Abstract
Until recently, the Kara Sea was a stable ecosystem unaffected by alien species invasions. However, at the beginning of the twenty-first century, the snow crab (Chionoecetes opilio) was detected. Studies conducted between 2013 and 2022 in Blagopoluchiya Bay (Novaya Zemlya Archipelago, Kara Sea) provided an opportunity to observe the establishment of a population of this species and its influence on benthic communities. Various sampling methods, such as trawl and grab surveys, as well as video observations, were used to study two main seabed habitats, one in the deep inner basin and the other at the sill at the bay’s exit. The study revealed significant changes in benthic ecosystems, including declines in integral benthic characteristics such as abundance, biomass, diversity, and shifts in dominant species. The response of megabenthos and macrobenthos varied between habitats, but in general, there was a sharp decline in the abundance of large bivalves and brittle stars. The observed changes were not related to environmental variability but most likely to the abundance and size structure of the snow crab. The taxonomic and size structure of the benthos changed as crab individuals increased in size, and the changes were faster and more pronounced in the area with higher crab abundance. These findings raise concerns about the potential long-term effects of the snow crab invasion on the Kara Sea ecosystem, including reduced biodiversity and changes in food webs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Arctic has historically been considered a low-risk region for biological invasions due to the limited access and severe environmental conditions that inhibit many species’ survival, growth and reproduction (Ruiz, Hewitt 2009). However, climatic shifts and anthropogenic impacts have facilitated the alteration of the Arctic's ecosystem, resulting in the introduction of various invasive species (Miller, Ruiz 2014; Renaud et al. 2015; Ricciardi et al. 2017; Ware et al. 2015). The beginning of the twenty-first century was marked by an expansion of the snow crab (Chionoecetes opilio O. Fabricius, 1788; Majoidea, Oregoniidae) on the Arctic shelf, a sub-Arctic species, which was previously absent in the European Arctic (Sokolov 2014; Spiridonov, Zalota 2017). This crab is naturally distributed in the cold waters of the northwestern Atlantic Ocean, in the northern Pacific Ocean northwards of the Aleutian Islands and the Sea of Japan, and the Chukchi Sea westward of the boundary with the East Siberian Sea and eastward to the Beaufort Sea (Ogata 1973; Sirenko et al. 2008; Slizkin 1998; Squires 1990). Outside its natural distribution range, it was found in the Barents Sea in 1996 (Kuzmin et al. 1999), but the origin of the snow crab in this area is still unclear. Recent genetic studies suggest that the presence of snow crab in the Barents Sea may be the result of natural range expansion (Dahle et al. 2022), while another view is that it was introduced with ballast water (Kuzmin et al. 1999). In any case, the Barents Sea crab population has grown rapidly in both geographic distribution and abundance since its initial discovery, and in 2012 snow crabs were found in the Kara Sea (Zimina 2014). Despite very detailed surveys in the Barents and Kara Seas, the snow crab has not been previously recorded in either region and thus meets one of the criteria for recognizing non-native species (Carlton, Schwindt 2024). Its larvae probably entered the Kara Sea from the Barents Sea, along with water masses from the north of the Novaya Zemlya Archipelago and through the Kara Gate Strait (Sokolov 2014; Zalota et al. 2020; Zimina 2014). Until the mid-2010s, these larvae played a key role in developing of the Kara Sea population of the snow crab, but more recent studies have suggested local reproduction of the species (Lipukhin et al. 2024; Zalota et al. 2019).
The Kara Sea was a relatively stable ecosystem practically unaffected by long-term transformations. The general pattern of distribution of benthic communities on the scale of the entire sea, their species structure and dominant species remained stable until the beginning of the twenty-first century (Antipova, Semenov 1989; Azovsky, Kokarev 2019; Filatova, Zenkevich 1957; Kozlovskiy et al. 2011). According to recent data, no significant changes in macrobenthic communities were observed, at least until 2013 (Gerasimova et al. 2021). However, studies conducted in the last decade have revealed the presence of sudden and dramatic changes in the benthic fauna in the southwestern part of the Kara Sea, particularly in Blagopoluchiya Bay, the fjord of the Novaya Zemlya Archipelago (Lepikhina et al. 2022).
The first comprehensive survey of benthic communities in Blagopoluchiya Bay was conducted in 2013 (Udalov et al. 2016). The study did not reveal the presence of snow crabs, but described the typical macrobenthic communities found in the Kara Sea fjord. Depth and sediment flux were the primary factors shaping the benthic fauna (Udalov et al. 2021, 2016). Juvenile Ch. opilio was first observed in Blagopoluchiya Bay in 2014. Since then, the number of individuals and total biomass of crabs in the bay has increased (Zalota et al. 2018, 2019). In 2020, the abundance of crabs in Blagopoluchiya Bay was up to 70 individuals per 100 square meters (Galkin et al. 2021), representing the highest density on the east coast of the Novaya Zemlya Archipelago. As expected, this increase in the crab population affected the benthos. A benthic survey conducted in 2020 revealed a significant decrease in the integral characteristics of macrobenthos, including abundance and biomass (Lepikhina et al. 2022). Additionally, the proportion of macro and meiobenthic abundance shifted, with an increase in the share of meiobenthos, indicating a significant change in trophic conditions and food web structure (Lepikhina et al. 2022).
Ch. opilio is an active predator that feeds on a variety of invertebrates, including bivalves, gastropods, polychaetes, ophiuroids, crustaceans, and sometimes even fish (Chuchukalo et al. 2011; Kolts et al. 2013; Squires 2003). Stomach content analyses of the specimens from the Barents Sea (Pavlov 2007; Zakharov et al. 2018) and from the Kara Sea (Burukovsky et al. 2022) assume that snow crabs consume almost all of the abundant local benthos resources. In the Barents Sea, the snow crabs themselves are a food target for several species feeding on benthos, such as haddock, wolffish, thorny and arctic skates, and especially the Atlantic cod, which is the main predator for this crab species (Dolgov, Benzik 2016; Eriksen et al. 2020; Holt et al. 2021), however in the Kara sea the Atlantic cod is absent (Mokievsky et al. 2016) and the information about the diet of the other species is scarce. Thus, almost the only possible predator for snow crab in the Kara Sea is the crab itself, and it is currently unclear whether the prey resources are sufficient to sustain an uncontrolled predator crab population.
The establishment of the snow crab in the Kara Sea is, therefore, a unique case that provides an opportunity to observe the development of an alien species and the ecosystem's response to it. Fjord ecosystems are characterized by a lower functional redundancy of macrofauna compared to the open sea, especially in the upper part with intensive runoff. Reduced functional complexity may indicate the high sensitivity of fjord ecosystems to various stressors (Włodarska-Kowalczuk et al. 2012), which is particularly important in the context of the ongoing invasion of snow crab (Zalota et al. 2019). The main objective of this study was to assess the nature and direction of the changes in the ecosystem of the Blagopoluchiya Bay fjord in two main habitats (the inner deep-water basin and the sill at the exit of the bay) from the time of the crab's establishment to the benthic communities using a variety of sampling methods (trawl and grab surveys, video observations). The obtained data will provide insight into the ecological impact of the snow crab on the intact ecosystem.
Materials and methods
Study area
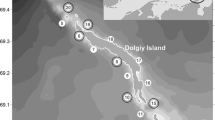
Blagopoluchiya Bay (75°39' N, 63°41' E) is located in the Kara Sea on the north-eastern coast of the Novaya Zemlya Archipelago (Fig. 1).
It is a 12 km long and 5 km wide fjord with an inner basin up to 180–200 m deep, separated from the outer part of the fjord by a 40 m deep sill. The outer basin gradually descends towards the Novaya Zemlya Trough, reaching a depth of about 200–350 m. Here and throughout the text, we refer to it as a "bay", as this is a widely accepted geographical name.
The functioning of the Blagopoluchiya Bay ecosystem is largely dependent on the dynamics of sea ice, in particular the timing and pattern of its melting. In the adjacent areas of the Kara Sea, ice melt begins in June–August. The timing of ice melt (and the onset of the hydrological spring, which determines water warming, runoff and primary production) varied significantly from 2013 to 2022 (Fig. 2). No direct temporal trend was observed; warm years with early ice melt in June were followed by cold years with ice melt in August.
Timing of sea ice melting in the outer part of Blagopoluchiya Bay and the adjacent Kara Sea (blue bars); surface water temperature (SWT, dashed lines) and near-bottom water temperature (NBWT, solid lines) at two stations in Blagopoluchiya Bay in 2013–2022 (August/September). Station S is shown in black, and station IB is shown in red. The timing of ice melting was assessed using open data maps from AARI [http://old.aari.ru/odata]
Two rivers originating from the Nalli Glacier provide significant runoff. The volume of glacial runoff, snowfall intensity, prevailing winds, and wave action influence the characteristics of the water column: temperature, salinity, and suspended sediment fluxes. Hydrological parameters vary considerably seasonally and between years. During the summer months, at station IB, the surface water temperature was + 1.5—+ 3℃, with a maximum of + 5℃ during the warmest summer (2022). A pronounced stratification of the water masses was observed at depths of 10—40 m, and the temperature in the thermocline decreased by + 2.5—+ 3℃, while the near-bottom layer reached a temperature of − 1.48 to − 0.49℃. There was a slight upward interannual trend in temperature: the near-bottom water temperature varied from − 1.49 to − 0.86℃ in 2013—2018 and reached − 0.49 and − 0.69℃ in 2020 and 2022, respectively. At station S, the structure of the water column was the same as at station IB, and the bottom water temperature was always negative, ranging from − 0.39 to − 1.29℃. Considering the interannual temperature variability, it can be concluded that there are no obvious linear trends (from 2013 to 2022) in surface and near-bottom water temperatures indicating warming or cooling of the water column in the Blagopoluchiya Bay in different years (Fig. 2).
The surface plume of desalinated water with a salinity of 24—30 extended to station S and, in some cases, far beyond the bay. Salinity gradually increased towards the thermocline and stabilized at around 34 in the near-bottom layer at both stations. Turbidity was generally higher in the inner part of the bay due to the river water discharge from the Nalli Glacier. However, at both stations, turbidity ranged from 0.07 to 1.2 FTU in the 0—20 m layer in different years, depending on wind strength and direction, time of year and river discharge. Near-bottom turbidity was relatively stable, ranging from 0.08 to 0.15 FTU at both stations. Dissolved oxygen saturation was high at station S (84—90%) and slightly lower at station IB (79—83%); oxygen depletion and eutrophication were absent throughout the study. No clear trends were observed in the variability of the biogenic elements; fluctuations were recorded both between years and between stations. The complete data set of hydrological and hydrochemical parameters of the water column is summarized in Supplementary Table S1.
The sediments in Blagopoluchiya Bay consist of silt and clay. In the deep inner basin at station IB, sediments were fine, with a modal particle diameter of 6 µm. The fine sand fraction (coarser than 63 µm) was less than 3%. At station S, the modal particle diameter was the same as at station IB (7 µm), but the fraction of fine sand was slightly higher (6%).
Sampling and sample processing
This study includes the data obtained during cruises of the R/V Professor Shtokman and the R/V Akademik Mstislav Keldysh in Blagopoluchiya Bay in 2013–2022 during August/September (Table 1). Two monitoring stations represent two main types of bottom topography (Fig. 1). The first station (INNER BASIN, IB) is located in the deepest part of the inner basin at 150—170 m. Studies of benthic communities were first conducted at this station in 2013 as a part of a large-scale survey of the entire bay (Udalov et al. 2016). The second station (SILL, S) was established in 2016 at the sill at a depth of 63—72 m. We used a variety of sampling techniques throughout the study period: grabs, trawls and video observations to investigate long-term trends in benthic communities.
Macrofauna samples were collected using an Ocean or Van Veen grab (0.1 m2 sampling area) (3 replicates per station); the collected samples were washed through a 0.5 mm mesh sieve. Megafauna and large macrofauna were sampled using a Sigsbee trawl with a frame size of 150 × 35 cm (with an inner mesh of 0.5 cm). Trawling was carried out at a drift speed of 0.4–0.6 knots. The trawling distance varied from 290 to 1016 m for navigational reasons but was generally around 550–600 m. The catch was washed through a set of 5 mm and 1 mm mesh sieves. After washing, trawl and grab samples were fixed in 6% buffered formalin diluted by seawater. In the laboratory, animals were sorted, identified to the lowest possible taxonomic level, counted, weighed (wet weight), and preserved in 70% ethanol. The resulting list of species was carefully checked for validity and standardized according to the World Registry of Marine Species database (WoRMS, https://www.marinespecies.org). Molluscs were weighed with shells, and polychaetes were weighed without tubes. All snow crabs from trawl samples were measured, and their sex was determined.
Since 2016, video surveys of the underwater landscape have been carried out before sampling. The video transects were conducted using a towed underwater vehicle (TUV) “Videomodule”, equipped with a navigation system, power supply, high-resolution video and still cameras, spotlights and two laser scale indicators with a fixed distance between them (60 cm or 20 cm in different modifications) (Anisimov et al. 2023; Pronin 2017). The TUV provided georeferenced (including depth), spatially oriented, and scaled images of the seafloor and the organisms inhabiting it. Video transects were conducted at a drift speed of 0.4–0.6 knots, and the observation distance was approximately 600 m. Each video was processed by analyzing high-quality, readable parts, and large organisms were identified and counted. Species densities were recalculated per 10 m2 using the scale of the laser pointers to obtain image width and navigation data to calculate track length.
Hydrological parameters (salinity, temperature, and turbidity profiles of the water column) were measured with the SBE911 CTD. Dissolved oxygen (mL/L, %) and nutrients (phosphate, dissolved inorganic silicon, nitrogen in ammonium, nitrite, and nitrate forms) were measured immediately after sampling in the on-board laboratory according to accepted methods (Parsons 2013). Samples for the granulometric composition of the sediments were taken by a tube core from an additional grab or box corer and analyzed using an Analysette 22 MicroTec Plus particle-size laser counter. Sea ice dynamics and the timing of ice melting were assessed using open data maps from AARI [http://old.aari.ru/odata].
Data analysis
Large species with high biomass represented in the grab samples by single individuals (sponges, ascidians, echinoderms) were excluded from the analysis as grabs with a small sampling area cannot adequately assess their distribution. Percentages were used to analyze trawl samples, as the volume of trawl catches varied considerably between stations. In addition, trawl samples were used for taxonomic studies to verify the video survey species list. There are difficulties in estimating the area of seafloor sampled by the trawl, as the exact time of contact with the seafloor surface is unknown. In addition, trawl sampling is ineffective in capturing mobile epibenthic organisms as they escape the gear.
We used species respiration rate R as a measure of abundance, estimated as R = ki*Ni0,25*Bi0,75, where Ni is the abundance of a species, Bi is the biomass, and ki is a taxon-specific coefficient. This measure provides a balanced contribution of small but common species and large (high biomass) rare species by combining their abundance and biomass (Azovsky et al. 2023; Vedenin et al. 2015). In addition, a number of taxa, total abundance, biomass, and univariate diversity measures (Shannon index (H’loge), Hurlbert rarefaction (ES100)) were calculated for each station (McCune et al. 2002). Statistical analyses were performed in PRIMER V7 (Clarke et al. 2015).
Results
Benthic communities
Grab data. A total of 128 species of macrobenthic organisms were recorded over the five surveys. While 112 species were recorded at station S, only 49 were recorded at station IB. The most diverse taxa were polychaetes (40 and 24 species at stations S and IB, respectively), followed by bivalves with 22 and 12 species and crustaceans with 17 and 3 species.
Quantitative characteristics and their dynamics differed between the two sites (Fig. 3). At station S, species diversity remained stable from 2013 to 2018 but then experienced a sharp decline in 2020, followed by a further 1.5–twofold decrease in 2022. In contrast, at station IB, diversity parameters changed insignificantly and generally were lower than at station S. Changes in diversity at both stations were accompanied by marked fluctuations in total abundance and biomass, which were high in 2013 and 2016 but became significantly lower by 2022 (Fig. 3).
Box plots (the crossbar indicates the median value) illustrating changes in the mean values of the integral characteristics of macrobenthos (grab samples) over the period from 2013 to 2022: abundance (ind/m2), biomass (g/m2), number of species per station (S), expected number of species per 100 individuals (ES(100)) and Shannon diversity index H'(loge)
At station IB, the bivalve Ennucula tenuis dominated throughout the study period (34—69% by R), except for 2018 when its abundance decreased sharply (to 23% by R), and the polychaete Scoletoma fragilis became dominant with a similar contribution (26% by R) (Table 2). In some years, the polychaetes Scoloplos aff. acutus, Aglaophamus malmgreni and Tharyx sp. were also among the subdominants. The abundance of the most common species decreased over time (Table 2). The bivalve Portlandia arctica was the only species that showed a significant increase in abundance in 2020 and 2022, which is probably the result of the settlement of juveniles, but further development of these newly settled bivalves is not yet clear. In contrast, station S exhibited a directional change in community composition between 2013 and 2022. From 2013 to 2016, the community was dominated by the bivalve Bathyarca glacialis (39–17% by R) and the sipuncula Golfingia margaritacea (40% by R) (Table 2). The abundance of bivalves decreased over time. Astarte spp. and Bathyarca glacialis disappeared completely by 2020, and G. margaritacea became the absolute dominant species by 2022, accounting for 74% of the total species respiration rate R and 94% of the biomass. However, its abundance and biomass remained the same as in previous years. Meanwhile, the abundance of the small protobranch Ennucula tenuis increased between 2018 and 2020, and this bivalve became the second dominant species (Table 2).
Thus, we observed a transition from the Golfingia—Bathyarca community to the Golfingia—Ennucula community at station S and Ennucula community dynamics with a rotation of subdominants (mainly polychaetes) at station IB.
Trawl data. A total of 168 taxa were identified during the entire observation period. The most abundant taxa at both stations were polychaetes (34 and 22 species at stations S and IB, respectively), followed by bivalves (21 and 18 species), crustaceans (16 and 18 species), and gastropods (18 and 12 species). Several taxa, especially fouling and filter-feeding organisms (cnidarians, bryozoans, sponges, ascidians), were more abundant at station S.
The highest species richness was observed at station IB in 2014 (72 species) and at station S in 2016 (110 species), with overall species diversity being higher at S than at IB in all years. The diversity components decreased sharply in 2018 (Fig. 4). Furthermore, species richness decreased continuously until 2022 at station S, while it returned to initial values at station IB. Evenness varied at both stations (Fig. 4).
Changes in taxonomic structure were quite pronounced both at the level of major taxa and at the species level (Fig. 5).
At the beginning of the observations, the large brittle star Ophiopleura borealis at station IB (36% in the total species respiration rate R) and the bivalve Bathyarca glacialis at station S (44%) dominated the trawl samples. In addition, the brittle stars O. borealis, Ophiacantha bidentata, and several Astarte species (Astarte borealis, Astarte elliptica) were highly abundant at station S (Table 3). Trawl catches at both stations changed dramatically in 2018. The snow crab became dominant, with its share in the total species respiration rate reaching 94% at station S and 72% at station IB. Subsequently, we observed a steady decline in the share of snow crabs to 43% in 2022 (Table 3), but it remained the dominant species at both stations. O. borealis was the second most abundant species at station IB in 2018 (21%), but by 2020, its share had decreased to 0.5%, and in 2022 no ophiuroids were found. At station S, the disappearance of ophiuroids was much faster, and they were practically absent in 2018 (Table 3). Besides crabs, the dominant species at station S were the bivalves Astarte spp. and the sea star Urasterias linkii in 2020, and the sea anemone Hormatia digitata and the gastropod Colus sabini at station IB in 2022.
Video data. Based on video observations, 25 megafauna taxa were recorded at both stations. The most diverse group was echinoderms, with 13 identified species. At station IB, the ophiuroid O. borealis was the dominant species in 2016 and 2018, with densities of 40.3 and 31.1 individuals per 10 m2, respectively. However, its abundance decreased significantly in 2020 (less than 5 ind./10 m2), and the species was no longer observed on the video transect in 2022. The second most abundant species was the snow crab, with a mean density ranging from 4.97 to 4.11 ind./10 m2 between 2016 and 2020. In 2022, the abundance of snow crabs decreased to 0.68 ind./10 m2. Actiniaria gen. sp. (presumably Hormatia digitata) and the starfish Urasterias lincki were also regularly observed throughout the study period (Table 4). The large isopod Saduria sabini was only recorded in 2016 (0.3 ind./10 m2).
As for station S, the ophiuroids O. borealis and O. bidentata dominated there in 2016. By 2018, their abundance had decreased tenfold, and a similar decrease occurred later at station IB in 2020 (Table 4). The abundance of snow crabs at station S was already very high in 2016 and 2018 (16—21 ind./10 m2). As the crabs grew, their density decreased, reaching 7.7 ind./10 m2 in 2020 and 4.12 ind./10 m2 in 2022. Soft corals of the genus Gersemia (mainly Gersemia fruticosa) and the sponge Polymastia sp. were observed in all years, although their density decreased with time (Table 4). Actiniaria gen. sp., the starfish Urasterias lincki, and the sea urchin Strongylocentrotus sp. were also among the subdominants. The large brittle star Gorgonocephalus arcticus, the starfishes Crossaster sp., Ctenodiscus crispatus, Pontaster tenuispinus, Icasterias panopla, Lophaster furcifer, Solaster sp., and the crinoid Heliometra glacialis were also recorded at both stations, but no patterns in their distribution and dynamics were detected. The screenshots from video tracks in different years are provided in Supplementary Fig. S1.
Snow crab population dynamics.
Snow crab densities varied considerably over the study period and among sampling sites. The video observations showed a higher abundance of snow crabs (2–5 times) at station S than at station IB in all years (Fig. 6). In 2016 and 2018 (no video observation was carried out in 2014), the average density at station S was 16—21 ind./10 m2. However, the small size of the crab individuals in 2016 most likely resulted in an underestimation of Ch. opilio abundance from the video data, and the actual population density should have been higher. Furthermore, their density decreased to 7.7 ind./10 m2 in 2020 and 4.1 ind./10 m2 in 2022. At station IB, the crab density was 4.97—4.11 ind./10 m2 in 2016—2020 and decreased to 0.68 ind./10 m2 in 2022 (Fig. 6).
Analyses of the size of the snow crab also showed significant changes in the snow crab population since its appearance in the fjord (Table 5). The first findings date back to 2014 at station IB when all specimens collected (21 ind.) were juveniles ranging in size from 4.5 to 5.2 mm. In 2016, the crabs became slightly larger, with the population consisting of 24% juvenile specimens ranging in size from 8 to 11 mm (mean 9.8 mm) and 76% adults represented by immature specimens (females and males) ranging in size from 11 to 38 mm (mean 15.1 mm). In 2018, crabs reached an average size of 31.5 mm and the first mature females were found. From 2018 to 2022, the crabs continued to grow, with the average size increasing to 45.7 mm (Fig. 6). The modal value increased with time, but no new juveniles were found. Thus, throughout the study period, we observed one main-size cohort that appeared in the bay in 2014–2015 and gradually matured and increased in size, with no new recruitment. It is also noteworthy that the average size of individuals at station IB is slightly larger than at station S (Fig. 6). A figure showing the size distribution of the snow crab in Sigsbee trawl samples in the different years is provided in Supplementary Fig S2.
Discussion
Following the snow crab invasion, we have observed changes in benthic communities at various levels. While changes in macrobenthos (grab samples) were relatively weak, changes in megabenthos community structure (trawl samples and video observations) were visually clear and apparent (Fig. 5). Video observations demonstrated a decline in the abundance of the dominant ophiuroids, not a single specimen was found in 2022 (Fig. 7). Additionally, a steady downward trend in the density of the snow crab was also observed. In trawl catches, the abundance of crabs decreased over time while their linear size increased (Fig. 6). Regarding macrobenthos, analyses of grab samples showed a change in subdominants in the Golfingia margaritacea community (the local extinction of Bathyarca glacialis and the increase in Ennucula tenuis abundance) at Station S and E. tenuis community dynamics with fluctuations in abundance of subdominant polychaetes at Station IB (Fig. 7). Changes in taxonomic structure at both stations were accompanied by a decline in total abundance and biomass by 2022 (Fig. 3). However, species diversity at station IB did not change significantly and was generally lower than at station S.
The observed shifts in the benthic communities cannot be attributed solely to environmental factors. While there are clear climate trends related to ice coverage, changes in ice extent and the lengthening of the ice-free period for the Arctic in general (Comiso et al. 2017; Kwok 2018; Serreze, Stroeve 2015; Wang, Danilov 2022), at regional scale, especially on temporal scales comparable to the duration of benthic invertebrates' life cycles, these patterns are not obvious. No consistent trends in ice melting were observed during the observation period in the north-western part of the sea along the coast of the Novaya Zemlya Archipelago and Blagopoluchiya Bay. Instead, we observed rotation between cold years with ice melting in early August and warm years with ice melting in June (Fig. 2). Abiotic parameters, such as turbidity, temperature, and nutrients, also did not exhibit any directional changes (Supplementary Table S1).
Here we postulate that changes in the benthic fauna of Blagopoluchiya Bay coincide with the establishment and subsequent development of the snow crab population. The settlement dynamics of snow crabs in the Kara Sea varied depending on introduction routes, environmental conditions, and prey resources (Zalota et al. 2018). The first findings of the snow crab in different parts of the Kara Sea date back to 2012–2014, but already in 2020, the area where the snow crab had spread was about 179 thousand km2, i.e. about 20% of the Kara Sea basin, and the density of large adult crabs in the western part of the sea was 100–500 ind./km2 (Bakanev, Pavlov 2020). The fjords of the Novaya Zemlya Archipelago were exposed to the crab invasion to varying degrees. In 2016–2018, we observed high densities of Ch. opilio in Blagopoluchiya Bay, reaching 21 ind/10 m2. In Tsivolki Bay, the highest density was 1.5 ind/10 m2, and in Oga Bay, 0.1 ind/10 m2 (Zalota et al. 2019). Both bays are about 150 km to the south of Blagopoluchiya Bay. Meanwhile, in the western part of the Kara Sea, crab densities rarely exceeded 1 ind/10 m2, except for the Kara Gate region, where they could reach 1.7—5.5 ind/10 m2 (Zalota et al. 2020, 2019). This is probably due to the geographical location of Blagopoluchiya Bay, the northernmost bay of the Novaya Zemlya Archipelago and the first bay on the way of possible larval migration from the Barents Sea. In certain years, the area is the first to become ice-free and the warm waters of the Barents Sea cause an increase in temperature. At the same time, ice cover and melt waters may still be present in the southern areas along the archipelago coast, and desalination, melting, and cold water may prevent the development and migration of crab larvae. It was shown that water temperature and salinity significantly affect the survival and development of snow crab larvae (Yamamoto et al. 2014, 2015).
The benthic communities that inhabited Blagopoluchiya Bay were described in detail prior to the crab invasion (Udalov et al. 2021, 2016). It was shown that macrofaunal biodiversity and quantitative characteristics decrease along the environmental gradient from the sea to the inner part of the bay due to the increase in terrigenous and glacial runoff, which is consistent with the patterns described in other studies of Arctic glacial fjords (Holte, Gulliksen 1998; Jordà Molina et al. 2019; Syvitski et al. 1989; Wlodarska-Kowalczuk, Pearson 2004). In the inner part of the bay, communities dominated by Ophiopleura borealis—Ennucula tenuis were found in deep areas or Portlandia arctica in shallow depths. The most abundant species in this specific biotope were surface and subsurface deposit feeders, omnivores, and predators tolerant of inorganic matter sedimentation and associated unstable and stressful conditions (Włodarska-Kowalczuk et al. 1998). These included polychaetes Scoletoma fragilis, Tharyx sp., Cossura longocirrata, Micronephthys minuta, and Scoloplos acutus, and bivalves such as Ennucula tenuis, Mendicula ferruginosa and Portlandia arctica. Communities on the sill were more diverse with a high proportion of large suspension-feeding bivalves such as Astarte spp. and Bathyarca glacialis along with the brittle stars Ophiacantha bidentata, soft corals Gersemia fruticosa and sipuncula Golfingia margaritacea. In addition, the rocky areas around the sill were characterized by a high density of epifaunal species that are not typical elsewhere in the Bay (Udalov et al. 2016). The predation of the snow crab likely caused the observed changes. Before the crab's appearance, large invertebrate predators were practically non-existent in the benthic communities of the Kara Sea shelf. In both the Barents and Kara Seas, Ch. opilio feeds on a wide range of benthic organisms. An analysis of the frequency of occurrence of food components revealed that bivalves, polychaetes, crustaceans, and ophiuroids were the most common organisms found in the crab's stomachs (Chuchukalo et al. 2011; Pavlov 2007; Zakharov et al. 2018, 2020). An analysis of the food spectra in the stomachs of the snow crab in Blagopoluchiya Bay showed that this species behaves as a non-selective epibenthic feeder with elements of cannibalism, consuming both plant and animal food (Burukovsky et al. 2022). In terms of abundance, the stomachs were dominated by brittle stars (more than one-third of the virtual food lump volume), detritus and plant debris, followed by bivalves and polychaetes. Although the crabs consumed almost all available macroepibenthos, feeding on deeply burrowing infauna species (for instance Sipuncula) was not observed (Burukovsky et al. 2022). This feeding intensity, combined with the high mobility of the snow crab and the lack of top-down control, led to the observed changes in benthic community structure.
It is important to note that there was only one prominent size cohort in Blagopoluchiya Bay during the study period (Zalota et al. 2019). Despite the larvae presence in the water column (Lipukhin et al. 2024), no newly settled juveniles have been observed since 2016 (Table 5). Crab densities were highest from 2016 to 2018, followed by a decrease in abundance and an increase in individual size. As crabs grew, their prey spectrum should shift over time, depending on the size of available prey. These size-dependent differences in prey accessibility were previously described in the Saint Lawrence Estuary (Lovrich, Sainte-Marie 1997) and in the northern Bering Sea, where snow crab diets included most of the prey species dominant in the study area (Kolts et al. 2013). As crabs mature, they display a preference for larger prey, such as hard-shelled bivalves, gastropods, and large polychaetes, which necessitate greater handling ability and claw strength. Juvenile crabs prefer softer prey that is easily accessible, such as amphipods and small bivalves with thin or incompletely calcified shells (Kolts et al. 2013; Lovrich, Sainte-Marie 1997; Squires 2003).
In Blagopoluchiya Bay, the main changes in the benthos were observed in 2018, when the crab reached a large abundance and size (30–40 mm carapace width) that it could feed on large molluscs and brittle stars. In the absence of large prey, the crab probably resorted to non-selective feeding. As a result, the abundance of common polychaete species such as Scoletoma fragilis, Tharyx sp. (Cirratulidae), Maldane sarsi and Aglaophamus malmgreni declined significantly by 2022, with some species disappearing completely. The number of crabs has also decreased considerably, possibly due to limited food resources. At the same time, we observed varying rates and intensities of changes in benthic communities across different areas of the bay, which can mainly be attributed to the initial abundance of crabs during their invasion. This may be explained by the fact that snow crabs exhibit different habitat preferences depending on their sex and life stage. For example, in Newfoundland waters, immature snow crabs have been found to inhabit relatively shallow rocky areas, and as they grow, they tend to move into deeper muddy habitats (Comeau et al. 1998). Studies from the eastern part of the Barents Sea indicate that this migration to deeper areas is predominantly the behavior of males, while females and juveniles typically occupy shallower areas (Zakharov et al. 2020). In the present study at station S at the 60 m depth sill and near the shallow zone of heterogeneous and rocky substrates with rich epifauna, the density of crabs at the beginning of the invasion (2016—2018) turns out to be significantly (4–5 times) higher than at station IB. Related to the above, the abundance of common macrobenthic species decreased significantly at station S by 2018, while at station IB, this occurred later, by 2020.
At both stations, the communities appeared to be highly sensitive to changes, which is most likely because the fjord communities are constantly under stressful conditions. The invasion of the snow crab into a system where a top predator was previously absent strongly destabilizes the system. Although there is little evidence of the impact of the snow crab in other invaded areas, similar processes have been previously observed for the red king crab invasion in the Barents Sea fjords. In particular, the loss of large prey species and changes in community composition have been reported (Falk-Petersen et al. 2011). Recent studies from Porsangerfjord (Fuhrmann et al. 2015), Kobbholmfjord (Oug et al. 2018), Kola Bay (Pavlova 2008), Motovsky Bay (Anisimova et al. 2005), Varangerfjord (Oug et al. 2011, 2018) confirmed that benthic quantitative characteristics decreased and species structure changed in the areas invaded by red king crabs. First of all, the abundance of soft-bottom epifauna and infauna decreased significantly. For example, in the Varangerfjord, echinoderms, immobile burrowing and tube-dwelling polychaetes, and most bivalves were reduced, while some small-sized polychaetes and small bivalves had increased (Oug et al. 2011).
Data from the Barents Sea show that the areas with the highest densities of snow crabs correspond to areas with high biomass of macrobenthos (Zakharov et al. 2018, 2020), there were described eleven communities, dominated mainly by bivalves, and infaunal bivalves are a preferred prey group for snow crabs (Gebruk et al. 2021; Zakharov et al. 2020). However (Holte et al. 2022) reported a low P/B ratio for this group, i.e. high infaunal biomass was attributed to organisms with low turnover rates (long-lived species with higher individual biomass), suggesting that infaunal bivalves may be more vulnerable to predation effects despite having relatively high stock biomass.
As for the top-down control, predators may commonly regulate decapods' abundance. Cod and thorny skate have been found to predate on snow crab in its natural habitat the Northeastern Atlantic (Chabot et al. 2008; Robichaud 1991). In the Barents Sea, the snow crab has rapidly increased within the cod diet since 2003 (Holt et al. 2021). However, unlike the Barents Sea, where the cod has the potential to regulate the snow crab population, no species predate on crabs in the Kara Sea except the crab itself. Cannibalism was proposed as one of the regulating factors controlling abundance in snow crab populations representing a major source of post-settlement mortality (Lovrich, Sainte-Marie 1997).
In addition to the interactions described above, decapods can indirectly modify habitat by preying on bivalves, leaving empty substrate for other organisms to settle using density-dependent mechanisms (Boudreau, Worm 2012). Our study provides evidence of such indirect effects through the mass settlement of the bivalves Ennucula tenuis and Portlandia arctica. Additional data on species-specific interactions are needed to clarify the mechanisms of cascading effects due to crab invasion. However, exclusion experiments supported the hypothesis of strong ecosystem effects of decapods on benthic fauna, such as molluscs and polychaetes, even though no trophic cascade was observed (Quijón, Snelgrove 2005). Therefore, the establishment of an abundant large predator in the benthic community has a significant impact on the entire ecosystem through both trophic and non-trophic interactions.
Data Availability
Data will be made available on request.
References
Anisimov IM, Zalota AK, Lesin AV et al (2023) Aspects of the towed underwater vehicle “videomodule”: utilization for surveying underwater objects and benthic fauna. Oceanology 63:733–743. https://doi.org/10.1134/s0001437023050028
Anisimova N, Berenboim B, Gerasimova O, Manushin I, Pinchukov M (2005) On the effect of red king crab on some components of the Barents Sea ecosystem. Report PINRO
Antipova T, Semenov V (1989) Composition and distribution of benthos in the southwestern areas of the typical marine water of the Kara Sea. Ecology bioresources of the Kara Sea. Kolskij nauchnyj tsentr Rossijskoj akademii nauk, Apatity 127–137
Azovsky AI, Kokarev VN (2019) Stable but fragile: long-term dynamics of arctic benthic macrofauna in Baydaratskaya Bay (the Kara Sea). Polar Biol 42:1307–1322. https://doi.org/10.1007/s00300-019-02519-y
Azovsky AI, Naumov AD, Savchenko ON (2023) Long-term dynamics of subarctic intertidal macrofauna: common trends and the role of local environment. Estuaries Coasts 46:740–756. https://doi.org/10.1007/s12237-023-01177-y
Bakanev SV, Pavlov VA (2020) Prospects of snow crab (Chionoecetes opilio) fisheries in the Kara Sea. Commercial fishery 21:332
Boudreau SA, Worm B (2012) Ecological role of large benthic decapods in marine ecosystems: a review. Mar Ecol Prog Ser 469:195–213. https://doi.org/10.3354/meps09862
Burukovsky RN, Syomin VL, Zalota AK et al (2022) Food spectra of Snow crabs (Chionoecetes opilio (O. Fabricius, 1788) (decapoda, oregoniidae), non-indigenous species of the Kara Sea. Oceanology 61:964–975. https://doi.org/10.1134/s0001437021060205
Carlton JT, Schwindt E (2024) The assessment of marine bioinvasion diversity and history. Biol Invasions 26:237–298. https://doi.org/10.1007/s10530-023-03172-7
Chabot D, Sainte-Marie B, Briand K et al (2008) Atlantic cod and snow crab predator–prey size relationship in the gulf of St. Lawrence. Can Mar Ecol Prog Ser 363:227–240. https://doi.org/10.3354/meps07384
Chuchukalo V, Nadtochiy V, Fedotov P et al (2011) Feeding habits and some aspects of biology of snow crab opilio (Chionoecetes opilio) in Chukchi Sea. Izvestia TINRO 167:197–206
Clarke K, Gorley RJP-E, Plymouth (2015) PRIMER Version 7.0. 12: User Manual/Tutorial.
Comeau M, Robichaud G, Starr M et al (1998) Mating of snow crab chionoecetes opilio (Ο. Fabricius, 1788) (decapoda, majidae) in the fjord of bonne bay. Nfld Crustac 71:925–941. https://doi.org/10.1163/156854098X00932
Comiso JC, Meier WN, Gersten R (2017) Variability and trends in the Arctic Sea ice cover: results from different techniques. J Geophys Res: Oceans 122:6883–6900. https://doi.org/10.1002/2017JC012768
Dahle G, Sainte-Marie B, Mincks SL et al (2022) Genetic analysis of the exploited snow crab (Chionoecetes opilio) in the Barents Sea—possibilities of origin. ICES J Mar Sci 79:2389–2398. https://doi.org/10.1093/icesjms/fsac172
Dolgov A, Benzik A. (2016). Snow crab as a component of fish diet in the Barents Sea. Snow crab Chionoecetes opilio in the Barents and Kara Seas, Ed. by K. M. Sokolov. PINRO Press. Murmansk
Eriksen E, Benzik AN, Dolgov AV et al (2020) Diet and trophic structure of fishes in the Barents Sea: The Norwegian-Russian program “Year of stomachs” 2015–Establishing a baseline. Prog Oceanogr 183:102262. https://doi.org/10.1016/j.pocean.2019.102262
Falk-Petersen J, Renaud P, Anisimova N (2011) Establishment and ecosystem effects of the alien invasive red king crab (paralithodes camtschaticus) in the Barents Sea–a review. ICES J Mar Sci 68:479–488. https://doi.org/10.1093/icesjms/fsq192
Filatova Z, Zenkevich L (1957) Quantitative distribution of the bottom fauna of the Kara Sea. Trudy Vsesoyuznogo Gidrobiologicheskogo Obshchestva 8:3–67
Fuhrmann MM, Pedersen T, Ramasco V et al (2015) Macrobenthic biomass and production in a heterogenic subarctic fjord after invasion by the red king crab. J Sea Res 106:1–13. https://doi.org/10.1016/j.seares.2015.09.003
SV Galkin, AK Zalota, AA Udalov, et al. (2021). Evaluating of the population density of alien crabs Chionoecetes opilio in the Kara Sea using underwater towed vehicle VIDEOMODULE Modern methods and equipment in oceanological research. Proceedings of the XVII All-Russian Scientific and Technical Conference. P.P.Shirshov Institute of Oceanology, Russian Academy of Sciences. 2:207–210
Gebruk A, Zalota AK, Dgebuadze P et al (2021) Trophic niches of benthic crustaceans in the Pechora Sea suggest that the invasive snow crab Chionoecetes opilio could be an important competitor. Polar Biol 44:57–71. https://doi.org/10.1007/s00300-020-02775-3
Gerasimova AV, Filippova NA, Lisitsyna KN et al (2021) Current state of macrobenthos in the southwestern Kara Sea. Cont Shelf Res. https://doi.org/10.1016/j.csr.2021.104452
Holt RE, Hvingel C, Agnalt A-L et al (2021) Snow crab (chionoecetes opilio), a new food item for North-east Arctic cod (Gadus morhua) in the Barents Sea. ICES J Mar Sci 78:491–501. https://doi.org/10.1093/icesjms/fsaa168
Holte B, Gulliksen B (1998) Common macrofaunal dominant species in the sediments of some north Norwegian and Svalbard glacial fjords. Polar Biol 19:375–382. https://doi.org/10.1007/s003000050262
Holte B, Fuhrmann MM, Tandberg AHS et al (2022) Infaunal and epifaunal secondary production in the Barents Sea, with focus on snow crab (Chionoecetes opilio) prey resources and consumption. ICES J Mar Sci 79:2524–2539. https://doi.org/10.1093/icesjms/fsac192
Jordà Molina È, Silberberger MJ, Kokarev V et al (2019) Environmental drivers of benthic community structure in a deep sub-arctic fjord system. Estuar, Coast Shelf Sci. https://doi.org/10.1016/j.ecss.2019.05.021
Kolts JM, Lovvorn JR, North CA et al (2013) Effects of body size, gender, and prey availability on diets of snow crabs in the northern Bering Sea. Mar Ecol Prog Ser 483:209–220. https://doi.org/10.3354/meps10292
Kozlovskiy V, Chikina M, Kucheruk NV et al (2011) Structure of the macrozoobenthic communities in the southwestern Kara Sea. Oceanology 51:1012–1020. https://doi.org/10.1134/S0001437011060087
Kuzmin S, Akhtarin S, Meni D (1999) The first findings of the snow crab Chionoecetes opilio (decapoda, majiidae) in the Barents Sea. Can Trans Fish Aquat Sci 5667:1–5
Kwok R (2018) Arctic sea ice thickness, volume, and multiyear ice coverage: losses and coupled variability (1958–2018). Environ Res Lett 13:105005. https://doi.org/10.1088/1748-9326/aae3ec
Lepikhina PP, Basin AB, Kondar DV et al (2022) Changes in the quantitative characteristics of macro- and meiobenthos in the Blagopoluchiya Bay from 2013 to 2020 (novaya zemlya, the Kara Sea). Oceanology 62:198–206. https://doi.org/10.1134/s0001437022020114
Lipukhin EV, Zalota AK, Mishin AV et al (2024) The origin of the snow crab Chionoecetes opilio in the Kara Sea. Oceanology 64:278–287. https://doi.org/10.1134/s0001437024020085
Lovrich GA, Sainte-Marie B (1997) Cannibalism in the snow crab, Chionoecetes opilio (O. Fabricius)(brachyura: majidae), and its potential importance to recruitment. J Exp Mar Biol Ecol 211:225–245. https://doi.org/10.1016/S0022-0981(96)02715-3
McCune B, Grace J, Urban DJO (2002) MJM Software Design. Analysis of ecological communities, Gleneden Beach
Miller AW, Ruiz GM (2014) Arctic shipping and marine invaders. Nat Clim Chang 4:413–416. https://doi.org/10.1038/nclimate2244
Mokievsky VO, Tsetlin AB, Sergienko LA et al (2016) Ecological atlas. Kara Sea Arctic research center, Moscow (in Russian)
Ogata T (1973) Studies on the population biology of the edible crab, Chionoecetes opilio O. Fabricius in the Japan Sea region. J Mar Sci Men 5:27–33
Oug E, Cochrane SK, Sundet JH et al (2011) Effects of the invasive red king crab (paralithodes camtschaticus) on soft-bottom fauna in Varangerfjorden, Northern Norway. Mar Biodivers 41:467–479. https://doi.org/10.1007/s12526-010-0068-6
Oug E, Sundet JH, Cochrane SKJ (2018) Structural and functional changes of soft-bottom ecosystems in northern fjords invaded by the red king crab (paralithodes camtschaticus). J Mar Syst 180:255–264. https://doi.org/10.1016/j.jmarsys.2017.07.005
Parsons TR (2013) A manual of chemical & biological methods for seawater analysis. Elsevier, Amsterdam, The Netherlands
Pavlov VA (2007) Feeding of snow crab Chionoecetes opilio (Fabricius, 1788) in the Barents Sea. Trudy VNIRO 147:99–107
Pavlova L (2008) Effect of juvenile red king crabs on zoobenthos in Kola Bay (Barents Sea). Springer, Doklady Biological Sciences. https://doi.org/10.1134/S0012496608050098
Pronin A (2017) Collection and representation data video movies of bottom surface in oceanological investigations with underwater towing equipment. Int J Appl Fundam Res. https://doi.org/10.17513/mjpfi.11980
Quijón PA, Snelgrove PV (2005) Differential regulatory roles of crustacean predators in a sub-arctic, soft-sediment system. Mar Ecol Prog Ser 285:137–149. https://doi.org/10.3354/meps285137
Renaud PE, Sejr MK, Bluhm BA et al (2015) The future of Arctic benthos: Expansion, invasion, and biodiversity. Prog Oceanogr 139:244–257. https://doi.org/10.1016/j.pocean.2015.07.007
Ricciardi A, Blackburn TM, Carlton JT et al (2017) Invasion science: a horizon scan of emerging challenges and opportunities. Trends Ecol Evol 32:464–474. https://doi.org/10.1016/j.tree.2017.03.007
Robichaud D (1991) Differential selection of crab chionoecetes opilio and hyas spp. as prey by sympatric cod gadus morhua and thorny skate Raja radiata. Fish Bull 89:669–680
Ruiz GM, Hewitt C. (2009). Latitudinal Patterns of Biological Invasions in Marine Ecosystems A Polar Perspective. Smithsonian at the Poles Contributions to International Polar Year Science edited by Krupnik, Igor, Lang, Michael A., and Miller, Scott E
Serreze MC, Stroeve J (2015) Arctic sea ice trends, variability and implications for seasonal ice forecasting. Philos Trans A Math Phys Eng Sci 37310.1098/rsta.2014.0159
Sirenko BI, Vassilenko SV, Sirenko BI. (2008). Crabs (crustacea, decapoda, brachyura) of the Chukchi Sea. Fauna and zoogeography of the Chukchi Sea. In: Sirenko BI, ed. Explorations of fauna of the sea
Slizkin A (1998) Distribution of snow crabs of the genus Chionoecetes and their habitat in the northern part of the Pacific Ocean. Canada Institute for Scientific and Technical Information
Sokolov AM (2014) The introduction of snow crab into the Kara Sea An example of further species adaptive strategy in Russian Arctic area (on the results of PINRO researches in 2013). Rybnoe Hozyaistvo 6:63–67
Spiridonov VA, Zalota AK (2017) Understanding and forecasting dispersal of non-indigenous marine decapods (Crustacea: Decapoda) in East European and North Asian waters. J Mar Biol Assoc UK 97:591–611. https://doi.org/10.1017/s0025315417000169
Squires HJ (2003) Stomach contents of snow crab (Chionoecetes opilio decapoda brachyura) from the Northeast Newfoundland Shelf. J Northwest Atl Fish Sci. https://doi.org/10.2960/J.v32.a2
Squires HJ (1990) Decapod Crustacea of the Atlantic coast of Canada. Canada: Department of the Environment Fisheries and Marine Service
Syvitski JP, Farrow GE, Atkinson RJA, Moore PG, Andrews JT (1989) Baffin Island fjord macrobenthos: bottom communities and environmental significance. Arctic. 42(3):232–247
Udalov AA, Vedenin AA, Simakov MI (2016) Benthic fauna of Blagopoluchiya Bay (novaya zemlya archipelago, Kara Sea). Oceanology 56:655–665. https://doi.org/10.1134/s0001437016050143
Udalov A, Chikina M, Chava A et al (2021) Patterns of benthic communities in arctic fjords (Novaya Zemlya Archipelago, Kara Sea): Resilience vs. Fragility. Front Ecol Evolut. https://doi.org/10.3389/fevo.2021.777006
Vedenin AA, Galkin SV, Kozlovskiy VV (2015) Macrobenthos of the ob bay and adjacent Kara Sea shelf. Polar Biol 38:829–844. https://doi.org/10.1007/s00300-014-1642-3
Wang Q, Danilov S (2022) A synthesis of the upper Arctic Ocean circulation during 2000–2019: understanding the roles of wind forcing and sea ice decline. Front Mar Sci 9:863204. https://doi.org/10.3389/fmars.2022.863204
Ware C, Berge J, Jelmert A et al (2015) Biological introduction risks from shipping in a warming Arctic. J Appl Ecol 53:340–349. https://doi.org/10.1111/1365-2664.12566
Włodarska-Kowalczuk M, Węsławski J, Kotwicki L (1998) Spitsbergen glacial bays macrobenthos–a comparative study. Polar Biol 20:66–73. https://doi.org/10.1007/s003000050277
Włodarska-Kowalczuk M, Renaud PE, Węsławski JM et al (2012) Species diversity, functional complexity and rarity in Arctic fjordic versus open shelf benthic systems. Mar Ecol Prog Ser 463:73–87. https://doi.org/10.3354/meps09858
Wlodarska-Kowalczuk M, Pearson TH (2004) Soft-bottom macrobenthic faunal associations and factors affecting species distributions in an Arctic glacial fjord (kongsfjord, spitsbergen). Polar Biol 27:155–167. https://doi.org/10.1007/s00300-003-0568-y
Yamamoto T, Yamada T, Fujimoto H et al (2014) Effects of temperature on snow crab (Chionoecetes opilio) larval survival and development under laboratory conditions. J Shellfish Res 33:19–24. https://doi.org/10.2983/035.033.0103
Yamamoto T, Yamada T, Fujimoto H et al (2015) Effects of salinity on snow crab (Chionoecetes opilio) larval survival and development under laboratory conditions. J Shellfish Res 34:499–504. https://doi.org/10.2983/035.033.0103
Zakharov D, Manushin I, Strelkova N et al (2018) Diet of the snow crab in the Barents Sea and macrozoobenthic communities in the area of its distribution. Trudy VNIRO 172:70–90
Zakharov DV, Manushin IE, Nosova TB et al (2020) Diet of snow crab in the Barents Sea and macrozoobenthic communities in its area of distribution. ICES J Mar Sci 78:545–556. https://doi.org/10.1093/icesjms/fsaa132
Zalota AK, Spiridonov VA, Vedenin AA (2018) Development of snow crab Chionoecetes opilio (crustacea: decapoda: oregonidae) invasion in the Kara Sea. Polar Biol 41:1983–1994. https://doi.org/10.1007/s00300-018-2337-y
Zalota AK, Zimina OL, Spiridonov VA (2019) Combining data from different sampling methods to study the development of an alien crab Chionoecetes opilio invasion in the remote and pristine Arctic Kara Sea. PeerJ 7:e7952. https://doi.org/10.7717/peerj.7952
Zalota A, Spiridonov V, Galkin S et al (2020) Population structure of alien snow crabs (Chionoecetes opilio) in the Kara Sea (trawl and video sampling). Oceanology 60:83–88. https://doi.org/10.1134/S0001437020010257
Zimina OL (2014) Finding the snow crab Chionoecetes opilio (O. Fabricius, 1788) (Decapoda: Majidae) in the Kara Sea. Russ J Mar Biol 40:490–492. https://doi.org/10.1134/s1063074014060224
Acknowledgements
The study was carried out within the framework of the program “Ecosystems of the Siberian Arctic Seas”. The authors would like to thank M.V. Flint—the leader and organizer of the expeditions and the crews of R/V “Professor Shtokmann” and “Akademik Mstislav Keldysh”. We greatly appreciate the help from the following colleagues: Andrey Vedenin (identifications of invertebrates) and Tina Molodtsova (graphical representation of animals). Finally, we are grateful for the valuable comments on the manuscript by the anonymous reviewers as well as the Editor in Chief, Evangelina Schwindt; their helpful suggestions have greatly improved our manuscript.
Funding
This research was funded by Russian Science Foundation (grant No №23–27-00028).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, data analysis, interpretation and visualization were performed by Udalov A. and Chikina M.; investigations and acquisition of data were performed by Anisimov I., Basin A., Borisenko G., Chikina M., Simakov M., Shchuka S., Udalov A.; project management was performed by Chikina M.; the first version of the manuscript was written by Udalov A. and Chikina M., all authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Udalov, A.A., Anisimov, I.M., Basin, A.B. et al. Changes in benthic communities in Blagopoluchiya Bay (Novaya Zemlya, Kara Sea): the influence of the snow crab. Biol Invasions 26, 3455–3473 (2024). https://doi.org/10.1007/s10530-024-03388-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-024-03388-1