Abstract
Biological invasions drive biodiversity loss and ecosystem change on tropical islands. However, we know little about the implications of species losses on the functional structure of both resident and novel communities. Herein, we examined the potential effect of a non-native palm species, Pinanga coronata, on the taxonomic and functional assemblages of understory plant species in a Fijian rainforest. We predicted that competition from this invasive species would lead to trait convergence according to the competitive hierarchy hypothesis. Using a trait-based approach, we sampled plant communities in 280 plots across a gradient of P. coronata densities. We measured five functional traits, including height and leaf traits related to nutrient acquisition. We found that an increase in P. coronata density is strongly correlated with a decrease in taxonomic diversity (i.e., about − 50% for species richness and − 33% for Shannon diversity index) and a decrease in functional richness. Community-weighted mean values of traits of resident species (i.e., excluding P. coronata) converged toward competitive strategies such as higher leaf nitrogen content (LNC), lower carbon-to-nitrogen (C:N) ratios and leaf dry matter content (LDMC), a pattern that is significantly non-random for LDMC and C:N. This study demonstrates that P. coronata might act as a strong biotic filter responsible for species loss and functional changes. Our findings suggest that in response to increasing competition with this invasive plant, resident and novel plant communities shift toward less diverse and more competitive assemblages. Nevertheless, the intensity of this filtering is habitat dependent (e.g. less filtering effect under mahogany trees). Lastly, changes in resource acquisition strategies (mainly nutrient-based) in particular in low nutrient status of rainforest soils, could lead to long-term impacts on tree regeneration, in turn causing large-scale changes in ecosystem properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical oceanic islands harbor many invasive alien plants (IAP) when compared to mainland areas (Russell et al. 2017; van Kleunen et al. 2015). In particular, Pacific islands are known to be highly vulnerable to biological invasions (Denslow et al. 2009; Kueffer et al. 2010; Keppel et al. 2014; Mueller-Dombois 2006). There exists a large body of work regarding invasive plant species on Pacific islands (e.g., Baruch et al. 1999; Daehler et al. 2004; Penuelas et al. 2010; Westerband et al. 2021). However, the rapid spread of IAP still requires great attention, especially in South Pacific islands (Meyer 2014). There, IAP-induced changes to ecosystems tend to readily manifest, given that the native biota is disharmonic, the functional redundancy is low, and food webs are simple. Recorded impacts on the biodiversity of Pacific island rainforests (e.g., by Dyer et al. 2018; Minden et al. 2010a) include the replacement of native understory species and the interruption of life cycles of foundation species (Boehmer et al. 2013; Keppel et al. 2014; Minden et al. 2010b; Mueller-Dombois and Boehmer 2013). Another notable impact of IAP is the alteration of habitat (e.g., a change in biogeochemical soil properties and microclimate) and ecosystem functioning (e.g., nutrient cycling and productivity) brought about as a consequence of species replacement and diversity loss (Pyšek et al. 2012, 2020; Vilà et al. 2011).
Plant functional traits are defined as any morphological, physiological, or phenological features measurable in individual plants that potentially affect their fitness (Violle et al. 2007) or their environment (Lavorel and Garnier 2002). Trait-based ecology has rapidly grown over the last decades and now offers a mechanistic means of predicting consequences of environmental changes upon plant communities (Violle et al. 2007; Adler et al. 2014). A large body of literature shows how plant traits relate to or influence ecological functions. Therefore, these traits have been used to estimate and understand the diversity of ecological and functional strategies of co-existing species (Burton et al. 2020). For example, species loss or extinction from a community may have scant consequences on functional diversity in the case of a high redundancy between species. Alternatively, it may lead to functional diversity erosion in the case of high functional complementarity between species (Cadotte et al. 2011; Hejda et al. 2017). Therefore, a loss of functional diversity may be observed regardless of taxonomic diversity variation, demonstrating the importance of considering the functional aspects of biodiversity (Tecco et al. 2010). Furthermore, community‐weighted mean (CWM, i.e., the average trait value of all species) of the traits of species within a community may help reveal how the functional structure of native communities is altered by invasion (Pavoine et al. 2017), while also giving an insight into the consequences of invasion for ecological processes and ecosystem functions (Grime 1998; Muscarella and Uriarte 2016). Previous studies comparing native and invasive plants have revealed that the latter tend to have fast resource acquisition strategies according to the leaf economics spectrum described by Wright et al. (2004). More precisely, IAP have higher trait values for height, specific leaf area (SLA), and leaf nitrogen content (LNC) and lower trait values for leaf dry matter content (LDMC; Montesinos 2022) than most native plants. These traits make invasive species particularly competitive. Conversely, native species in invaded communities often display more conservative strategies (Jakobs et al. 2004; Liao et al. 2008; Van Kleunen et al. 2010).
Many hypotheses consider competition a major mechanism that determines the success of an invasion (Cleland et al. 2011; Vilà and Weiner 2004). A strongly competitive IAP can act as a biotic filter influencing native species. According to the limiting similarity theory (Abrams 1983; MacArthur and Levins 1967), competition from IAP can limit or even exclude native species that share similar traits. In turn, IAP competition is expected to have little or no impact on native species with dissimilar traits (Gallien and Carboni 2017; MacDougall et al. 2009). According to this hypothesis, through competitive interactions, an IAP can potentially lead to trait divergence in the resident community (i.e., overdispersion). This mechanism promotes the coexistence of different strategies and may limit the reduction in functional richness of the resident community following an invasion (Fried et al. 2019; Hejda et al. 2017). Alternatively, the competitive hierarchy hypothesis in the context of plant invasion, stipulates that the spread of a highly competitive invasive plant will exclude less competitive species while native species with a similar or a higher fitness will coexist with the invader (Fried et al. 2019; Hejda et al. 2017). As a consequence, IAP can diminish both species richness and functional richness (Dı́az and Cabido 2001; Ebeling et al. 2018) as well as trait convergence (Sodhi et al. 2019). However, such changes in native assembly rules and native coexistence (trait convergence/divergence) as a consequence of an invasion, are generally, under-studied (however, see Sodhi et al. 2019). Instead, most studies on functional traits focus only on differences in traits between native communities and exotic species (e.g., Leffler et al. 2014). Ultimately, invasive species, through trait convergence/divergence and indirect effects (i.e., changes in abiotic properties), might induce character/trait displacement and thus act as evolutionary forces that shape plant communities (Beans et al. 2014). Studying the functional changes in native communities following an invasion may allow us to make inferences both about the mechanisms underpinning species losses and the consequences for ecosystem functioning and evolution. A functional approach is also particularly helpful in ecological restoration projects of invaded sites, either for selecting plant species mixtures with desired properties or function, and for influencing both plant and invertebrate species assemblages (e.g. Ostertag et al. 2015, 2020 in Hawaiian forests).
In Fiji, approximately 390 invasive alien species have been identified (Lenz et al. 2022). Among these, the non-native ivory cane palm Pinanga coronata (Blume ex Mart.) Blume (Arecaceae family) has considerable potential to fundamentally change the structure and diversity of the archipelago’s rainforests (Dyer et al. 2018, 2019; Forey et al. 2021; Keppel and Watling 2011). In forest habitats, P. coronata has a strong advantage over understory species in competing for light due to its taller stature and architectural adaptation to shaded conditions (Kimura and Simbolon 2002). Consequently, it can potentially eliminate some understory species, leaving only mature preexisting canopies of dominant trees in place. Additionally, like most invasive palm trees (Fehr et al. 2020), P. coronata has the capacity to produce and disperse fruits and to spread clonally (Kimura and Simbolon 2002). Introduced to the country as an ornamental in the 1970s, the palm’s invasive potential was first observed in the early 1990s (Watling and Chape 1993). By 2011, it had already been reported that the species formed a monodominant understory within the Colo-i-Suva Rainforest Reserve, which is north of Suva, Fiji’s largest city. It was, at that time, deemed “the biggest threat to the native biodiversity of rainforests in Fiji” (Keppel and Watling 2011, p. 44). In recent years, it has been observed that P. coronata is preventing the establishment of native tree ferns (Dyer et al. 2018). However, no detailed ecological research has been carried out so far, and little is known about the ecological impacts of P. coronata on native plant communities. As a consequence, no invasion management actions have been initiated. Thus P. coronata in Fiji represents both a perfect practical case to study the functional consequences of invasion-induced species losses and an urgent conservation problem.
To understand and highlight the taxonomic effect of P. coronata on Fiji's forests together with the functional consequences, we compared understory plant communities along a gradient of P. coronata densities, from uninvaded plots to monostands of P. coronata. Given the highly competitive nature of this invasive plant and its relatively recent invasion of native plant communities, we expect to observe evidence supporting the competitive hierarchy hypothesis. Firstly, we predict that both the taxonomic and functional diversity of invaded plant communities will decrease along the gradient of increasing P. coronata density. Secondly, we predict that invaded plant communities will turn into more competitive plant assemblages (with high SLA, LNC, and height; and low LDMC and C:N) in response to increasing P. coronata density and competition in a closed forest habitat in comparison to non-invaded communities. In other words, we expect that the invasion by P. coronata will induce community trait convergence rather than expected at random (null models), in turn leading to possible important changes in ecosystem functioning.
Methodology
Study area
The Republic of Fiji is an archipelago consisting of 330 islands and approximately 500 islets and lies within a 570,000 km2 area on the South Pacific Ocean (Clark and Anderson 2009). This study was conducted in Colo-i-Suva (CIS) Forest Park, a government-protected reserve of Fiji’s largest island, Viti Levu, and located approximately 12 km north of Suva, the capital city, in the southeastern area of the island. The Park has an altitudinal range of 120–240 m above sea level, while the climate is described as tropical wet to super-wet (Keppel et al. 2005; Richards 1996). According to the Fiji Meteorological Service (2006), the windward coasts of the main islands (i.e., those in the southeast) receive approximately 3,000 mm of rainfall annually—a figure that increases with altitude to produce an approximate average annual rainfall of ca. 4,000 mm in the study area (Watling 2005). The mean annual temperature, which is 24 °C, varies by only 2 °C between February and July—the warmest and coolest months, respectively (Kay 1986).
The CIS forest park was established in 1952, having being originally a lowland tropical rainforest, which was logged in the 1940s and 1950s (Paine 1991). In 1960, this reserve was inter-planted with South American mahogany (Swietenia macrophylla), an introduced tree used for timber production, which meant that there was clearing of the native rainforest in order to facilitate the establishment of the plantation (Tuiwawa 1998). The native and dominant trees mixed with mahogany are Garcinia myrtiflora, Garcinia myrtiflora, Psychotria amoeba, Cryptocarya constricta and Haplolobus floribundus. CIS is now classed as an old growth mahogany plantation given that it has not been logged since its establishment (Tuiwawa and Keppel 2012).
Sampling design
Within the 5 km2 Colo-i-Suva Rainforest Park, starting in the northeast and moving in a southwesterly direction, 280 plots of 5 m × 3 m area were regularly distributed along a gradient to investigate the extent to which they had been invaded. This revealed a gradient of P. coronata density at the community level, which ranged from 0 individuals per 15 m2 to 49 individuals per 15 m2. Plots were purposely selected close to each other in order to minimize climatic variations while retaining a range of P. coronata density. The 15 m2 plot areas facilitated correlations between P. coronata and understory plant species abundance, with a well-adapted sampling of understory species of tropical forest (Sutherland 2006).
For each plot, P. coronata density was measured by recording the number individuals, producing values that were strongly correlated with P. coronata relative cover within the plots (r = 0.7, p-value < 0.001). Thus, the population of P. coronata was recorded for each plot as a proxy for the extent of invasion (Appendix 1). Of the 280 plots examined, 61 did not contain any P. coronata individuals.
Vegetation sampling and measurements
The density (i.e., the number of individuals) of each herb and shrub understory species (i.e. plants less than 5 m in height including tree saplings) was recorded in each 15 m2 plot in order to obtain plant relative abundance. A total of 120 vascular plant species were identified (Appendix 2). Of the 120 species recorded, nine were exotics (Appendix 2). We cannot rule out that some of these species could have already influenced the resident plant communities, but due to their low density and frequencies, these species are not currently considered to be threats. It should be noted that throughout this study we use the term “resident community” instead of “native community” due to the presence of these other exotic species in the native understory community. Additionally, the presence of mahogany (an exotic tree) was monitored within the 15m2 plot. Several studies highlighted that such exotic trees could influence native plant diversity (Richardson et al 2015; Brundu et al. 2020). Regarding mahogany, it is unclear whether this exotic species can invade adjacent habitats and displace local plant species (Norghauer et al. 2011). We overall found mahogany within only 91 plots (33%) out of the 280 investigated.
For trait measurements, it is recommended to select the most abundant species that collectively make up 80% of cumulative standing biomass (Garnier et al. 2004; Pérez-Harguindeguy et al. 2003). Such a concentration of standing biomass is a good representation of the studied plant community and provides sufficient information to scale-up the values of traits to the plant community level (Cornelissen et al. 2003). Thus, for functional characterization, we sampled the 31 most abundant species across all plots (all belonging to shrubs or short-stature tree species). When the contribution of P. coronata (i.e., the invasive species) in every plot was excluded, we reached a threshold of 81.6 ± 14.2% (mean ± standard deviation) of cumulative relative abundance per plot. When the abundance of P. coronata in each plot was included, we reached a threshold of 92.9 ± 8.4%. The list of the 31 most abundant plant species used for the functional approach is provided in Appendix 3. In addition, in order to have a more comprehensive estimation of plant biomass and, subsequently, plant functions per plot (Garnier et al. 2004), plant density was converted to plant volume as follows: Volume (m3) = max height (m) x line intercept cover (m) x width (m). For shrub and short-stature tree species, such an allometric model of volume provides an accurate prediction of aboveground biomass (Flade et al. 2020). For each species, the volume was measured on 20 randomly distributed individuals over the entire gradient. Lastly, the total volume of each species within a plot was calculated by multiplying the volume by the population density.
Five functional plant traits (Table 1) of the 31 selected species were directly measured for individuals collected in the field. Plant height (in cm) was measured for five different individuals of each species. After that, eight mature leaf samples of five individuals per species were collected. An exception was made for particularly large leaves—those that were greater in size than A3 paper. In such cases, only one sample per species was collected. Leaves were placed in a plastic container and transported to the laboratory in cool boxes. We rehydrated the fresh leaves overnight before leaf measurements (Cornelissen et al. 2003). Fresh leaves of each sample were weighed, photocopied for further analysis, and dried before grinding. LDMC was obtained by dividing the oven-dry mass of a leaf by its fresh mass (mg.g−1). For SLA (mm2.mg−1), leaf areas were calculated by analyzing photocopied leaves using WinFOLIA™ software (Regent Instruments Inc., Canada). SLA was calculated by dividing the area of one side of a fresh leaf by its oven-dry mass, producing a value expressed in cm2.g−1. Using ground samples (n = 3 per species), leaf nitrogen (LNC) and carbon content values were calculated, from which C:N ratios were computed using a ThermoFisher Flash Analyzer 2000 in Ecodiv Laboratory (Université de Rouen, France). Both LNC (mg.g−1) and leaf CN ratio (C:N) were used for functional analyses.
Data analyses
The indices of taxonomic diversity (species richness, Shannon diversity, and evenness) for each plot were calculated using the vegan package of R software (Aylward 2016) for the entire data set (120 species). Taxonomic indices were also calculated on the reduced data set (31 species) and we found similar patterns (data not shown). Then, indices of functional diversity were obtained using the reduced data set concentrating on the 31 most abundant species. Functional diversity was assessed using three complementary indices: functional richness (FRic), functional evenness (FEve), and the Rao quadratic entropy index (RaoQ) for functional dispersion. FRic relates to the amount of niche space occupied by each species within a community, totaling to a measure of the cumulative functional space occupied in the community (Villéger et al. 2008). Functional evenness (FEve) relates to the way in which the biomass of a community is distributed in a niche/functional space, which in turn appertains to the effective utilization of the available resources (Villéger et al. 2008). Lastly, RaoQ was used to calculate the mean distance of all individual pairs based on their functional traits, thus representing a measure of functional dispersion (Botta-Dukát, 2005). These indices were calculated using the dbFD function in FD package of R (Laliberté and Legendre 2010). Pinanga coronata density (number of individuals) was used to assess the gradient of the species invasion. To test our first hypothesis (i.e., that P. coronata could lead to both taxonomic and functional loss in native communities), we used linear regression to examine the relationships between the measured diversity indices and the gradient of P. coronata invasion. Finally, to account for the possible influence of the exotic tree mahogany in the plots, we performed linear separate regression for the whole set of plots and both subsets with and without mahogany.
The CWM of each trait per plot was calculated using functcomp function in the FD package for all plots, and for plots with or without mahogany. CWMs of trait correspond to the mean value of a trait across species (i.e., community) weighted by species abundance (Garnier et al. 2004; Violle et al. 2007). Trait CWMs are used to determine properties and processes within ecosystems. For example, a high CWM for LNC is likely to lead to high soil N input and fast decomposition rates (Reich 2014). These CWM trait values were calculated at the plant scale both for resident communities (i.e., without including the functional contribution of P. coronata) and for novel communities (i.e., with the functional contribution of P. coronata). In other words, analyses without P. coronata do not refer to plots that truly did not contain P. coronata, but just to the way in which we analyzed the data a posteriori.
To test our second hypothesis and link community trait variation to the gradient of P. coronata density, each CWM trait was regressed on the P. coronata gradient. Because p-values in these regressions can be biased by non-independence among the sampling points arising from the application of a species’ mean trait value to each plot (Zelený 2018), we used the fourth-corner approach (Brown et al. 2014). It has been argued that this method is superior to conventional methods of trait CWM correlation and species niche centroid correlation in testing for trait–environment correlations (Peres-Neto et al. 2017). To get an overview of the overall functional strategies in communities along the invasion gradient, we performed a Principal Components Analysis (PCA) of the CWM of traits.
Lastly, we assessed non-random trait-convergence and trait-divergence assembly patterns on resident communities (i.e., without P. coronata) using null models as proposed by the “TCAP/TDAP” method of Pillar et al. (2009). According to Pillar et al. (2009), a trait-convergence assembly pattern (TCAP) can be observed when communities contain species with similar traits and when trait variation is related to an ecological (e.g., invasion) gradient beyond random expectations, suggesting that gradient is involved in filtering species within communities based on their traits. Based on the work by Pillar et al. (2009), we assessed TCAP by matrix correlation ρ(TE) between the trait CWMs (T) and the invasion gradient (E) and compared it to random expectations by permutation against a null model. Conversely, a trait-divergence assembly pattern (TDAP) can be identified when the turnover in community traits is also related to the gradient, but communities along the gradient increasingly contain species that do not share similar traits. Here, to quantify invasion-related TDAP as suggested by Pillar et al. (2009), we correlated communities weighted by their trait similarities (X) with the invasion gradient (correlation ρ(XE), which may indicate either TCAP or TDAP), and partialled out the TCAP component (partial correlation ρ(XE.T)). The observed correlation ρ(XE) for TDAP/TCAP and the partial correlation ρ(XE.T) for TDAP were also tested by permutation against null models (see Pillar et al. (2009) for details). Additionally, an iterative process (10,000 permutations) was employed to identify the optimal trait combinations that maximized convergence, divergence, or both (see Pillar and Sosinski 2003). All analyses relative to TCAP and TDAP were performed using the ‘SYNCSA’ software (Debastiani and Pillar 2012).
Results
Effect of P. coronata on taxonomic and functional diversity
All indices of taxonomic diversity are strongly correlated with the gradient of density of P. coronata (Fig. 1). Although the variability was high, species richness (r = − 0.53, p < 0.001) and Shannon diversity index (r = − 0.38, p < 0.001) significantly decreased with increasing P. coronata density. The species richness and Shannon diversity indices showed an average loss of 50% and 33%, respectively along the gradient of P. coronata (i.e., from uninvaded communities to communities containing the greatest abundance of P. coronata). Conversely, Shannon evenness slightly increased along the gradient of P. coronata density (r = 0.14, p < 0.05).
Variation of taxonomic (left column) and functional biodiversity (right column) of plant communities along the gradient of P. coronata densities. Three regression lines are given: “all plots” (black regression) and two subsets “no mahogany” and “with mahogany” canopy (grey regressions). Taxonomic indices were calculated on the entire data set (120 species) and functional indices on the dominant 31 species. Regarding taxonomic data, the same patterns were observed when using the sub-set of 31 species (data not shown). Each point represents a plot. The total number of plots for taxonomic indices was N = 280. For functional indices N = 249. The difference of the number of N values between taxonomic and functional data was due to a lack of available values in plots with less than three species, which prevents the computation of functional diversity. R2 is the coefficient of determination and asterisks indicate significance levels of the linear regression line: ***P < 0.001; **P < 0.01; *P < 0.05, ns = not significant
The increasing presence of P. coronata led to a 50% decrease in the overall functional richness (− r = 0.23, p < 0.001) and RaoQ (r = − 0.07, p < 0.001). There was no notable change in functional evenness (r = 0.10, p = 0.11) along the gradient. Note that these functional measures include the functional contribution of P. coronata in communities.
When analyzing plots with or without mahogany, P. coronata density was still negatively correlated with taxonomic and functional richness (Fig. 1). These correlations became non-significant for evenness (Fig. 2).
Changes in CWM traits of resident plant communities (i.e., without P. coronata contribution) along the gradient of P. coronata densities for the five functional traits. Three regression lines are given: “all plots” (black regression) and two subsets “no mahogany” and “with mahogany” canopy (grey regressions). Invaded communities comprise 30 sampled dominant species, with P. coronata traits excluded from analyses. Each point represents a plot using N = 280. R2 is the coefficient of determination and asterisks indicate significance levels of the linear regression line: ***P < 0.001; **P < 0.01; *P < 0.05, ns = not significant
Effect of P. coronata on functional traits of resident plant communities
When P. coronata was excluded (i.e. by excluding the contribution of P. coronata from the data set), the gradient of invasion significantly influenced four CWM traits of resident species. LNC (r = 0.14, p < 0.001) and SLA (r = 0.19) increased significantly—up to + 17% on average—along the gradient of P. coronata density. Conversely, LDMC (r = − 0.14, p < 0.05) and C:N (r = − 0.26, p < 0.001) decreased by, on average, 8% and 22%, respectively, between uninvaded communities and communities facing the greatest abundance of P. coronata invasion (Fig. 2). Plant heights did not change significantly along the gradient (p = 0.056). When analyzing plots with or without mahogany, the correlation between P. coronata and CWM traits was only significant in plots without mahogany (Fig. 2).
The P. coronata density gradient induced patterns of trait convergence (TCAP, ρ(TE)) and overall changes in species similarity within communities (TCAP/TDAP, ρ(XE)) of the full set of traits. The maximum convergence pattern was obtained in a subset of traits containing only LDMC and C:N (ϱ(TE) = 0.33, p < 0.01). This same subset of traits also maximized the combined effect of convergence and divergence (ϱ(XE) = 0.32, p < 0.01; Table 2), thus suggesting that traits diverged at the beginning of the gradient and then converged at the end of the gradient (Fig. 2).
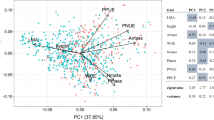
The first three principal components of the PCA performed on CWM traits (without P. coronata contribution) accounted for 45%, 28%, and 15% of the variance, respectively. Axis 1 of the PCA (Fig. 3) represents a relative gradient of nutrient acquisition from conservative strategies in the negative part (high C:N and LDMC values) to more acquisitive species (high LNC) in the positive part. The second axis of this PCA is, in comparison, linked to a strategy to optimize light, with species either characterized as having a high SLA or being particularly tall. Thus, communities in less invaded plots (negative area of axis 1) tend to be characterized by more conservative strategies (nutrient and light acquisition) than those in invaded plots.
Multivariate analyses (PCA from axis 1–2) without the contribution of P. coronata, showing the effect of P. coronata on functional traits in resident plant communities using five plant functional traits. SLA = specific leaf area. LDMC = leaf dry matter content. LNC = leaf nitrogen content. H = height. C:N = leaf CN ratio
Functional characteristics of novel communities (including P. coronata)
When including P. coronata in the calculation of functional traits, it was revealed that all CWM traits in highly invaded plots converge to those of P. coronata (Fig. 4). This is a result of the strong contribution of P. coronata to the novel plant communities. In plots where there are at least 10 individuals present, P. coronata accounts for more than 40% of the relative abundance of the novel community (Appendix 4). Thus, along the gradient of P. coronata, communities converge through higher SLA (SLAP. coronata = 16.7 mm2.mg−1), LDMC (LDMCP. coronata = 356 mg.g1), C:N (C:NP. coronata = 29.5), and height (HP. coronata = 280 cm) and have a lower LNC (LNCP. coronata = 1.38%) when compared with less invaded plots (Fig. 4). It can be noted that LNC for plots without mahogany, and SLA and LDMC for plots with mahogany were not significantly correlated with the gradient of P. coronata density (Fig. 4).
Changes in community-weighted mean (CWM) traits of novel plant communities (i.e. with P. coronata contribution) along the gradient of P. coronata densities for the five functional traits. Three regression lines are given: “all plots” (black regression) and two subsets “no mahogany” and “with mahogany” canopy ( grey regressions). Novel communities comprise a total of 31 sampled dominant species. N = 280. R2 is the coefficient of determination and asterisks indicate significance levels of the linear regression line: ***P < 0.001; **P < 0.01; *P < 0.05, ns = not significant
Discussion
Effect of P. coronata on taxonomic and functional diversity of resident plant species
Plant invasions can have harmful impacts on native vegetation, primarily by reducing species richness, diversity, and evenness, thus emphasizing the strong correlation between the dominance of invasive plants and the decline of native plants (Didham et al. 2005; Hulme et al. 2008). This case study in a Fijian rainforest supports this idea by demonstrating that plant species richness and diversity are strongly and negatively correlated with the abundance of P. coronata, an invasive palm tree. In this CIS forest park, the exotic mahogany trees that were planted before the introduction of P. coronata could also be a potential confounding factor. Indeed, according to the ‘Invasional meltdown hypothesis’, the presence of invasive species in an ecosystem facilitates the invasion of other non-native species (Simberloff and Von Holle 1999). Nevertheless, our study does not provide evidence of invasional meltdown. Indeed, the majority of plots (two-thirds) were without mahogany canopy suggesting that mahogany did not directly facilitated P. coronata. Secondly, negative relationships observed between P. coronata and plant diversity were maintained independently on the presence or not of mahogany, with a tendency to have weaker correlations under mahogany canopy.
Thus, P. coronata might be a notable threat to Fiji´s unique assemblage of flora, particularly considering that approximately 50% of the country’s land area is covered by tropical lowland rainforest (Mueller-Dombois and Fosberg 1998)—an ecosystem within which P. coronata clearly thrives. Biodiversity in these forests is extremely high, containing over 99% of the national endemic flora and fauna (Keppel et al. 2010; Olson et al. 2010). This rainforest ecosystem is among the most diverse in the Pacific, being classed as species-rich because 1,350 of the 1,769 different native vascular species are found within the forest environment (Mueller-Dombois and Fosberg 1998). Ecological studies on Fiji are scarce and, although the country has 390 recorded invasive alien species, this is the first study to quantify the potential effect of an invasive alien plant on the flora of Fiji.
Studies conducted by Daehler and Baker (2006) and Meyer et al. (2008) showed that although P. coronata displayed signs of becoming invasive in Hawaii and Tahiti, the species was not, at that time, viewed as a threat. However, later studies conducted by Parker and Parsons (2012) categorized the species as an invasive species in Hawaii. In Fiji, this study has presented ample evidence that P. coronata is a highly invasive species, forming populations with density and overall cover that are significantly greater than those of native species.
In addition to changing species composition and reducing plant diversity, fast-growing invasive species may also affect the functional diversity of vegetation (Michelan et al. 2010). The increasing abundance of P. coronata also led to a decline in FRic, suggesting that this invasive plant may cause a loss of functions or the original combination of traits found in understory resident species. Conversely to other functional indices, FRic measures the total functional range covered by a community and thus has the advantage of conveying information beyond species richness (Legras et al. 2018). Our results thus indicate that a P. coronata invasion is linked not only to a decrease in species richness but also to a decrease in the breadth of ecological strategies in rainforest communities.
Pinanga coronata ‘s effect on plant traits and strategies of resident communities
This study demonstrated that dominant resident species in invaded plots (i.e., communities without a P. coronata functional contribution) tend to have a high SLA and LNC and a low LDMC and C:N. This change in CWM traits was mainly driven by species that persisted along the gradient as opposed to the arrival of new dominant native species into the communities. Such a combination of leaf traits characterizes species that are adapted to competition and/or to disturbance. Thus, in the case of the invasion by P. coronata, traits of resident plants are filtered to form competitive understory communities in order to cope with competition from P. coronata for light and/or nutrients. Additionally, using the “TCAP/TDAP” method (Pillar et al. 2009), we were able to show that these patterns are beyond random expectations and that the gradient of P. coronata density led to greater trait convergence of resident species than would be expected by chance, most notably for LDMC and C:N. This trait convergence of plant communities (tested against null models) is supposedly due to the extent of habitat selection and not the consequence of a decrease in plant richness (Pilar et al. 2009). Such convergence suggests that the density of P. coronata can acts as a strong selective biotic filter that could reduce the diversity of trait values towards more competitive strategies, a feature also demonstrated by other invasive species (Sodhi et al. 2019). These results support the competitive hierarchy hypothesis.
In community assembly rules, convergence is usually explained by abiotic factors (see, for example, Bernard-Verdier et al. 2012). In a complementary experiment performed in the same mature forests in Fiji, we found that soil moisture and photosynthetically active radiation (i.e., light)—two important determinants for plant growth—were not influenced by P. coronata density along the gradient of invasion (Gopaul, pers. comm). Subsequently, we suggest that P. coronata outcompetes species by dominating the space and/or through higher soil nutrient uptake or storage, as opposed to water and/or light competition. Although not directly investigated in our study, the trait convergence observed when P. coronata is abundant may result from biotic interactions, such as competition. Alternatively, it is possible that P. coronata acts as an ecosystem engineer by changing ecosystem functioning and affecting other trophic levels which, in turn, filters plants in the ecosystem. For example, Forey et al. (2021), in the same forest, demonstrated that P. coronata led to a change in soil fauna communities (i.e., Collembola) and suggested a decrease in plant–soil interactions under P. coronata invasion.
Lastly we found, that the intensity of P. coronata filtering seemed to be modulated by the presence or absence of mahogany. Indeed, when considering only plots under mahogany, no CWM traits changes were observed suggesting that the canopy of mahogany might contribute to mitigate the effect of P. coronata. Further abiotic measurements (e.g. soil fertility, pH, water and light availability) are necessary to understand and hierarchize the different filters that act on resident understory communities.
Functional properties of novel plant communities
This study indicated that P. coronata influences the novel plant communities' functional assemblages, and as a result, the remaining species within the understory plant community tend to have traits that are related to fast resource acquisition. Indeed, when including P. coronata in the assessment of functional assemblage, it was found that the considerable dominance of this invasive plant results in CWMs of traits converging towards those of the invasive plant. When compared with the traits of resident species, P. coronata tends to have a more conservative strategy (higher LDMC and lower LNC) for nutrients but a more competitive strategy for light (high SLA) (Albert et al. 2010). Funk (2013), in a meta-analysis, demonstrated that invasive species in low-nutrient systems tend to have a higher SLA than native species, but this does not always translate into a shorter leaf lifespan than natives. Thus, it is likely that the apparent competition for nutrients between P. coronata and resident species is rather due to a greater ability to conserve nutrients (high biomass and long lifespan of palm leaves) rather than a faster nutrient uptake and plant growth. Additionally, P. coronata have a better competitive strategy for light than do resident species (Kimura and Simbolon 2002), and this allows the invasive to dominate the other species under a canopy of trees. Similar results were found by Pattison et al. (1998) in the understory of Hawaiian rainforests, where it was demonstrated that relative photosynthetic growth rates of invasive species growing in the sun and in a partial shade were significantly higher than those for native species.
The traits of invaders depend on the characteristics of the invaded habitats (Funk 2013), thus making it difficult to identify a suite of general traits explaining invasiveness. Nevertheless, according to the “try harder” hypothesis (Dainese and Bragazza 2012; Tecco et al. 2010), IAP should differ from resident species through traits that allow them to better deal with local conditions when compared with the resident species. More precisely, Funk and Vitousek (2007) suggested that, in low-resource environments, IAP tend to have more conservative functional traits (i.e., have long-lived, thick, tough leaves with high concentrations of defense compounds and a low nutrient content) than resident species. Conversely, in resource-rich environments, IAP should show more acquisitive characteristics – such as possessing thin, soft, and nutrient-rich leaves with a high SLA and short lifespan (Tecco et al. 2010) – than resident species. In this study, the nutrient conservation strategy of P. coronata is in agreement with studies that investigate plant growth strategies in tropical rainforest with low-nutrient soils (e.g., Bakker et al. 2010) and, in particular, the very low soil phosphorus concentrations limiting microbial processes (Camenzind et al. 2018).
The plant community composition subsequent to P. coronata introduction, with traits or rather markers of a fast resource acquisition strategy, can be detrimental to ecosystem resources as it will require more nutrients for growth and maintenance. This change in species dominance brought about by the presence of the invasive P. coronata is inducing a considerable change in the Fijian rainforest, thus inflicting wholesale changes in the structure and functioning of the ecosystem (Denslow 2007). In turn, these changes may have serious consequences for a number of important variables, including trophic structure, the nutrient content of the soil, decomposition, rare species, ecosystem services, and the overall productivity and diversity of the invaded habitat (Denslow 2007).
Conclusion
This study has highlighted the potentially negative effect that an invasive alien palm species (P. coronata) could have on the resident plant community and, indeed, on the overall ecosystem of the Colo-i-Suva Forest Park. Firstly, we demonstrated that the invasion of P. coronata was strongly correlated with a reduction in species richness and diversity but also with a drecrease of the functional diversity of resident plant communities. Secondly, using a functional approach and null models, we revealed that an invasion by P. coronata induced trait community convergence towards a fast resource acquisition strategy according to the competitive hierarchy hypothesis. Such functional changes of plant communities into competitive plant assemblages could notably alter the resilience of an ecosystem and its functioning. Additionally, it should be borne in mind that this study concerns a single invasive plant species, but interactions with other invasive species (i.e., a “cocktail” effect) are highly probable in insular ecosystems, and this might hamper our understanding of functional invasion consequences (Bruno et al. 2005; Johnson et al. 2009). Lastly, our study, like most in-situ invasive species studies, is correlative and we are aware that correlations are not causations and observed effects may be caused by unmeasured or confounding variables (Warren et al. 2017). Such observations should be confirmed by experimental studies.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abrams P (1983) The theory of limiting similarity. Annu Rev Ecol Evol Syst 14(1):359–376
Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel S (2010) A multi-trait approach reveals the structure and the relative importance of intra-vs. interspecific variability in plant traits. Funct Ecol 24:1192–1201
Aylward J (2016) Understory plant community structure in forests invaded by garlic mustard (Alliaria petiolata), Masters thesis, viewed 16 May 2019, University of Massachusetts Amherst
Bakker MA, Carreño-Rocabado G, Poorter L (2011) Leaf economics traits predict litter decomposition of tropical plants and differ among land use types. Funct Ecol 25:473–483
Baruch Z, Goldstein G (1999) Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121:183–192
Beans CM (2014) The case for character displacement in plants. Ecol Evol 4:862–875
Bernard-Verdier M, Navas ML, Vellend M, Violle C, Fayolle A, Garnier, (2012) Community assembly along a soil depth gradient: contrasting patterns of plant trait convergence and divergence in a Mediterranean rangeland. J Ecol 100:1422–1433
Boehmer HJ, Wagner HH, Gerrish GC, Jacobi JD, Mueller-Dombois D (2013) Rebuilding after Collapse: evidence for long-term cohort dynamics in a monodominant tropical rainforest. J Veg Sci 24:639–650
Botta-Dukát Z (2005) Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J Veg Sci 16:533–540
Brown AM, Warton DI, Andrew NR, Binns M, Cassis G, Gibb H (2014) The fourth-corner solution – using predictive models to understand how species traits interact with the environment. Methods Ecol Evol 5:344–352
Brundu G, Pauchard A, Pyšek P, Pergl J, Bindewald A, Brunori A, Richardson D (2020) Global guidelines for the sustainable use of non-native trees to prevent tree invasions and mitigate their negative impacts. NeoBiota 61:65–116
Bruno JF, Fridley JD, Bromberg KD, Bertness MD (2005) Insights into biotic interactions from studies of species invasions. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer press, Sunderland, pp 13–40
Burton JI, Perakis SS, Brooks JR, Puettmann KJ (2020) Trait integration and functional differentiation among co-existing plant species. Am J Bot 107:628–638
Cadotte MW, Carscadden K, Mirotchnick N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48:1079–1087
Camenzind T, Hättenschwiler S, Treseder KK, Lehmann A, Rillig MC (2018) Nutrient limitation of soil microbial processes in tropical forests. Ecol Monogr 88:21
Clark GR, Anderson A (2009) The early prehistory of Fiji, vol 31. ANUE Press, Downey
Cleland EE, Clark CM, Collins SL, Fargione JE, Gough L, Gross KL, Pennings SC, Suding KN (2011) Patterns of trait convergence and divergence among native and exotic species in herbaceous plant communities are not modified by nitrogen enrichment. J Ecol 99:1327–1338
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, Ter Steege H, Morgan HD, Van Der Heijden MGA, Pausas JG (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Eco 51:335–380
Daehler CC, Baker RF (2006) New records of naturalized and naturalizing plants around Lyon Arboretum, Mānoa Valley, O’ahu’. Bish Mus Occas Pap 87:3–18
Dainese M, Bragazza L (2012) Plant traits across different habitats of the Italian Alps: a comparative analysis between native and alien species. Alp Bot 122:11–21
Debastiani VJ, Pillar VD (2012) SYNCSA—R tool for analysis of metacommunities based on functional traits and phylogeny of the community components. Bioinformatics 28:2067–2068
Denslow JS, Space JC, Thomas PA (2009) Invasive exotic plants in the tropical Pacific islands: patterns of diversity. Biotropica 41:162–170
Denslow JS (2007) Managing dominance of invasive plants in wildlands. Curr Sci 93:1579–1586
Dı́az S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ (2005) Are invasive species the drivers of ecological change? Trends Ecol Evol 20:470–474
Dyer MJ, Keppel G, Tuiwawa M, Vido S, Boehmer HJ (2018) Invasive alien palm Pinanga coronata threatens native tree ferns in an oceanic island rainforest. Austr J Bot 66:647–656
Dyer MJ, Keppel G, Tuivava M, Vido S, Boehmer HJ (2019) Using expert knowledge and field surveys to guide management of an invasive alien palm in a Pacific Island lowland rainforest. In: Veitch CR (ed) Island Invasives: Stepping up to the Challenge. Gland, Switzerland, IUCN, Dundee, Scotland. pp 417–422
Ebeling A, Rzanny M, Lange M, Eisenhauer N, Hertzog LR, Meyer ST, Weisser WW (2018) Plant diversity induces shifts in the functional structure and diversity across trophic levels. Oikos 127:208–219
Fehr V, Buitenwerf R, Svenning JC (2020) Non-native palms (Arecaceae) as generators of novel ecosystems: a global assessment. Divers Distrib 26:1523–1538
Fiji Meteorological Service (2006) The climate of Fiji. Inf Sheet 35:28
Flade L, Hopkinson C, Chasmer L (2020) Allometric equations for shrub and short-stature tree aboveground biomass within boreal ecosystems of Northwestern Canada. Forests 11:1207
Forey E, Lodhar SYF, Gopaul S, Boehmer HJ, Chauvat M (2021) A functional trait-based approach to assess the impact of an alien palm invasion on plant and soil communities on a South Pacific island. Austr Ecol 46:398–410
Fried G, Carboni M, Mahaut L, Violle C (2019) Functional traits modulate plant community responses to alien plant invasion. Perspect Plant Ecol Evol Syst 37:53–63
Funk JL (2013) The physiology of invasive plants in low-resource environments. Conserv Physiol 1:1–17
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Gallien L, Carboni M (2017) The community ecology of invasive species: where are we and what’s next? Ecography 40:335–352
Garnier E, Cortez J, Billès G, Navas ML, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637
Grime JP (1998) Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol 86:902–910
Hejda M, Hanzelka J, Kadlec T, Štrobl M, Pyšek P, Reif J (2017) Impacts of an invasive tree across trophic levels: species richness, community composition and resident species’ traits. Divers Distrib 23:997–1007
Hulme PE (2008) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18
Jakobs G, Weber E, Edwards PJ (2004) Introduced plants of the invasive Solidago gigantea (Asteraceae) are larger and grow denser than conspecifics in the native range. Divers Distrib 10:11–19
Johnson PTJ, Olden JD, Solomon CT, Vander Zanden MJ (2009) Interactions among invaders: community and ecosystem effects of multiple invasive species in an experimental aquatic system. Oecologia 159:161–170
Kay R (1986) Fiji, a travel survival kit. Lonely Planet Publications, Berkeley
Keppel G, Watling D (2011) Ticking time bombs – current and potential future impacts of four invasive plant species on the biodiversity of lowland tropical rainforests in south-east Viti Levu, Fiji. South Pac J Nat Appl Sci 29:43–45
Keppel G, Navuso JC, Naikatini A, Thomas NT, Rounds IA, Osborne TA, Batinamu N, Senivasa E (2005) Botanical diversity at Savura, a lowland rain forest site along the PABITRA gateway transect, Viti Levu, Fiji. Pacific Sci 59:175–191
Keppel G, Morrison C, Meyer JY, Boehmer HJ (2014) Isolated and vulnerable: the history and future of Pacific Island terrestrial biodiversity. Pac Conserv Biol 20:136–145
Kimura M, Simbolon H (2002) Allometry and life history of a forest understory palm Pinanga coronata (Arecaceae) on Mount Halimun, West Java. Ecol Res 17:323–338
Kueffer C, Daehler CC, Torres-Santana CW, Lavergne C, Meyer JY, Otto R, Silva L (2010) A global comparison of plant invasions on oceanic islands. Perspect Plant Ecol Evol Syst 12:145–161
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
Leffler AJ, James JJ, Monaco TA, Sheley RL (2014) A new perspective on trait differences between native and invasive exotic plants. Ecology 95:298–305
Legras G, Loiseau N, Gaertner JC (2018) Functional richness: overview of indices and underlying concepts. Acta Oecol 87:34–44
Lenz MI, Galvin S, Keppel G, Gopaul S, Kowasch M, Watling D, Dyer MJ, Lodhar S, Hanson GC, Erasmi S, Boehmer HJ (2022) Where to invade next: inaction on biological invasions threatens sustainability in a Small Island developing state of the tropical South Pacific. In: Low PS (ed) Sustainable development – Asia-Pacific perspectives. University Press, Cambridge
Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177:706–714
MacArthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385
Meyer JY (2014) Critical issues and new challenges for research and management of invasive plants in the Pacific Islands. Pacific Conserv Biol 20:146–164
Meyer JY, Lavergne C, Hodel DR (2008) Time bombs in gardens: invasive ornamental palms in tropical islands, with emphasis on French Polynesia (Pacific Ocean) and the Mascarenes (Indian Ocean). Palms 52:71–83
Michelan TS, Thomaz SM, Mormul RP, Carvalho P (2010) Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshw Biol 55:1315–1326
Minden V, Henneberg KJ, Porembski S, Boehmer HJ (2010a) Invasion and management of alien Hedychium gardnerianum (kahili ginger, Zingiberaceae) alter plant species composition of a montane rainforest on the island of Hawai’i’. Plant Ecol 206:321–333
Minden V, Jacobi JD, Porembski S, Boehmer HJ (2010b) Effects of invasive alien kahili ginger (Hedychium gardnerianum) on native plant species regeneration in a Hawaiian rainforest. Appl Veg Sci 13:5–14
Montesinos D (2022) Fast invasives fastly become faster: Invasive plants align largely with the fast side of the plant economics spectrum. J Ecol 110:1010–1014
Mueller-Dombois D (2006) Pacific island forests: successionally impoverished and now threatened to be overgrown by aliens? Pac Sci 62:303–309
Mueller-Dombois D, Boehmer HJ (2013) Origin of the Hawaiian rainforest ecosystem and its transition states in long-term primary succession. Biogeosciences 10:5171–5182
Mueller-Dombois D, Fosberg R (1998) Vegetation of the tropical Pacific Islands. Springer, New York
Muscarella R, Uriarte M (2016) Do community-weighted mean functional traits reflect optimal strategies? Proc R Soc Lond 283:20152434
Norghauer JM, Martin AR, Mycroft EE, James A, Thomas SC (2011) Island invasion by a threatened tree species: evidence for natural enemy release of mahogany (Swietenia macrophylla) on Dominica. Lesser Antilles Plos One 6:e18790
Ostertag R, Warman L, Cordell S, Vitousek PM (2015) Using plant functional traits to restore Hawaiian rainforest. J Appl Ecol 52:805–809
Ostertag R, Sebastián-González E, Peck R, Hall T, Kim J, DiManno N, Uowolo A (2020) Linking plant and animal functional diversity with an experimental community restoration in a Hawaiian lowland wet forest. Food Webs 25:e00171
Paine JR (1991) IUCN commission on national parks and protected areas. IUCN, South Pacific Regional Environment Programme
Parker JL, Parsons B (2012) New plant records from the big island for 2010–2011. Bish Mus Occas Pap 113:65–74
Pattison R, Goldstein G, Ares A (1998) Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecologia 117:449–459
Pavoine S, Bonsall MB, Dupaix A, Jacob U, Ricotta C (2017) From phylogenetic to functional originality: guide through indices and new developments. Ecol Indic 82:196–205
Penuelas J, Sardans J, Llusià J, Owen SM, Carnicer J, Giambelluca TW, Niinemets Ü (2010) Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Glob Chang Biol 16:2171–2185
Peres-Neto PR, Dray S, ter Braak CJF (2017) Linking trait variation to the environment: critical issues with community-weighted mean correlation resolved by the fourth-corner approach. Ecography 40:806–816
Pérez-Harguindeguy N, Díaz S, Vendramini F, Cornelissen JH, Gurvich DE, Cabido M (2003) Leaf traits and herbivore selection in the field and in cafeteria experiments. Austr Ecol 28:642–650
Perez-Harguindeguy N, Diaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Cornelissen JHC (2016) Corrigendum to: new handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 64(8):715–716
Pillar VD, Sosinski EE Jr (2003) An improved method for searching plant functional types by numerical analysis. J Veg Sci 14:323–332
Pillar VD, Duarte LDS, Sosinski EE, Joner F (2009) Discriminating trait-convergence and trait-divergence assembly patterns in ecological community gradients. J Veg Sci 20:334–348
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol 18:1725–1737
Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Richardson DM (2020) Scientists’ warning on invasive alien species. Biol Rev 95:1511–1534
Reich PB (2014) The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J Ecol 102:275–301
Richards PW (1996) The tropical rain forest. An ecological study. University Press, Cambridge
Richardson DM, Le Roux JJ, Wilson JR (2015) Australian acacias as invasive species: lessons to be learnt from regions with long planting histories. South For: J Forest Sci 77:31–39
Russell JC, Meyer JY, Holmes ND, Pagad S (2017) Invasive alien species on islands: impacts, distribution, interactions and management. Environ Conserv 44:359–370
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Sodhi DS, Livingstone SW, Carboni M, Cadotte MW (2019) Plant invasion alters trait composition and diversity across habitats. Ecol Evol 9:6199–6210
Sutherland WJ (2006) Ecological census techniques: a handbook. Cambridge University Press, Cambridge
Tecco PA, Díaz S, Cabido M, Urcelay C (2010) Functional traits of alien plants across contrasting climatic and land-use regimes: do aliens join the locals or try harder than them? J Ecol 98:17–27
Tuiwawa SH, Keppel G (2012) Species diversity, composition and the regeneration potential of native plants at the Wainiveiota Mahogany Plantation Viti Levu, Fiji Islands. South Pac J Nat Appl Sci 30:51–57
Tuiwawa M (1998) Technical Group 7-Priority Locations for Biodiversity Conservation-The locations and justification of priority sites for the conservation of Fiji’s botanical biodiversity, Fiji Biodiversity Strategic and Action Plan, Fiji Department of Environment, Suva, Fiji
Van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245
Van Kleunen M, Dawson W, Essl F, Pergl J, Winter M, Weber E, Kreft H, Weigelt P, Kartesz J, Nishino M, Antonova LA (2015) Global exchange and accumulation of non-native plants. Nature 525:100
Vilà M, Weiner J (2004) Are invasive plant species better competitors than native plant species?–evidence from pair-wise experiments. Oikos 105:229–238
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Villéger S, Mason NW, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecol 89:2290–2301
Violle C, Navas ML, Vile DK, E, Fortunel C, Hummel I, Garnier E, (2007) Let the concept of trait be functional! Oikos 116:882–892
Warren RJ II, King JR, Tarsa C, Haas B, Henderson J (2017) A systematic review of context bias in invasion biology. PLoS ONE 12(8):e0182502
Watling D (2005) Palms of the Fiji Islands. Environmental Consultants (Fiji) Ltd., Suva
Watling D, Chape SA (1993) Environment Fiji: the National State of the Environment Report. IUCN - Government of Fiji, Suva
Westerband AC, Knight TM, Barton KE (2021) Intraspecific trait variation and reversals of trait strategies across key climate gradients in native Hawaiian plants and non-native invaders. Ann Bot 127:553–564
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Zelený D (2018) Which results of the standard test for community weighted mean approach are too optimistic? J Veg Sci 29:953–966
Acknowledgements
We thank Gunnar Keppel, Dick Watling, and Nicholas Rollings for their advice and assistance. Sainivalati Vido and Panapasa for their training and guidance in the field. Michael J. B. Dyer for support and assistance. The Ministry of Fisheries and Forestry Fiji for granting permission to conduct our research and the Faculty of Science Technology and the Environment (FSTE) of the University of the South Pacific for providing the funding to carry out this research. Also, to the European Union through which the Caribbean-Pacific Intermobility Scheme (CARPIMS) scholarship was granted for S.L, G.C.H. and S.G. to live and study in Fiji was made available. We also thank the European Commission for the attribution of the Erasmus Mundus Grant to E.F. that allowed this international research cooperation project. Lastly, we sincerely thank the three anonymous reviewers and the editor for their advice and feedback on this manuscript.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Estelle Forey and Sherri Y. F. Lodhar are co-first author.
Appendices
Appendix 1
Cumulative ranked abundance curves of P. coronata density in the 280 plots (15m2) used for this study.

Appendix 2
List of the 120 species recorded in the 280 plots. Information based on the Global Plants Database operated by JSTOR. https://plants.jstor.org/
Species name | Family | Native/Exotic | Species name | Family | Native/Exotic |
|---|---|---|---|---|---|
Acanthephippium vitiensis | ORCHIDACEAE | Native | Gymnostoma vitiense | CASUARINACEAE | Native |
Agathis vitiensis | ARAUCARIACEAE | Native | Haplolobus floribundus | BURSERACEAE | Native |
Aglaia elegans | MELIACEAE | Native | Heliconia psittacorum | HELICONIACEAE | Exotic |
Aglaia sp. | MELIACEAE | Native | Heritiera ornithocephala | STERCULIACEAE | Native |
Aglaia vitiensis | MELIACEAE | Native | Hernandia olivacea | HERNANDIACEAE | Native |
Alstonia pacifica | APOCYNACEAE | Native | Homalium vitiense | FLACOURTIACEAE | Native |
Alstonia vitiensis | APOCYNACEAE | Native | Ixora pelagica | RUBIACEAE | Native |
Amaroria soulameiodes | SIMAROUBACEAE | Native | Kingiodendron platycarpum | FABACEAE | Native |
Anacolosa lutea | OLACACEAE | Native | Koelreuteria elegans | SAPINDACEAE | Native |
Ardisia crispa | MYRSINACEAE | Exotic | Macaranga graeffeana | EUPHORBIACEAE | Native |
Astronidium confertiflorum | MELASTOMATACEAE | Native | Macaranga harveyana | EUPHORBIACEAE | Native |
Atuna racemosa | CHRYSOBALANACEAE | Native | Macaranga magna | EUPHORBIACEAE | Native |
Baccaurea seemannii | EUPHORBIACEAE | Native | Maesa insularis | MYRSINACEAE | Native |
Balaka microcarpa | ARECACEAE | Native | Maesopsis eminii | RHAMNACEAE | Exotic |
Barringtonia edulis | LECYTHIDACEAE | Native | Medusanthera vitiensis | ICACINACEAE | Native |
Barringtonia seaturae | LECYTHIDACEAE | Native | Melicope cucullata | RUTACEAE | Native |
Buchanania attenuata | ANACARDIACEAE | Native | Melochia degeneriana | STERCULIACEAE | Native |
Calophyllum cerasiferum | CLUSIACEAE | Native | Metroxylon vitiense | ARECACEAE | Native |
Calophyllum vitiense | CLUSIACEAE | Native | Myristica castaneifolia | MYRISTICACEAE | Native |
Canarium harveyi | BURSERACEAE] | Native | Myristica chartacea | MYRISTICACEAE | Native |
Cliderma hirta | MELASTOMATACEAE | Exotic | Myristica gillespieana | MYRISTICACEAE | Native |
Cerbera manghas | APOCYNACEAE | Native | Myristica grandifolia | MYRISTICACEAE | Native |
Citronella vitiensis | ICACINACEAE | Native | Neuburgia corynocarpa | LOGANIACEAE | Native |
Cordyline fruticosa | ASPARAGACEAE | Native | Pagiantha thurstonii | APOCYNACEAE | Native |
Cordyline terminalis | ASPARAGACEAE | Native | Palaquium horneii | SAPOTACEAE | Native |
Crossostylis seemann ii | RHIZOPHORACEAE | Native | Palaquium porphyreum | SAPOTACEAE | Native |
Cryptocarya constricta | LAURACEAE | Native | Palaquium vitilevuense | SAPOTACEAE | Native |
Cyathocalyx insularis | ANNONACEAE | Native | Pandanus tectorius | PANDANACEAE | Native |
Cynometra insularis | FABACEAE | Native | Parinari insularum | CHRYSOBALANACEAE | Native |
Decaspermum vitiense | MYRTACEAE | Native | Pinanga coronata | ARECACEAE | Exotic |
Dillenia biflora | DILLENIACEAE | Native | Pittosporum arborescens | PITTOSPORACEAE | Native |
Dolicholobium latifolium | RUBIACEAE | Native | Pittosporum pickeringii | PITTOSPORACEAE | Native |
Dysoxylum lenticellare | MELIACEAE | Native | Planchonella garberi | SAPOTACEAE | Native |
Dysoxylum quercifolium | MELIACEAE | Native | Podocarpus neriifolius | PODOCARPACEAE | Native |
Dysoxylum richii | MELIACEA | Native | Polyscias multijuga | ARALIACEAE | Native |
Elaeocarpus kambi | ELAEOCARPACEAE | Native | Pommetia pinnata | SAPINDACEAE | Native |
Emmenosperma micropetalum | RHAMNACEAE | Native | Psychotria amoena | RUBIACEAE | Native |
Endiandra elaeocarpa | LAURACEAE | Native | Psychotria cf. archboldiana | RUBIACEAE] | Native |
Endiandra gillespiei | LAURACEAE | Native | Psidium guajava | MYRTACEAE | Exotic |
Endospermum macrophyllum | EUPHORBIACEAE | Native | Samanea saman | FABACEAE | Exotic |
Ervatamia obtusiuscula | APOCYNACEAE | Native | Schefflera seemanniana | ARALIACEAE | Native |
Ficus barclayana | MORACEAE | Native | Scirpodendron ghaeri | CYPERACEAE | Native |
Ficus fulvo-pilosa | MORACEAE | Native | Semecarpus vitiensis | ANACARDIACEAE | Native |
Ficus pritchardii | MORACEAE | Native | Storckiella vitiensis | LEGUMINOSAE | Native |
Ficus theophrastoides | MORACEAE | Native | Swietenia marcophylla | MELIACEAE | Exotic |
Ficus vitiensis | MORACEAE | Native | Symplocos leptophylla | SYMPLOCACEAE | Native |
Garcinia myrtifolia | CLUSIACEAE | Native | Syzygium cornynocarpum | MYRTACEAE | Native |
Garcinia pseudoguttifera | CLUSIACEAE | Native | Syzygium curvistylum | MYRTACEAE | Native |
Garcinia sessilis | CLUSIACEAE | Native | Syzygium malaccense | MYRTACEAE | Native |
Gardenia storckii | RUBIACEAE | Native | Syzygium sp. | MYRTACEAE | Native |
Geissois superba | CUNONIACEAE | Native | Syzygium spp. | MYRTACEAE | Native |
Geniostoma macrophyllum | LOGANIACEAE | Native | Tapeinosperma ligulifolium | MYRSINACEAE | Native |
Geniostoma rupestre | LOGANIACEAE | Native | Tapeinosperma megaphyllum | MYRSINACEAE | Native |
Gironniera celtidifolia | ULMACEAE | Exotic | Terminalia pterocarpa | COMBRETACEAE | Native |
Glochidion seemannii | PHYLLANTHACEAE | Native | Timonius affinis | RUBIACEAE | Native |
Glochidion vitiense | EUPHORBIACEAE | Native | Turrillia vitiensis | PROTEACEAE | Native |
Gmelina vitiensis | LAMIACEAE | Native | Vavaea amicorum | MELIACEAE | Native |
Gnetum gnemon | GNETACEAE | Native | Vavaea harveyi | MELIACEAE | Native |
Gonystylus punctatus | GONYSTYLACEAE | Native | Veitchia joannis | ARECACEAE | Native |
Guioa chrysea | SAPINDACEAE | Native | Xylopia pacifica | ANNONACEAE | Native |
Appendix 3
Trait values of the 5 functional traits measured on the 31 selected dominant plant species in Colo-i-Suva Park (Fiji). Abbreviations and description of the five functional traits (LDMC, SLA, C:N, LNC & Height) are defined in Table 1.
Plant species | SLA mm2.g−1 | LDMC mg.g−1 | N % | C/N | Height cm |
|---|---|---|---|---|---|
Aglaia elegans | 12.9 | 316.4 | 2.023 | 21.44 | 140.4 |
Aglaia vitiensis | 11.76 | 317.5 | 1.739 | 27.66 | 158 |
Amaroria soulameiodes | 13.74 | 221.9 | 2.22 | 19.39 | 413.8 |
Anacolosa lutea | 13.11 | 268.5 | 2.467 | 16.48 | 180.2 |
Astronidium confertiflorum | 11.84 | 237.7 | 1.075 | 31.08 | 183.6 |
Atuna racemosa | 16.11 | 335.1 | 1.367 | 32.53 | 215 |
Barringtonia edulis | 21.07 | 206.3 | 1.526 | 27.77 | 92.6 |
Citronella vitiensis | 13.31 | 235.1 | 0.902 | 49.05 | 162.4 |
Clidema hirta | 23.67 | 345.5 | 1.671 | 24.76 | 79.3 |
Crossostylis seemannii | 10.95 | 236.9 | 1.11 | 34.6 | 219.4 |
Cryptocarya constricta | 11.52 | 420.5 | 1.363 | 32.83 | 270.2 |
Cynometra insularis | 10.61 | 478.4 | 1.389 | 31.81 | 233.6 |
Dysoxylum quercifolium | 14.9 | 295 | 1.271 | 37.32 | 153.2 |
Endiandra elaeocarpa | 9.46 | 416.4 | 1.301 | 35.7 | 170.4 |
Garcinia myrtiflora | 9.15 | 386.3 | 0.739 | 63.28 | 158 |
Garcinia sessilis | 14 | 234.3 | 1.502 | 28.13 | 262.6 |
Glochidion seemannii | 17.62 | 307.2 | 1.584 | 25.06 | 101.4 |
Glochidion vitiensis | 14.35 | 282.3 | 1.435 | 29.86 | 94.5 |
Gnetum gnemon | 12.15 | 293.7 | 2.372 | 18.78 | 217.4 |
Haplolobus floribundus | 13.44 | 357.5 | 1.345 | 33.83 | 229.2 |
Heliconia psittacorum | 20.37 | 207.7 | 2.035 | 20.38 | 85 |
Myristica castaneifolia | 11.14 | 327.6 | 1.342 | 35.41 | 177.2 |
Myristica gillespieana | 11.72 | 286.7 | 1.579 | 30.45 | 158.4 |
Palaquium horneii | 10.3 | 345.1 | 1.02 | 45.84 | 198.4 |
Pinanga coronata | 16.72 | 356.2 | 1.384 | 29.56 | 280 |
Psychotria amoena | 12.06 | 205.3 | 1.852 | 22.21 | 295.4 |
Swietenia marcophylla | 29.6 | 263.4 | 1.486 | 29.43 | 134.8 |
Syzygium cornynocarpum | 15.09 | 297.1 | 1.098 | 40.55 | 150.4 |
Syzygium curvistylum | 8.79 | 366.9 | 0.701 | 65.03 | 184.2 |
Vavaea amicorum | 12.08 | 277.9 | 0.943 | 43.63 | 223 |
Xylopia pacifica | 14.29 | 331.4 | 1.461 | 29.73 | 138.8 |
Appendix 4
Relationship between the relative abundance and density of P. coronata for each plot.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Forey, E., Lodhar, S.Y.F., Galvin, S.D. et al. Alien palm invasion leads to selective biotic filtering of resident plant communities towards competitive functional traits. Biol Invasions 25, 1489–1508 (2023). https://doi.org/10.1007/s10530-022-02991-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02991-4