Abstract
Deployment of mouse carcasses laced with acetaminophen has become a common management tool to control invasive brown tree snakes (Boiga irregularis; BTS) on Guam. Additionally, anticoagulant rodenticides may be used to control invasive rats (Rattus spp.) if their populations increase due to predator release in the wake of BTS eradication. However, there has been little research examining how scavengers on Guam could be incidentally exposed to toxicants by scavenging carcasses of animals that die from these population control strategies. Furthermore, there is a limited understanding of how the proliferation of invasive species on Guam has influenced the composition of the scavenger community. We investigated these topics by examining scavenger consumption of mouse, rat, and BTS carcasses on Guam in both a coastal and upland site during the wet (May–Aug 2016) and dry season (Jan–Apr 2017). We documented carcass consumption by 9 species, which scavenged 48% of carcasses. Interactions between season, habitat, and carcass type influenced probability of scavenging, and appeared to be driven by consumption by the two main scavenger species, BTS and cane toads (Rhinella marina), both of which are invasive on Guam. Baiting programs should consider the potential for toxin exposure to land crabs (Coenobita spp., Birgus latro), native species that scavenged at every combination of carcass type, habitat, and season. Overall, 60% of scavenging events were attributed to species considered pests that are recent introductions to Guam. Invasive species on Guam are the primary scavengers of small vertebrate carrion, suggesting a substantial role in trophic dynamics that extends beyond predation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Islands contain a disproportionate share of global biodiversity (Courchamp et al. 2014). Invasive species, however, threaten the viability of island wildlife populations and constitute the primary cause of island vertebrate extinctions (Bellard et al. 2016; Doherty et al. 2016). In the years following World War II, ships salvaging war material from islands in the western Pacific inadvertently introduced brown tree snakes (Boiga irregularis; BTS) to Guam, the largest of the Mariana Islands (Richmond et al. 2015; Rodda et al. 1992). The dietary flexibility of BTS coupled with a lack of predators spawned dramatic population increases following their introduction (Savidge 1988). By 1970, BTS occurred across the entire island with densities reaching as high as 100 individuals per ha (Rodda et al. 1992). Today, BTS densities on Guam (8.3–22.4 snakes/ha) are among the highest recorded for any reptile worldwide (Smith et al. 2016).
The ecological consequences of BTS on Guam have been profound (Clark et al. 2017). Predation by BTS has reduced abundances of many vertebrates, resulting in the extinction or extirpation of 20 native bird species and several endemic lizard species (Wiles 1987; Wiles et al. 2003). As a result, there are currently portions of Guam that are largely devoid of native vertebrates (Rodda and Savidge 2007). Extirpations of these species have in turn suppressed key ecosystem functions such as pollination and seed dispersal with subsequent effects to plant community structure (Mortensen et al. 2008; Rodda and Savidge 2007). Additionally, the cost of repairing infrastructure damages caused by BTS on Guam and investment in eradication efforts collectively exceeds 4 million US dollars annually (Pimental 2007). Based on their biological and economic impact, BTS thus rank among the world’s most damaging invasive species (Invasive Species Specialist Group 2021).
Despite their success at invading Guam and decimating native wildlife, BTS possess vulnerabilities that have been exploited in eradication efforts. Acetaminophen is toxic to BTS and an 80-mg dose is fatal to the majority of individuals (Siers et al. 2021). BTS readily scavenge mouse carcasses, and deployment of neonatal mouse carcasses laced with acetaminophen is an effective management tool, reducing the presence of BTS by 80–85% (Clark and Savarie 2012). By deploying baits aerially, BTS populations can be reduced over large inaccessible areas (Siers et al. 2019). Using oral toxicants is also more cost effective than other techniques such as trapping and has been determined to pose little risk to other wildlife (Clark et al. 2012). As a result, control efforts implementing toxic baits are part of an integrated approach to BTS management on Guam (Engeman et al. 2018).
Although the ecological benefits can be immense, invasive species reduction can also have unpredictable negative consequences termed ‘surprise effects’ (Courchamp et al. 2003). In the case of predators such as BTS, a potential ‘surprise effect’ is an increase in other invasive species resulting from predator release (Courchamp et al. 2003). In New Zealand, for example, invasive Pacific rat (Rattus exulans) populations increased after feral cat (Felis cattus) removal, leading to greater native bird mortality as the result of greater rat predation (Rayner et al. 2007). Invasive Pacific rats and black rats (Rattus rattus) constitute important prey items for BTS on Guam, raising the potential for increased populations of these invasive rodents following BTS reduction (Fritts and Rodda 1998). Therefore, control efforts for BTS may in turn necessitate control of rats, which is often accomplished with baits containing anticoagulant rodenticides such as brodifacoum (Pitt et al. 2011).
In contrast to techniques such as trapping where the carcass is contained, broadcast applications of toxic baits can result in the diffusion of carcasses across the landscape. Such widespread carrion availability may have consequences for other wildlife because many vertebrates are facultative scavengers and invertebrates often scavenge as well (DeVault et al. 2003). Thus, there is the potential for incidental exposure of nontarget wildlife to toxicants as the result of their scavenging behavior. This may occur through primary ingestion when scavengers consume toxic baits intended for invasive species, or through secondary ingestion by scavenging carcasses of animals that have ingested toxic baits (Lujan et al. 2010). Although BTS are arboreal, their carcasses frequently come to rest on the ground after dying from toxicant ingestion, where they are potentially available to many vertebrate scavengers (Smith et al. 2016). There has been little research, however, into the extent to which invasive species management on Guam may expose scavengers to toxicants. Although many native vertebrates have been extirpated from the island, invertebrate scavengers such as hermit crabs are still abundant and could experience toxicant exposure. Understanding patterns in carcass consumption is important for predicting the risk of invasive species management programs to native fauna as the result of scavenging. Additionally, with many native animals having been extirpated, investigating carrion consumption would provide insight into trophic interactions on an island where native animals have been largely replaced by invasive species (Abernethy et al. 2016).
Scavenging is widespread among vertebrates, but its prevalence is impacted by a variety of factors including climatic conditions, habitat type, location, and carcass species (Beasley et al. 2015; Olson et al. 2016; Smith et al. 2017; Turner et al. 2017). As such, consumption of toxic bait carcasses (defined as both baits laced with toxicants [i.e., mice] and animals dying from the consumption of toxic baits [i.e., rats and BTS]) may vary based on season, as well as the habitat where carcasses are located. During warmer seasons, vertebrate scavenging is often reduced because the rapid activity by microbes results in a narrow window during which carcasses are palatable (DeVault et al. 2004; Hill et al. 2018b; Turner et al. 2017). Habitats with less understory may also have greater scavenging activity due to increased visual detection and accessibility of carcasses by scavengers (Selva et al. 2005; Turner et al. 2017). Additionally, carcass type may influence scavenging patterns because some scavengers avoid particular species of carrion (Butler-Valverde et al. 2022; Moleón et al. 2017; Olson et al. 2016).
To investigate the potential for toxicant exposure to scavengers resulting from toxic bait carcass consumption and further understand scavenging dynamics on Guam, we experimentally placed mouse, rat, and BTS carcasses in two habitats (coastal and upland) in both wet and dry seasons. We tested the hypothesis that carcass type, habitat, and season influenced scavenging behavior. We predicted that carcasses would be consumed more quickly, and a greater proportion of carcasses would be scavenged in the coastal compared to the upland site due to less understory vegetation. We also predicted that more carcasses would be consumed in the dry season compared to the wet season due to lower temperatures prolonging the period of carcass availability.
Methods
Study site
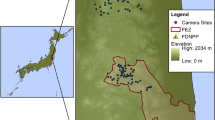
We carried out this study on Guam, a 543 km2 island in the western Pacific Ocean. Trials were conducted in two 0.25 km2 research sites (coastal and upland) located ~ 3.2 km apart on Andersen Air Force Base (13.587° N, 144.924° E, Fig. 1). The coastal site was an old growth, historical coconut (Cocos nucifera) plantation with primarily coconut palms in the overstory and little understory vegetation. The site was located within 1 km of the ocean and elevation ranged from 22 to 41 m. The upland site was in a secondary mixed limestone forest-plateau approximately 150-m above sea-level. Vegetation was characterized by a dense forest of short trees with relatively open canopy that permitted extensive understory growth. Common plant species in the undergrowth included Ficus prolixa, Guamia mariannae, Aglaia mariannensis and Vitex parviflora. We carried out this study during both a wet season (May–August 2016) and a dry season (January–April 2017). Mean monthly temperature was 29.1 °C during the wet season and 27.8 °C during the dry season. Mean monthly precipitation during the wet season was 235.9 mm and during the dry season was 159.9 mm (NOAA 2021).
Experimental design
We carried out 1376 trials (i.e., unique carcasses monitored). Trials were run for 5 days because we estimated that all carcasses would be fully scavenged or decomposed (defined as the time when there appeared to be no edible flesh remaining) over this duration given the size of the carcasses and environmental conditions (Abernethy et al. 2016). In the dry season we performed 11 consecutive sessions of concurrent trials (60 trials/session), and during the wet season we performed 10 sessions (72 trials/session). During each session, trials were evenly divided among the three carcass types and both habitats. After each session, we moved the cameras and removed all traces of carcass remains to reduce the likelihood of scavengers becoming habituated to carcass locations. Following the 6th session in each season, there was a 7-day pause before beginning the 7th session to decrease the chances of scavengers becoming habituated to carcasses along transects (Abernethy et al. 2016).
In each habitat, we established 5 transects located 50-m inland from roads that measured 1 km in length and were spaced 0.1 km apart. Carcasses were placed in randomized sequences along each transect such that active cameras were buffered by 150 m from other active cameras on a given transect and a minimum of 100 m from cameras on neighboring transects (Abernethy et al. 2016). These distances were based on spatial constrains within the focal habitats. For each trial, we baited the camera with either a mouse, rat, or BTS carcass. Mice were used to examine potential scavenging of acetaminophen-laced mouse carcasses by non-target animals. Rats were used to examine impacts on scavengers of potential rat increases following BTS eradication. BTS carcasses were used to examine scavenger consumption and subsequent toxin ingestion of these carcasses resulting from control efforts. Dark colored, frozen mice (13.0–18.0 g) and rats (40.0–165.0 g) were acquired from RodentPro.com (RodentPro.com, LLC, Inglefield, IN) and PerfectPrey.com (PerfectPrey.com LLC, Loxahatchee, FL), respectively. We acquired frozen BTS carcasses from lethal control operations on Guam conducted by the US Department of Agriculture. BTS carcasses varied in size (28.0–270.0 g) depending on availability of euthanized captures at the time of use. All carcasses were stored frozen and thawed to ambient temperature prior to deployment. The masses of these carcasses were equivalent to what would be available to scavengers following BTS management efforts.
We manipulated carcass placement along transects to mimic the natural conditions where carcasses would be likely to occur, standardizing carcass placements across different canopy covers. Small mammal carcasses often are obstructed from view and are not visually conspicuous (DeVault and Rhodes Jr 2002; DeVault et al. 2003). Therefore, we placed half of the mouse and rat carcasses on the ground uncovered and the other half inside burrows. BTS carcasses were placed uncovered (92%), inside a burrow (4%), or approximately 1.5–2.0 m in a tree (4%) to imitate typical carcass locations of BTS that die from acetaminophen consumption (Smith et al. 2016). We constructed burrows above ground using available natural materials such as rocks, vegetation and sticks because site restrictions prevented us from digging burrows in the ground.
We set carcasses 0.3–1 m away from the camera lens and programmed cameras (Reconyx infrared PC900 Hyperfire™ cameras, Reconyx, Inc., Holmen, WI) to record a single image every 6 min as well as a burst of 5 images when triggered by motion and/or by an external trigger. We equipped cameras paired with rodent carcasses with a water resistant, external trigger system (FB Engineering, Hilo, HI) constructed with a 0E2385HX-snap action switch (ZF Electronics Corporation, Pleasant Prairie, WI) and base, made from acrylic sheets, such that when the rodent was removed from the switch, the camera would be triggered (DeVault et al. 2004) (Fig S1). To prevent rodents from falling off external switches, we loosely tied them with thread as to not inhibit scavengers from taking carcasses. When we returned to carcass sites at the end of every trial, we estimated the percent of flesh that remained to determine whether the carcass had been fully scavenged. We analyzed camera images to document which scavengers consumed carcasses. We also determined the amount of time elapsed between carcass placement and when the carcass had been fully scavenged.
In addition to the scavenging trials, we conducted a series of decomposition trials to assess decomposition of carcasses in the absence of vertebrate scavenging. The goal of this was to understand how long carcasses would be available to vertebrate scavengers. In these trials, we placed a wire cage with 2.54 cm by 1.00 cm mesh over the carcass to prohibit access by vertebrates. There were 10 trials of each of the 3 carcass types per season per habitat for 120 total decomposition trials. We programed cameras to take a single picture every 15 min until decomposition occurred. Decomposition trials were conducted concurrently with scavenging trials but outside the standard spatial buffer of the scavenging trials so that they would remain independent of the scavenging experiments.
Statistical analysis
We performed all analyses in R version 4.0.4 (R Core Team 2022). Our scavenging models contained combinations of these three independent variables with the following levels: season (wet or dry); habitat (coastal or upland); and carcass type (mouse, rat, or BTS). We did not include carcass mass as a variable because each of the three carcass types differ in mass (mouse < rat < BTS), so carcass mass is already accounted for to some extent. Additionally, variation within carcass types is not pertinent to our objectives as these carcasses are meant to represent types that would potentially be available as the result of BTS control efforts, and in reality there would be a range of weights within a carcass type as we have in our study.
We examined probability of carcasses being scavenged using a generalized linear model with binomial distribution with the response variable scavenged or not scavenged. We also examined consumption by the four most common scavengers: BTS, cane toads (Rhinella marina), monitor lizards (Varanus indicus), and hermit crabs (Coenobita spp.). Invertebrate scavengers included ants and flies, but we did not document them frequently enough to analyze their scavenging behavior. The response for each model was presence or absence of that species scavenging at each carcass. For the BTS model, we excluded BTS carcasses because they were only scavenged by BTS on one occasion. We assessed species richness of scavengers (i.e., number of species scavenging a carcass) using a generalized linear model with Poisson distribution, including only carcasses that were scavenged.
We analyzed time to carcass consumption using an accelerated failure time Weibull regression with the package ‘survival’ (Therneau et al. 2020), which is commonly used to study carcass persistence in scavenging studies (Bispo et al. 2013). Stratified Kaplan–Meier curves indicated that the model was appropriate for our data (Zhang 2016). Time to consumption was calculated as the time elapsed between carcass placement and complete consumption by scavengers. Carcasses that were not fully scavenged were right-censored at 5 days. We used the same model to assess time to decomposition for the decomposition trials.
For the overall probability of scavenging model, species richness model, and scavenging survival model, we considered the 3-way interaction and all possible two-way interactions. If the 3-way interaction was significant, we evaluated the 2-way interactions organized by the third factor (Abernethy et al. 2016; Turner et al. 2017). For the species-specific scavenging models, we examined main effects only due to the smaller frequency of scavenging across every treatment level. For the decomposition survival model, we included carcass type, season, and habitat as in the other models, but also incorporated the carcass weight. We also only assessed main effects in this model due to the smaller sample sizes.
We ranked all possible model combinations using sample size corrected AICc, selecting that with the lowest AICc as the best performing model and making inferences from this top model. When carcass type as a main effect or interactions were included in the top model, we assessed pairwise comparisons with α = 0.05 to determine differences using the package ‘emmeans’ (Lenth et al. 2019) for the generalized linear models and the package ‘rarredd/rawr’ for the survival models (Redd 2021).
Results
We determined carcass fate in 1305 of the trials (94%), of which 622 carcasses were scavenged (48%; Table 1). During the dry season, 46% of carcasses were scavenged, and 49% were scavenged during the wet season. Fifty two percent of carcasses in coastal habitat were scavenged and 44% were scavenged in the upland site. Across carcass species, 63% of mouse, 49% of rat, and 30% of snake carcasses were scavenged. We were able to identify all scavengers in 580 (93%) of the trials. We observed scavenging by 9 species (Table 2), the most common of which were cane toads, documented at 217 carcasses (16.6% of all carcasses), followed by BTS (161 carcasses, 12.3%), monitor lizards (108 carcasses, 8.2%), and hermit crabs (100 carcasses, 7.6%). The remaining species were all documented at 1% or less of carcasses.
Our top model for probability of scavenging included all three main effects and the carcass × habitat and habitat × season interactions (AICc = 1681.9, LL = − 832.91, wi = 0.68; Table S1). There was no difference in probability of scavenging between coastal and upland for mouse (adjusted p value = 0.139) or snake carcasses (adjusted p value = 0.349), but rat carcasses were approximately 20% more likely to be scavenged in coastal than upland habitat (adjusted p value < 0.0001, Fig. 2). In coastal habitat, there was no difference in probability of scavenging between seasons (adjusted p value = 0.335), but carcasses were 11% less likely to be scavenged in the dry season compared to the wet season in upland habitat (adjusted p value = 0.004, Fig. 2).
Panel A Predicted proportion of mouse, rat, and brown tree snake carcasses scavenged across a coastal and upland site in Guam (2016–2017). Panel B Predicted proportion of carcasses (mouse, rat and brown tree snake combined) scavenged in a coastal and upland site on Guam during the wet season (May–August 2016) and dry season (January–April 2017)
The top model for BTS scavenging included only habitat (AICc = 741.8, LL = − 368.89, wi = 0.43; Table S2) and predicted a 25% increase in frequency of scavenging in the coastal compared to the upland site. The top model for cane toad scavenging included all three variables (AICc = 1004.8, LL = − 497.40, wi = 1.00; Table S2). The model predicted a 12% increase in cane toad scavenging in wet season compared to dry season, and 18% increase in scavenging in the upland compared to the coastal site. Cane toads were more likely to consume mice than snakes or rats (p < 0.001 for both cases), but there was no difference in frequency of rat and snake consumption (p = 0.959).
For monitor lizards, the top model included habitat and season (AICc = 644.3, LL = − 319.112 wi = 0.53; Table S2). It predicted a 17% increase in scavenging in upland compared to coastal habitat, and an increase of 6% in the dry season compared to the wet season. The top model for hermit crab scavenging included carcass and habitat (AICc = 684.9, LL = − 338.42, wi = 0.42; Table S2) and predicted a 7% increase in scavenging by hermit crabs in the upland compared to the coastal site. Although the top model included carcass type, there was no difference in frequency of carcass consumption based on pairwise comparisons (p values: mouse–rat = 0.965; mouse–snake = 0.083; rat–snake = 0.133).
Mean species richness overall was 1.07; one scavenger species was documented at 580 carcasses, 2 species at 40 carcasses, and 3 species at 2 carcasses. The top model for species richness was the null model (AICc = 1305.6, LL = − 651.79, wi = 0.43; Table S3). For the scavenging survival analysis, the top model included all three independent variables as well as the carcass × habitat and habitat × season interactions (AICc = 2691.0, LL = − 1336.43, wi = 0.57; Table S4). For mouse carcasses, there was no difference in time to carcass consumption between habitats (p = 0.321), but time to consumption was shorter in the coastal habitat compared to the upland habitat for snakes (p = 0.029) and rats (p < 0.001; Fig. 3). Although the habitat × season interaction was included in our top model, pairwise comparisons indicated no significant differences in time to consumption between seasons in the coastal (p = 0.441) or upland habitats (p = 0.121).
After removing 7 trials due to camera failure, our sample size for the decomposition analysis consisted of 113 trials. The median time to decomposition was 3.96 days (range 1.66–8.64 days; Fig. 4). The top model for the decomposition survival analysis included carcass type and season (AICc = 1083.7, LL = − 536.55, wi = 0.42; Table S5). Predicted median time to decomposition was 3.67 ± 0.18 days for carcasses in the wet season compared to 4.48 ± 0.21 days for carcasses in the dry season (Fig. 5). There was no difference in time to decomposition between mouse and rat carcasses (p = 0.118), but both types decomposed more quickly than BTS carcasses (p < 0.001 both cases; Fig. 5).
Discussion
Invasive species consume the majority of small vertebrate carrion on Guam. Similarly, invasive vertebrates were the primary scavengers of carcasses in Hawaii (Abernethy et al. 2016), and invasive red foxes on Australian beaches outcompeted native birds for carrion to become the dominant scavenger (Brown et al. 2015). Scavenging behavior can play an important role in vertebrate range expansions, possibly contributing to invasion success by increasing dietary niche breadth (Blázquez et al. 2016; Clergeau and Yésou 2006). Invasive species sometimes fail to establish because they are unable to exploit novel prey, but this pitfall may be somewhat circumvented by utilization of carrion in destination landscapes (Mack et al. 2000; Preiszner et al. 2020; Sih et al. 2010).
As they become the dominant scavengers, these invasive species likely have a substantial effect on the detrital pathway of food webs because more energy is transferred via scavenging links than predation links (Wilson and Wolkovich 2011). Invasive species can affect the rate of scavenging and competitively exclude native species from an important resource (Brown et al. 2015). Scavenging by invasive animals likely also influences the spatial distribution of nutrients across the landscape with subsequent effects to plant communities (Abernethy et al. 2016). Thus, the impacts of invasive species on food webs and ecological functions may be much greater when their scavenging behavior is considered. On Guam, BTS and cane toads probably have such an effect considering they collectively were responsible for over half of all scavenging events. Furthermore, the dominance of scavenging by these invasive animals on Guam also raises the possibility of positive feedback loops contributing to invasion meltdown, wherein greater abundance of these species increases their carrying capacity via provisioning of resources in the form of carrion (Abernethy et al. 2016; Bump et al. 2009; Simberloff and Von Holle 1999).
Invasive species were largely responsible for the relationships between scavenging and habitat, carcass type and season on Guam. As a result, variation in scavenging by these species across treatments accounts for the lack of full support for our predictions. BTS, for example, are trapped in the upland site but not the coastal site, resulting in densities at the coastal site that are nearly three times higher (Smith et al. 2016). These differences in abundance of a major scavenger likely contributed to the interaction between habitat and carcass, with a pronounced decline in scavenging of rat carcasses, but not other carcass types, in the upland site. Cane toads, the other major scavenger, consumed rats less frequently and thus did not compensate for reduced scavenging of rats by BTS in the upland site. Additionally, reduced activity by BTS in the upland site may have led to scavenging by a greater variety of species due to reduced competition, as all three of the other major scavengers (i.e., cane toads, hermit crabs, and monitor lizards) individually consumed more carcasses in the upland than in the coastal site.
Cane toads were introduced to Guam in the 1930s to control agricultural pests (Rodda et al. 1991) and were the most common scavenger overall, documented at every combination of carcass type, habitat, and season. The interaction between habitat and season can be attributed to cane toads because their activity levels increase with greater amounts of rainfall, which led to increased scavenging during the wet season in the upland site (Muller et al. 2018; Schwarzkopf and Alford 2002). The lack of increase in scavenging in the coastal site by cane toads during the wet season adds further support for a dampening effect of BTS on activity by other scavengers in this habitat. More frequent consumption of mice compared to rats and BTS by cane toads was likely the result of mouse carcasses sizes, which were more similar in size to cane toads’ prey and likely easier to consume (Reed et al. 2007).
Scavenging behavior of BTS also influenced the lower overall consumption of BTS carcasses compared to rats and mice because BTS scavenged conspecifics on just one occasion. A review of scavenging by snakes found only one instance of scavenging a conspecific (DeVault and Krochmal 2002). Aversion to intraspecific scavenging (i.e., scavenging carrion of the same species) has also been reported for birds and mammals (Butler-Valverde et al. 2022; Muñoz-Lozano et al. 2019; Olson et al. 2016). This behavior may be adaptive as species that are more closely related are more likely to be affected by the same pathogens (Moleón et al. 2017). As such, the risk of contracting disease may outweigh the benefits of scavenging, particularly when other food sources are available (Moleón et al. 2017). The minimal potential for secondary ingestion of acetaminophen by BTS through scavenging indicates that toxic baiting programs must rely on primary bait consumption because scavenging of conspecifics is virtually nonexistent.
More frequent scavenging by monitor lizards during the dry season may reflect a seasonal increase in carrion consumption due to decreased availability of their major prey items such as gastropods and earthworms (Dryden 1965; Wikramanayake and Dryden 1988). Additionally, the slower decomposition times of carcasses in the dry season may have resulted in a larger window in which carcasses were palatable to monitor lizards. Consumption of mouse and BTS carcasses could pose a risk to monitor lizards because acetaminophen can kill juveniles of this species (Dorr et al. 2016). However, scavenging at these carcass types was relatively infrequent, documented at 6% of mouse and 9% of snake carcasses. The ecological consequences of potential monitor lizard mortality from toxic bait carcass consumption are unclear, as the status of the species as native or introduced has been debated, and they have been subject to various removal efforts on Guam (Weijola et al. 2020). Because there was no seasonal difference in carcass consumption by BTS, concentrating baiting efforts during the wet season may reduce the potential for nontarget mortality of monitor lizards without sacrificing the efficiency of control efforts.
Hermit crabs scavenged extensively and were the only species besides cane toads to scavenge at every treatment level. Hermit crabs can survive 80-mg doses of acetaminophen (Johnston et al. 2002), but thresholds for toxicity have not been established, nor have potential sublethal effects of acetaminophen exposure. Brodifacoum even at high doses is not toxic to crabs and is likely to pose little risk to invertebrates generally (Pain et al. 2000). Widespread scavenging by hermit crabs suggests the potential for substantial toxicant exposure, which warrants further study to understand their effects on hermit crabs, especially considering their important ecological role on Pacific islands (Nigro et al. 2017; Page and Willason 1983). Additionally, although they were less common scavengers, potential effects of carcass consumption on coconut crabs is also an area where additional research is needed, as coconut crabs are economically and culturally important on Guam.
Scavenging behavior of hermit crabs could expose other vertebrates to toxicants through trophic transfer. Birds in particular are susceptible to poisoning by brodifacoum and hermit crabs are consumed by some critically endangered species found on Guam, such as Mariana crows (Corvus kubaryi) (Faegre et al. 2020). Native avifauna could therefore be at risk of exposure to toxicants through predation of scavengers. Additionally, with more than half of carcasses not scavenged by vertebrates or crabs, there was likely considerable scavenging by insects, which can also store brodifacoum in their tissues and could serve as a pathway for toxicant exposure (Brooke et al. 2013). Indeed, we sometimes observed skinks consuming maggots that were present on carcasses. Brodifacoum does not generally persist in the environment or invertebrate tissues for long periods of time, so overall risk of secondary poisoning may be low (Brooke et al. 2013; Wegmann et al. 2019), but it has been implicated in the mortality of nestling birds (Masuda et al. 2014). The potential transfer of toxicants from scavengers to predators warrants further investigation to ensure toxic bait carcasses do not incidentally harm species that occupy higher trophic levels.
Scavenging activity at less than half of all carcasses was likely in part the result of the small carcass sizes and environmental conditions. Carcasses of larger species such as large ungulates compared to small birds or rodents are more likely to be detected and consumed as the result of increased visual and chemical cues (Moleón et al. 2015; Turner et al. 2017). Because carcass size is positively associated with scavenger species richness, the small vertebrate carcasses also resulted in low species richness, similar to other studies using small vertebrate carcasses (DeVault et al. 2011; Inger et al. 2016; Sebastián-González et al. 2020; Turner et al. 2017). The small carcass sizes we used also enabled scavengers to often consume the entire carcass at once, resulting in largely similar patterns between frequency of carcass consumption and time to carcass removal.
Additionally, the high temperatures of our study site led to rapid carcass decomposition, resulting in a narrow time frame in which carrion is palatable to most scavengers (DeVault et al. 2004; Hill et al. 2018a; Turner et al. 2017). Our decomposition trials indicated that on average, carcasses decomposed within 4 days, but in some cases as little as 1 day. For many facultative scavengers, carrion consumption is more prevalent when other resources are scarce, and alternative food availability may have led to low scavenging rates (Jędrzejewski and Jędrzejewska 1992; Jędrzejewski et al. 1993). Indeed, we captured images of wild pigs consuming coconuts in the background, but not scavenging the carcass. Rapid carcass decomposition coupled with availability of other food resources thus contributed to the observed consumption rates of small vertebrate carcasses on Guam.
Scavenging by invasive animals on Guam raises potential issues with invasive species control efforts. Toxic baiting programs could increase food availability to invasive vertebrates, but successful eradication efforts would produce a single pulse of carrion that may not benefit invasive species over the long term. However, if management practices result in reductions of BTS but not complete eradication, continued control efforts could provide a more long-term source of carrion for invasive species, albeit at a lower quantity. The dominance of cane toad scavenging in the site with lower BTS densities suggests that they would be the species most likely to benefit from increases in carrion availability resulting from BTS eradication. Thus, there is the potential for the elimination of one invasive species to benefit another via reduced competition for resources, another ‘surprise effect’ of invasive species eradication (Courchamp et al. 2003). However, since BTS are arboreal and cane toads are not, there is not complete trophic overlap because many BTS prey are inaccessible to cane toads. As such, the functional response of cane toads due to BTS eradication is unclear. Nevertheless, an integrated approach to invasive species management on Guam will likely necessitate strategies to reduce cane toad abundances (e.g., Muller, Schwarzkopf 2018) to ensure that BTS eradication does not result in trading one invasive species for another.
Data availability
The datasets generated during the current study are included in its supplementary information files.
References
Abernethy EF, Turner KL, Beasley JC et al (2016) Carcasses of invasive species are predominantly utilized by invasive scavengers in an island ecosystem. Ecosphere 7:e01496
Beasley JC, Olson ZH, DeVault TL (2015) Ecological role of vertebrate scavengers. In: Benbow ME, Tomberlin JK, Tarone AM (eds) Carrion ecology, evolution, and their applications. CRC Press, Boca Raton
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. Biol Lett 12:20150623
Bispo R, Bernardino J, Marques TA et al (2013) Modeling carcass removal time for avian mortality assessment in wind farms using survival analysis. Environ Ecol Stat 20:147–165
Blázquez MC, Delibes-Mateos M, Vargas JM et al (2016) Stable isotope evidence for Turkey vulture reliance on food subsidies from the sea. Ecol Indic 63:332–336
Brooke MdL, Cuthbert R, Harrison G et al (2013) Persistence of brodifacoum in cockroach and woodlice: implications for secondary poisoning during rodent eradications. Ecotoxicol Environ Saf 97:183–188
Brown MB, Schlacher TA, Schoeman DS et al (2015) Invasive carnivores alter ecological function and enhance complementarity in scavenger assemblages on ocean beaches. Ecology 96:2715–2725
Bump JK, Peterson RO, Vucetich JA (2009) Wolves modulate soil nutrient heterogeneity and foliar nitrogen by configuring the distribution of ungulate carcasses. Ecology 90:3159–3167
Butler-Valverde M, DeVault TL, Beasley JC (2022) Trophic interactions at avian carcasses. Do scavengers feed on vulture carrion? Food Webs 31:e00230
Clark L, Savarie PJ (2012) Efficacy of aerial broadcast baiting in reducing brown treesnake numbers. Hum Wildl Interact 6:212–221
Clark L, Savarie PJ, Shivik JA et al (2012) Efficacy, effort, and cost comparisons of trapping and acetaminophen-baiting for control of brown treesnakes on Guam. Hum Wildl Interact 6:222–236
Clark L, Clark C, Siers S (2017) Brown tree snakes: methods and approaches for control. In: Pitt WC, Beasley J, Witmer GW (eds) Ecology and management of terrestrial vertebrate invasive species in the United States. CRC Press, Boca Raton, pp 107–134
Clergeau P, Yésou P (2006) Behavioural flexibility and numerous potential sources of introduction for the sacred ibis: Causes of concern in western Europe? Biol Invasions 8:1381–1388
Courchamp F, Chapuis J-L, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383
Courchamp F, Hoffmann BD, Russell JC et al (2014) Climate change, sea-level rise, and conservation: keeping island biodiversity afloat. Trends Ecol Evol 29:127–130
DeVault TL, Krochmal AR (2002) Scavenging by snakes: an examination of the literature. Herpetologica 58:429–436
DeVault TL, Rhodes OE Jr (2002) Identification of vertebrate scavengers of small mammal carcasses in a forested landscape. Acta Theriol 47:185–192
DeVault TL, Rhodes OE Jr, Shivik JA (2003) Scavenging by vertebrates: behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 102:225–234
DeVault TL, Brisbin IL, Rhodes OE Jr (2004) Factors influencing the acquisition of rodent carrion by vertebrate scavengers and decomposers. Can J Zool 82:502–509
DeVault TL, Olson ZH, Beasley JC et al (2011) Mesopredators dominate competition for carrion in an agricultural landscape. Basic Appl Ecol 12:268–274
Doherty TS, Glen AS, Nimmo DG et al (2016) Invasive predators and global biodiversity loss. Proc Natl Acad Sci USA 113:11261–11265
Dorr BS, Clark CS, Savarie PJ (2016) Aerial application of Acetaminophen-treated baits for control of Brown treesnakes. US Department of Defense, Alexandria
Dryden G (1965) The food and feeding habits of Varanus indicus on Guam. Micronesica 2:73–76
Engeman RM, Shiels AB, Clark CS (2018) Objectives and integrated approaches for the control of brown tree snakes: an updated overview. J Environ Manag 219:115–124
Faegre SK, Nietmann L, Hannon P et al (2020) Age-related differences in diet and foraging behavior of the critically endangered Mariana Crow (Corvus kubaryi), with notes on the predation of Coenobita hermit crabs. J Ornithol 161:149–158
Fritts TH, Rodda GH (1998) The role of introduced species in the degradation of island ecosystems: a case history of Guam. Annu Rev Ecol Syst 29:113–140
Hill JE, DeVault TL, Beasley JC et al (2018a) Effects of vulture exclusion on carrion consumption by facultative scavengers. Ecol Evol 8:2518–2526
Hill JE, DeVault TL, Beasley JC et al (2018b) Roads do not increase carrion use by a vertebrate scavenging community. Sci Rep 8:16331
Inger R, Per E, Cox DT et al (2016) Key role in ecosystem functioning of scavengers reliant on a single common species. Sci Rep 6:1–5
Invasive Species Specialist Group (2021) Gobal invasive species database. IUCN. http://www.iucngisd.org/gisd/100_worst.php
Jędrzejewski W, Jędrzejewska B (1992) Foraging and diet of the red fox Vulpes vulpes in relation to variable food resources in Biatowieza National Park. Pol Ecography 15:212–220
Jędrzejewski W, Zalewski A, Jędrzejewska B (1993) Foraging by pine marten Martes martes in telation to food resources in Białowieża National Park, Poland. Acta Theriol 38:405–426
Johnston J, Savarie P, Primus T et al (2002) Risk assessment of an acetaminophen baiting program for chemical control of brown tree snakes on Guam: evaluation of baits, snake residues, and potential primary and secondary hazards. Environ Sci Technol 36:3827–3833
Lenth R, Singmann H, Love J et al (2019) Package ‘emmeans’
Lujan DT, Vice DS, Guerrero JP et al (2010) Rodent eradication on Cocos Island, Guam: integrating wildlife damage management, resort operations, and non-target concerns. In: Proceedings of the vertebrate pest conference
Mack RN, Simberloff D, Mark Lonsdale W et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Masuda BM, Fisher P, Jamieson IG (2014) Anticoagulant rodenticide brodifacoum detected in dead nestlings of an insectivorous passerine. N Z J Ecol 38:110–115
Moleón M, Sánchez-Zapata JA, Sebastián‐González E et al (2015) Carcass size shapes the structure and functioning of an african scavenging assemblage. Oikos 124:1391–1403
Moleón M, Martínez-Carrasco C, Muellerklein OC et al (2017) Carnivore carcasses are avoided by carnivores. J Anim Ecol 86:1179–1191
Mortensen HS, Dupont YL, Olesen JM (2008) A snake in paradise: disturbance of plant reproduction following extirpation of bird flower-visitors on Guam. Biol Conserv 141:2146–2154
Muller BJ, Schwarzkopf L (2018) Relative effectiveness of trapping and hand-capture for controlling invasive cane toads (Rhinella marina). Int J Pest Manag 64:185–192
Muller BJ, Cade BS, Schwarzkopf L (2018) Effects of environmental variables on invasive amphibian activity: using model selection on quantiles for counts. Ecosphere 9:e02067
Muñoz-Lozano C, Martín-Vega D, Martínez-Carrasco C et al (2019) Avoidance of carnivore carcasses by vertebrate scavengers enables colonization by a diverse community of carrion insects. PLoS ONE 14:e0221890
Nigro KM, Hathaway SA, Wegmann AS et al (2017) Stable isotope analysis as an early monitoring tool for community-scale effects of rat eradication. Restor Ecol 25:1015–1025
NOAA (2021) Climate data online. In: National oceanic and atmospheric administration. http://www.ncdc.noaa.gov/cdo-web/datasets. Accessed 4 Jan 2021
Olson ZH, Beasley JC, Rhodes OE Jr (2016) Carcass type affects local scavenger guilds more than habitat connectivity. PLoS ONE 11:e0147798
Page H, Willason S (1983) Feeding activity patterns and carrion removal by terrestrial hermit crabs at Enewetak Atoll, Marshall Islands. Pac Sci 37:151–155
Pain DJ, Brooke MdL, Finnie J et al (2000) Effects of brodifacoum on the land crab of Ascension Island. J Wildl Manag 64:380–387
Pimental D (2007) Environmental and economic costs of vertebrate species invasions into the United States. Manag Vertebr Invasive Species 38:1–8
Pitt WC, Driscoll LC, Sugihara RT (2011) Efficacy of rodenticide baits for the control of three invasive rodent species in Hawaii. Arch Environ Contam Toxicol 60:533–542
Preiszner B, Czeglédi I, Boros G et al (2020) Scavenging behaviour and size-dependent carcass consumption of the black bullhead (Ameiurus melas). J Fish Biol 97:1113–1119
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rayner MJ, Hauber ME, Imber MJ et al (2007) Spatial heterogeneity of mesopredator release within an oceanic island system. Proc Natl Acad Sci USA 104:20862–20865
Redd R (2021) raredd/rawr version 1.0.1
Reed RN, Bakkegard KA, Desy GE et al (2007) Diet composition of the invasive cane toad (Chaunus marinus) on Rota, Northern Mariana Islands. Pac Conserv Biol 13:219–222
Richmond JQ, Wood DA, Stanford JW et al (2015) Testing for multiple invasion routes and source populations for the invasive brown treesnake (Boiga irregularis) on Guam: implications for pest management. Biol Invasions 17:337–349
Rodda GH, Savidge JA (2007) Biology and impacts of pacific island invasive species. 2. Boiga irregularis, the brown tree snake (Reptilia: Colubridae) 1. Pac Sci 61:307–324
Rodda G, Fritts T, Reichel J (1991) The distributional patterns of reptiles and amphibians in the Mariana Islands. Micronesica 24:195–210
Rodda GH, Fritts TH, Conry PJ (1992) Origin and population growth of the brown tree snake, Boiga irregularis, on Guam. Pac Sci 46:46–57
Savidge JA (1988) Food habits of Boiga irregularis, an introduced predator on Guam. J Herpetol 22:275–282
Schwarzkopf L, Alford RA (2002) Nomadic movement in tropical toads. Oikos 96:492–506
Sebastián-González E, Morales‐Reyes Z, Botella F et al (2020) Network structure of vertebrate scavenger assemblages at the global scale: drivers and ecosystem functioning implications. Ecography 43:1143–1155
Selva N, Jędrzejewska B, Jędrzejewski W et al (2005) Factors affecting carcass use by a guild of scavengers in european temperate woodland. Can J Zool 83:1590–1601
Siers S, Pitt W, Eisemann J et al (2019) In situ evaluation of an automated aerial bait delivery system for landscape-scale control of invasive brown treesnakes on Guam. In: Veitch C, Clout MN, Martin A, Russell JC, West C et al (eds) Island invasives: scaling up to meet the challenge. IUCN, Gland, pp 348–355
Siers SR, Goetz SM, Volsteadt RM et al (2021) Evaluating lethal toxicant doses for the largest individuals of an invasive vertebrate predator with indeterminate growth. Manag Biol Invasions 12:476–494
Sih A, Bolnick DI, Luttbeg B et al (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: Invasional meltdown? Biol Invasions 1:21–32
Smith JB, Turner KL, Beasley JC et al (2016) Brown tree snake (Boiga irregularis) population density and carcass locations following exposure to acetaminophen. Ecotoxicology 25:1556–1562
Smith JB, Laatsch LJ, Beasley JC (2017) Spatial complexity of carcass location influences vertebrate scavenger efficiency and species composition. Sci Rep 7:1–8
Therneau T, Lumley T, Atkinson E et al (2020) Survival: survival analyses. R package version 3-2-3
Turner K, Abernethy E, Conner LM et al (2017) Abiotic and biotic factors modulate carrion fate and vertebrate scavenging communities. Ecology 98:2413–2424
Wegmann A, Howald G, Kropidlowski S et al (2019) No detection of brodifacoum residues in the marine and terrestrial food web three years after rat eradication at Palmyra Atoll, Central Pacific. In: Veitch C, Clout MN, Martin A, Russell JC, West C et al (eds) Island Invasives: scaling up to meet the challenges. IUCN, Gland, pp 600–603
Weijola V, Vahtera V, Koch A et al (2020) Taxonomy of micronesian monitors (Reptilia: Squamata: Varanus): endemic status of new species argues for caution in pursuing eradication plans. R Soc Open Sci 7:200092
Wikramanayake ED, Dryden G (1988) The reproductive ecology of Varanus indicus on Guam. Herpetologica 44:338–344
Wiles GJ (1987) The status of fruit bats on Guam. Pac Sci 41:148–157
Wiles GJ, Bart J, Beck RE Jr et al (2003) Impacts of the brown tree snake: patterns of decline and species persistence in Guam’s avifauna. Conserv Biol 17:1350–1360
Wilson EE, Wolkovich EM (2011) Scavenging: how carnivores and carrion structure communities. Trends Ecol Evol 26:129–135
Zhang Z (2016) Parametric regression model for survival data: Weibull regression model as an example. Ann Transl Med 4:1–8
Funding
This study was conducted via agreement 14-7439-1097 between the USDA National Research Center and Naval Facilities Engineering Command, Marianas. Funding was also provided by the Department of Energy Office of Environmental Management under Award number DE-EM0004391.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hill, J.E., Turner, K.L., Smith, J.B. et al. Scavenging dynamics on Guam and implications for invasive species management. Biol Invasions 25, 1845–1858 (2023). https://doi.org/10.1007/s10530-023-03014-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03014-6