volume 1: bryophytes). Science Press, Beijing
Jiang Y, de Bie CAJM, Wang T, Skidmore AK, Liu X et al (2013) Hyper-temporal remote sensing helps in relating epiphyllous liverworts and evergreen forests. J Veg Sci 24:214–226
Kier G, Mutke J, Dinerstein E, Ricketts TH, Küper W et al (2005) Global patterns of plant diversity and floristic knowledge. J Biogeogr 32:1107–1116
Medina NG, Draper I, Lara F (2011) Biogeography of mosses and allies: does size matter. In: Fontaneto D (ed) Biogeography of microscopic organisms: is everything small everywhere. Cambridge University Press, Cambridge, pp 209–233
Medina NG, Lara F, Mazimpaka V, Hortal J (2013) Designing bryophyte surveys for an optimal coverage of diversity gradients. Biodivers Conserv 22:3121–3139
Möls T, Vellak K, Vellak A, Ingerpuu N (2013) Global gradients in moss and vascular plant diversity. Biodivers Conserv 22:1537–1551
Mutke J, Geffert JL (2010) Keep on working: the uneven documentation of regional moss floras. Trop Bryol 31:7–13
O’Malley MA (2007) The nineteenth century roots of ‘everything is everywhere’. Nat Rev Microbiol 5:647–651
Oliver M, Tuba Z, Mishler BD (2000) The evolution of vegetative desiccation tolerance in land plants. Plant Ecol 151:85–100
Patiño J, Guilhaumon F, Whittaker RJ, Triantis KA, Gradstein SR et al (2013) Accounting for data heterogeneity in patterns of biodiversity: an application of linear mixed effect models to the oceanic island biogeography of spore-producing plants. Ecography 36:904–913
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Pócs T (1996) Epiphyllous liverworts diversity at worldwide level and its threat and conservation. An Inst Biol Univ Nac Auton Mex Bot 67:109–127
Porley R, Hodgetts N (2005) Mosses and liverworts. Harper Collins, London
Proctor MC, Oliver MJ, Wood AJ, Alpert P, Stark LR et al (2007) Desiccation-tolerance in bryophytes: a review. Bryologist 110:595–621
Qiu YL, Cho Y, Cox JC, Palmer JD (1998) The gain of three mitochondrial introns identifies liverworts as the earliest land plants. Nature 394:671–674
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rangel TFLVB, Diniz-Filho JAF, Bini LM (2006) Towards an integrated computational tool for spatial analysis in macroecology and biogeography. Glob Ecol Biogeogr 15:321–327
Rangel TFLVB, Diniz-Filho JAF, Bini LM (2010) SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33:46–50
Rice SK, Collins D, Anderson AM (2001) Functional significance of variation in bryophyte canopy structure. Am J Bot 88:1568–1576
Shaw AJ, Cox CJ, Goffinet B (2005) Global patterns of moss diversity: taxonomic and molecular inferences. Taxon 54:337–352
Telford RJ, Vandvik V, Birks HJB (2006) Dispersal limitations matter for microbial morphospecies. Science 312:1015
Vanderpoorten A, Engels P (2003) Patterns of bryophyte diversity and rarity at a regional scale. Biodivers Conserv 12:545–553
Vanderpoorten A, Goffinet B (2009) Introduction to bryophytes. Cambridge University Press, Cambridge
Virtanen R (2014) Diaspore and shoot size as drivers of local, regional and global bryophyte distributions. Glob Ecol Biogeogr 23:610–619
von Konrat M, Renner M, Söderström L, Hagborg A, Mutke J (2008) Chapter nine: early land plants today: liverwort species diversity and the relationship with higher taxonomy and higher plants. Fieldiana Bot 47:91–104
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Willig MR, Kaufman DM, Stevens RD (2003) Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu Rev Ecol Evol Syst 34:273–309
Willott SJ (2001) Species accumulation curves and the measure of sampling effort. J Appl Ecol 38:484–486
Wood AJ (2007) The nature and distribution of vegetative desiccation-tolerance in hornworts, liverworts and mosses. Bryologist 110:163–177
Yang W, Ma K, Kreft H (2013) Geographical sampling bias in a large distributional database and its effects on species richness–environment models. J Biogeogr 40:1415–1426
Zhang J, Nielsen SE, Grainger TN, Kohler M et al (2014) Sampling plant diversity and rarity at landscape scales: importance of sampling time in species detectability. PLoS ONE 9:e95334. doi:10.1371/journal.pone.0095334
Zhu RL, So ML (2001) Epiphyllous liverworts of China. Nova Hedwig 121:1–418
Acknowledgments
We thank financial support from the National Key Technology R&D Program (2012BAC01B08) and the Special Public Science and Technology Research Program for Environmental Protection (201209027).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dirk Sven Schmeller.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, S., Ferry Slik, J.W., Mao, L. et al. Spatial patterns and environmental correlates of bryophyte richness: sampling effort matters. Biodivers Conserv 24, 593–607 (2015). https://doi.org/10.1007/s10531-014-0838-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0838-8