Abstract
Long-term population dynamics across heterogeneous habitats can be a major factor in determining species’ ability to expand their ranges and persist in novel environments, though this is largely unstudied in relation to invasion success. While largely restricted to disturbed semi-shaded microhabitats in its home range, the invasive herb Alliaria petiolata (garlic mustard) successfully invades intact forest understories – a novel microhabitat – in its introduced range. To test the hypothesis that source-sink metapopulation dynamics may be promoting A. petiolata’s incursion into the forest understory, we conducted two multi-season field surveys approximately a decade apart to evaluate trait variation, biomass allocation, and long-term population demographics of A. petiolata growing at the forest edge, within the intact forest understory, and in the intermediate transition zone between the two. Our results show that plants in the edge microhabitat had highest performance and reproductive capacity, as well as the highest density across most life stages in both survey periods. Populations in all microhabitats were predicted to grow (λ > 1) at the onset of the study. A decade later, declines in population size were only predicted in the forest understory (λ < 1). Since edge patches had the highest densities of adult plants which produced the most fruit and had larger reproductive biomass, edge populations may have sustained the lower-density forest populations through source-sink dynamics. Within a management context, eradication of A. petiolata populations in edge microhabitat could thus be an effective management strategy for reducing populations in the forest microhabitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding changes in the dynamic connections between the core and marginal habitats of a species’ range, as well as between habitats of differing suitability over time at the local scale is critical to predicting establishment and population growth in new habitat types (Kawecki 2008; Holt and Barfield 2011). Large, high-quality habitats can support source populations which produce high propagule numbers that then disperse into adjacent lower quality environments (Hodgson et al. 2011). Thus, these larger areas of high-quality habitat can be key determinants of whether species can shift and expand their geographic ranges outside of core habitat (Hodgson et al. 2011). Small-scale environmental and ecological variability is also a critical component of the rates and patterns of species’ range expansion (Bell and Lechowicz 1991; Stratton 1994; Baack et al. 2006; Bennie et al. 2013).
Temporal patterns of source-sink dynamics, metapopulation structure, and dispersal of propagules from patches of varying qualities are important, but relatively unstudied, topics in relation to range expansion of invasive species (Thomson 2007; Koehncke et al. 2013; Wallace and Prather 2013). Invasive species tend to establish at sites of disturbance, such as roadsides, where soil is disturbed and there is high anthropogenic activity (Burke and Grime 1996; Christen and Matlack 2006; Mortenson et al. 2009). However, geographic expansion outside of the core range of a species, such as sites of disturbance for invasive species, can lead to establishment within novel, peripheral habitats and possibly larger-scale range expansion over time (Kirkpatrick and Barton 1997). Improved understanding of how species-specific population dynamics affect range expansion into novel habitat types will have important implications for understanding future range shifts and developing the most effective long-term strategies for the management of invasive species (Biswas and Wagner 2015; Merow et al. 2017).
Alliaria petiolata (garlic mustard (Bieb.) Cavara & Grande) is a biennial Eurasian forb that is currently invading both forest edge and intact forest understory microhabitats across North America. In its native range, A. petiolata usually occupies disturbed semi-shaded forest edge microhabitats (Grime et al. 1988). In North America, A. petiolata readily invades disturbed areas with high to low shade conditions (Cavers et al. 1979) and has been increasingly invading intact woodland ecosystems – a novel microhabitat for this species (Nuzzo 1999, 2000). What determines whether and how forest understory colonization is successful for A. petiolata is not well understood (Rodgers et al. 2008; Stinson and Seidler 2014), and few other studies have captured more than a short snapshot of the invasion process for this species.
Given that higher-quality wooded understories have different environmental conditions than those at forest edges where A. petiolata originated, how do temporal patterns of microhabitat patch dynamics influence this species’ invasion into and persistence in a novel microhabitat type? Our study examines the role of trait variation and long-term population dynamics in relation to expansion into novel microhabitats, and more broadly, the potential for species to establish in novel microhabitat types of varying quality. Long-term monitoring of invasions is rare, but can provide vital insight into the invasion process, impacts on ecosystem processes, and community structure (Blossey 1999). To our knowledge, no other studies have investigated long-term changes in closely associated populations of A. petiolata across multiple growth microhabitats: the forest edge, forest understory, and the transition zone between the two. In this study, we investigate: (1) if growth microhabitat type significantly affects reproductive capacity, growth, and biomass allocation in A. petiolata, (2) if there are demographic differences among populations of A. petiolata growing in three distinct microhabitat types over a decadal time scale, and (3) which life-stage transitions are most important for sustained population growth within each microhabitat. We hypothesized that varying environmental conditions, namely light availability, across the three microhabitat types contribute to variable plant traits and patch dynamics that influence population growth rates and stability over time (Smith and Reynolds 2014; Stinson and Seidler 2014). Specifically, we predicted that population densities, biomass, and reproductive capacity of A. petiolata would be highest in the disturbed, semi-shaded edge sites and depressed in the intact forest understories.
Materials and methods
Study species
Alliaria petiolata was first documented in the United States on Long Island in 1868 (Nuzzo 2000), though this species has likely been introduced to the U.S. multiple times (Durka et al. 2005). Since its initial introduction in the nineteenth century, A. petiolata has become established across North America with large populations in the Northeast, Midwest, and Northwest United States, and sporadic populations established elsewhere (Nuzzo 2000). A member of the Brassicaceae, A. petiolata is non-mycorrhizal and produces multiple phytotoxic secondary compounds (Renwick 2002) that have been shown to disrupt North American plant-arbuscular mycorrhizal fungi (AMF) associations even in low concentrations (Callaway et al. 2004; Cantor et al. 2011). Along with producing novel phytotoxic chemicals (Barto et al. 2010), A. petiolata is highly invasive due to such attributes as early spring phenology (Engelhardt and Anderson 2011), high propagule pressure (Eschtruth and Battles 2009), and release from herbivores in its invaded range (Rodgers et al. 2008).
Alliaria petiolata has a biennial life-cycle. Seedlings emerge in the spring and then develop into basal rosettes over the first growing season. Basal rosettes overwinter and in the second growing season, adult plants form stalks which support the maturation of reproductive organs (flowers, fruits) before subsequently dying. Flowers are primarily self-pollinated, though cross pollination has been documented (Cruden et al. 1996; Durka et al 2005). Seed production per plant can vary due to factors such as environmental conditions and population density, but individual plants have been shown to produce up to approximately 8000 seeds under robust conditions (Nuzzo 2000). The majority of seeds (95%) disperse only short distances (≤ 1.14 m) from the maternal plant, though it is possible for seeds to be dispersed longer distances through epizoochory (Loebach and Anderson 2018), anthropogenic activities, flooding, or other mechanisms (Nuzzo 2000).
Study site and experimental design
To investigate A. petiolata growth, performance, reproduction, and long-term population dynamics across different microhabitats, we conducted a long-term observational study within the Harvard Forest Long-Term Ecological Research (LTER) site in Petersham, Massachusetts, USA (42.5°N Latitude; 72°W Longitude) where A. petiolata was growing across our focal microhabitats: disturbed forest edge microhabitats near trails, roadsides, and houses (hereafter: edge), the understory of a mature mixed-deciduous forest (hereafter: forest understory), and the transition zones between the forest edge and understory which had signs of semi-recent disturbance (hereafter: intermediate). Alliaria petiolata has been present at the Harvard Forest since at least 1979 (Jenkins et al. 2008). The forest canopy at this site is dominated by species such as red maple (Acer rubrum), red oak (Quercus rubra), birch (Betula sp.), American beech (Fagus grandifolia), and white pine (Pinus strobus) (Jenkins et al. 2008). Mean annual air temperature at the Harvard Forest is 7 °C, with temperatures ranging between 32 °C in the summer to − 25 °C in the winter. Total annual mean precipitation, including the water equivalent of snow, is 110 cm (Contosta et al. 2011).
Since we were interested in whether populations in different microhabitats demonstrated differential phenotypic and demographic properties, we sampled plants from three replicate populations growing within edge microhabitat, three replicate populations within forest understory microhabitat, and three replicate populations within intermediate microhabitat. This yielded a sampling design of 3 replicate populations per focal microhabitat (edge, intermediate, and forest understory; N = 3). We conducted two multi-year population samplings between 2003 and 2006 (sampling 1) and 2015–2016 (sampling 2) to monitor demographic and individual plant performance of A. petiolata populations.
The exact area of A. petiolata invasion varied for each replicate population. If the area of A. petiolata invasion exceeded 20 × 20 m for each replicate, a 20 × 20 m area was marked. To accomplish random sampling of the population, we then established five equally spaced transects across the entire length of the replicate sampling location. In 2004, we used a random number generator to determine placement of two 1-m2 quadrats on each transect for a total of ten quadrats per population to further facilitate random sampling of population density. In 2015, we re-established the transects and quadrats at the same sampling locations using the same methods. We also established one additional intermediate and forest understory population sampling locations, with 3 transects (six quadrats) in each microhabitat. All sampling areas were within approximately 275 m of each other.
Microhabitat environmental variation
To characterize environmental variation within the microhabitats, we periodically collected data (described below) on light availability, canopy cover, soil moisture, soil temperature, and nutrient availability between June 2003 and July 2016. Consistent methods were used for each respective type of data collection throughout the study period.
Light availability and canopy cover—We measured photosynthetically active radiation (PAR) on two days in June 2003, once in June 2004, and once in July 2004 at a height of 100 cm above the ground in the middle of each quadrat using a LI-COR 185A photometer (LI-COR Inc., Lincoln, Nebraska, USA). We captured hemispherical canopy cover photos on a single day in July 2005 and two days in July 2016 after canopy leaf out using a Nikon CoolPix 5000 camera with a Nikon FC-E8 fisheye lens converter (Nikon, Inc., Melville, New York, USA). We determined percent canopy openness from the hemispherical canopy photos using the “Sky” package (Bachelot 2016) in R version 3.4.1 (R Core Team 2016).
Soil moisture, temperature, and nutrient availability—We measured soil moisture on single days in each month of the following years: 2003 (June), 2004 (June and July), 2005 (April, May, and June), 2015 (September), and 2016 (July) using a ThetaProbe ML2x Soil Moisture Sensor (Delta-T Devices Ltd, Houston, Texas, USA) and soil temperature on single days in each month of the following years: 2003 (June), 2004 (July), 2005 (April and May), 2015 (September), and 2016 (May and July) using a Weber Probe instant-read digital thermometer (Weber-Stephen Products LLC, Palatine, Illinois, USA). We recorded soil temperature and soil moisture at a point nearest to the middle of every quadrat at all sampling locations. Temperature measurements were taken at a depth of approximately 3.5 cm.
In order to characterize possible differences in nutrient availability between microhabitats, we collected two 10 cm deep soil cores at opposite ends of each of the microhabitat patches in July 2016. We dried the soil cores in a laboratory drying oven at 105 °C for a minimum of 72 h. We then sieved a random subsample of each replicate to remove large debris and ground it to a fine powder using a Spex Sample Prep Grinder (SPEX SamplePrep, New Jersey, USA). We analyzed the subsamples for percent soil carbon (C), percent soil nitrogen (N), and soil C:N ratio with an Elemental Analyzer vario Micro Cube (Elementar Analysensysteme GmbH, Germany).
Individual plant trait measurements
We randomly selected five adult plants closest to each transect in every microhabitat patch for individual plant performance data. If there were fewer than twenty-five plants within a microhabitat patch (e.g. 5 plants × 5 transects), all identified adult plants within the microhabitat patch were included in data collection. Our transects therefore are not units of replication within a population, but rather a field method for selecting target plants for trait measurements without bias in our selection. We recorded the following traits in 2003, 2004, 2005, and 2016: (1) height to tip of plant from root collar, (2) number of branches per plant, and (3) number of siliques (fruits) per plant. Functional and fitness trait data were recorded for each plant when biomass was harvested at the point of reproductive maturity (between late June and late August) prior to fruit dehiscence and senescence. We then divided individuals into root, shoot, and reproductive organs, dried tissues at 60 °C for 10 days in a drying oven, and measured dry biomass for each plant.
We estimated the number of seeds produced per plant for the 2004 and 2016 seasons by obtaining the total weight of all seeds produced per plant and then dividing by the average weight of a single seed for that plant. Average seed weight was calculated by weighing 10 randomly selected seeds and dividing by 10. If a plant produced fewer than ten seeds, we counted the exact number of seeds produced.
Population demography surveys
In each quadrat, we recorded the number of individuals in each life stage: seedlings, first-year rosettes, second-year rosettes, and reproductive adults. Surveys occurred twice per year, once during the spring (April to early May) and once in the summer (late June to late July) of every study year. The specific survey periods varied from year to year because of differences in phenology timing. Spring surveys occurred at the approximate peak of seedling germination in the spring and summer surveys occurred before any considerable adult plant senescence. Sampling period 1 (hereafter: sampling 1) was in 2003, 2004, and 2005 and sampling period 2 (hereafter: sampling 2) was in 2015 and 2016.
Statistical analyses
For all responses, we constructed linear or generalized linear mixed models using the ‘lme4’ package in R version 3.4.1 (R Core Team 2017). For all environmental data, Gaussian error structures were used. We report all other model error structures in supplementary file 1. All models with a Gamma error structure used a log link. All models included microhabitat as a fixed factor and population (i.e. sampling location) as a random factor. For environmental, trait, and biomass data, year was also included as a random factor, except for soil C, N, and C:N because all data were collected within a single year. When we detected significant fixed effects from Analysis of Variance or Likelihood Ratio Tests (Whitlock and Schluter 2014), we used Tukey’s HSD post hoc test to determine pairwise comparisons between categories using the ‘glht’ function in R.
We checked for outliers in our models using Cook’s Distance plots. Two models (total biomass and elasticity of growth life stages) each had a single extreme data point (Cook’s distance > 1), and thus those data points were removed prior to final analysis. For total reproductive biomass and the ratio of reproductive biomass to vegetative biomass (non-reproductive root and shoot tissues), 11 of 784 observations were zero. Since Gamma error structures cannot include zeros, a miniscule number equal to one one-hundredth of the next smallest observation for reproductive biomass (0.0005) and the ratio of reproductive to vegetative biomass (0.019443) was added to the zero observations.
We determined the population growth rates (lambda; λ) for each of the microhabitat patches using life stage structured population matrix models following Caswell (2001). We constructed projection matrices for each of the microhabitat patches for the 2003–2004, 2004–2005, and 2015–2016 growing seasons in R version 3.2.3 (R Core Team 2015). The matrices consisted of transitions between the following life stages: seed to seedling, seedling to second-year rosette, second-year rosette to fruiting adult, and fruiting adult to seed (Fig. 1). We also included a seed to seed transition (i.e. ungerminated seeds that remained in the seed bank). Germination rates and seed to seed transitions rates were calculated from a previous germination experiment conducted at the same sampling locations at the Harvard Forest (Stinson et al. 2019). We were not able to collect data on plant fecundity in 2005. In order to estimate seed production for plants growing during the 2004–05 cycle, we averaged the mean number of seeds produced per plant in each microhabitat using 2004 and 2016 data. From 29 matrices (3 or 4 populations × 3 microhabitats × 3 life cycles), we obtained values for λ, transition sensitivities, and transition elasticities using the ‘popbio’ package (Stubben and Milligan 2007) in R version 3.2.3 (R Core Team 2015). Due to the linear life-stage transitions of A. petiolata, the elasticity values that we calculated represent two “loops”. The first loop consists of growth stage transitions from seed to seedling; seedling to rosette; and rosette to reproductive adult. The second loop consists of a single transition from seed back into the seed bank (e.g. seed to seed). We prioritize the sensitivity results in this paper (e.g., Kalisz et al. 2014; Stinson et al. 2019), but also report the elasticity values for comparison.
Results
Environmental variation across microhabitats
The growth microhabitats differed in light availability, canopy cover, soil moisture, and soil temperature (P < 0.05; Tables 1, 2). Soil C, N, and C:N were not affected by microhabitat (P > 0.05; Tables 1, 2). As expected, the edge microhabitats had higher photosynthetically active radiation (PAR) and canopy openness than both the intermediate and forest microhabitats, which were statistically similar to each other. In the spring, soil in the intermediate and edge microhabitats was warmest and soil in the forest was coolest. During the summer, soil was warmest in the edge and coolest in the forest, with all microhabitats different from each other (Table 2, Fig. 2). Unlike other environmental characteristics, soil moisture did not show a directional gradient from forest edge to forest interior. During both the spring and summer, the intermediate microhabitat was the wettest and the edge microhabitat was the driest. Later in the growing season, soil moisture in the forest was similar to both the edge and intermediate soil moisture, though the edge soil was significantly drier compared to the intermediate soil (Tables 1 and 2, Fig. 2).
Trait and biomass variation across microhabitats
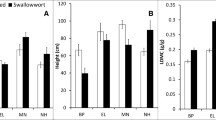
Adult A. petiolata plants differed significantly in their height, number of branches, number of fruits, total biomass, reproductive biomass, and reproductive: vegetative tissue ratio across the microhabitats (P < 0.05; Table 3, Figs. 3, 4). Surprisingly, the only non-significant difference in biomass allocation was for root:shoot ratio, which was similar for plants growing in all three microhabitats (P > 0.05; Table 3). Plants growing in the edge microhabitat were taller, produced more branches and fruits, and had more total and reproductive biomass compared to plants in the intermediate and forest microhabitats (Figs. 3, 4). Plants growing in the edge and intermediate microhabitats had similar reproductive: vegetative tissue ratios, which were significantly higher than for plants in the forest (Fig. 3). Plants growing in the forest produced fewer branches and fruits and had less total and reproductive biomass compared to both the edge and intermediate plants (Figs. 3, 4). However, the intermediate and forest microhabitats supported plants of similar height (Fig. 4). Plants growing in the edge produced more and heavier seeds than plants growing in the intermediate or forest microhabitats (Table 4, Fig. 5). While there was no interactive effect of year and microhabitat (P > 0.05), there was a significant effect of year on both seed weight and number, with all microhabitats showing a decline in both seed number and seed weight between 2004 and 2016 (Table 4, Fig. 5).
Alliaria petiolata (garlic mustard) biomass allocation of adult plants: total biomass (g; top panel), total reproductive biomass (g; middle panel), and the reproductive:vegetative tissue ratio (bottom panel) in three growth microhabitat types (edge, intermediate, and forest). Data were collected in 2003, 2004, 2006, and 2016. Population level means and standard errors (SEM) are shown categorized by microhabitat. Non-matching letters indicate significant differences between microhabitat categories
Alliaria petiolata functional and fitness traits of second-year plants: plant height (cm; top panel), number of fruits per plant (middle panel), and number of branches per plant (bottom panel) in three growth microhabitat types (edge, intermediate, and forest). Data were collected in 2003, 2004, 2006, and 2016 (height and fruit number) and 2003, 2006, and 2016 (branch number). Population level means and standard errors (SEM) are shown categorized by microhabitat. Non-matching letters indicate significant differences between microhabitat categories
Long-term population dynamics across microhabitats
We constructed demographic models to assess variation in the density of three life stages, population growth, and the contribution of each life stage to population growth, depending on microhabitat, sampling period, and the growth microhabitat × sampling period interaction.
Microhabitat, sampling period, and their interaction affected all life stage densities and λ (Pmicrohabitat < 0.05, Psampling period < 0.05, and Pmicrohabitat × sampling period < 0.05; Tables 4, 5). The sensitivity of two life stage transitions (rosette to adult and adult to seed transitions) was affected by microhabitat (Pmicrohabitat < 0.05; Table 5). Additionally, the adult to seed transition sensitivity was also affected by sampling period (Table 5). Sampling period only significantly affected transition elasticities during growth stages, i.e. the second “loop” (Table 5). None of the transition sensitivities or elasticities had a significant microhabitat × sampling period interaction (Table 5).
The edge consistently supported higher densities (plants per m2) of seedlings and first-year rosettes (Fig. 6). Early life stage densities (seedlings and first-year rosettes) have remained stable over time within the edge and intermediates microhabitats, while the forest populations have shown declines (Fig. 6). Fruiting adult densities (plants per m2) have declined in all microhabitats between the two sampling periods, with the forest microhabitat consistently supporting the lowest number adult plants (Fig. 6).
Alliaria petiolata life stage density per m2: mean number of seedlings (top panel), mean number of first-year rosettes (middle panel), and the mean number of fruiting adults (bottom panel) in three growth microhabitats (edge, intermediate, and forest understory) in two sampling periods. Data were collected in spring 2003, 2004, 2005, and 2016 for seedling density; summer 2003, 2004, 2005, 2015, and 2016 for first-year rosettes and fruiting adults. The error bars represent SEM. Population-level variation can be seen in supplementary file 3
Population growth (i.e. rate of replacement) was consistent across time and slightly above 1 (i.e. stable population size with minimal growth) within the intermediate microhabitat (Fig. 7). The population growth rates within the edge and forest understory microhabitats declined over time, with the forest populations sharply declining to a population growth rate below 1 in sampling period 2 (Fig. 7). There was also a significant effect of microhabitat on the contributions of different life stages to λ (Tables 5, 6). Microhabitat significantly affected the contribution of the rosette à adult transition and the adult to seed transition to λ. Edge populations showed higher sensitivity of λ to the rosette to adult transition than the intermediate and forest populations, while the forest populations had the strongest sensitivity to reproduction (adult to seed). Within each growth microhabitat, the importance of each transition to population growth was consistent, with the germinant to rosette transition sensitivity being highest for all three microhabitats. While there was no significant effect of microhabitat on transition elasticity, populations in the intermediate microhabitat had higher elasticity for the seed to seed transition, while the edge and forest populations had higher elasticity for the growth stage transitions (Table 6).
Mean population growth rates (λ) for Alliaria petiolata across three microhabitats (edge, intermediate, and forest understory) across time. Lambda was calculated for each microhabitat during 6 growing seasons (e.g. three life cycles) in 2003–2004 and 2004–2005 and 2015–2016. The grey horizontal line represents stable population size (λ = 1). Error bars represent SEM. Population-level variation can be seen in supplementary file 4
Discussion
We monitored A. petiolata in 2003–06 and again a decade later in 2015–16 across three growth microhabitats to investigate plant performance and the long-term population dynamics contributing to this species’ invasion into the intact forest understory in its introduced range. Since A. petiolata shows suppressed growth and reproductive output in reduced light environments and little evidence for genetic divergence across habitats (Meekins and McCarthy 2000, 2001; Myers et al. 2005; Stinson and Seidler 2014), we hypothesized that plants from the edge microhabitat would produce the most robust plants (tallest, branchiest, most fruits and seeds) in the highest densities and that this pattern would persist over time. We found that plants performed best and had the most reproductive output in the highest light edge microhabitat and plants in the forest understory performed the worst. Further, A. petiolata demographic performance (density and rate of replacement) was also highest in the edge microhabitat and lowest in the forest understory – and this trend was consistent over a decadal time scale. Population level means did not vary substantially from each other for each microhabitat. Together, this data supports the hypothesis that populations growing at the forest edge may be supporting forest understory invasion through source-sink metapopulation dynamics.
Source-sink dynamics and variation across microhabitats
Over the past several decades, A. petiolata’s range has expanded drastically not only in disturbed habitats, but also within intact, low disturbance woodland ecosystems (Nuzzo 1999, 2000) and is one of only a few successful invaders of forest understories across North America (Nuzzo 2000). However, the mechanisms behind the success of A. petiolata’s invasion into North American woodland understories is not clear (Stinson and Seidler 2014). Because A. petiolata grows within multiple microhabitats in its invaded range, it is important to understand how population dynamics contribute to invasion and microhabitat expansion of this species over time (e.g., Thomson 2007; Wallace and Prather 2013). At our study site, A. petiolata has been present in various microhabitats since the late 1970s, but is most commonly found in anthropogenically disturbed areas along roads, trails, and houses at the edge of the forest (Jenkins et al. 2008; Stinson et al. 2019). The forest populations in our study showed the lowest plant and population performance, confirming our prediction that forest understory is the lowest quality microhabitat. While intermediate microhabitats supported slower plant growth and rates of replacement compared to populations within the edge microhabitats, the intermediate populations showed consistent and sufficient reproductive capacity and population growth, indicating that these populations were not self-limiting (Figs. 3, 4, 5, 6, 7). The edge populations showed significantly higher and better provisioned reproductive output and may be sourcing propagules into the low reproductive capacity forests where there was high sensitivity to perturbations in adult fecundity (Table 6, Figs. 3, 4, 5 and 7). Since high propagule pressure can be a driving factor in the ability of exotic species to invade novel habitats (Colautti et al. 2006; Warren et al. 2012), our results indicate that edge microhabitat may be important long-term drivers of forest invasion.
Despite spatially close proximity to one another, we found that environmental characteristics, A. petiolata population attributes, and individual plant traits within each of the microhabitats do not strictly follow a directional gradient from forest edge to understory. Surprisingly, the three microhabitats did not represent a clear light, soil temperature, and soil moisture gradient, but rather a complex suite of environmental variables throughout the season (Table 2, Fig. 1), which may inhibit genetic divergence across microhabitats. Consistent with our hypothesis, our findings supported that the edge microhabitat maintained the most robust plants and population densities and forest populations were the most suppressed. Elsewhere, light availability has been shown to be one of the most important factors in the growth and reproduction of A. petiolata, with plants having suppressed growth and reproductive output in reduced light environments (Meekins and McCarthy 2000, 2001; Myers et al. 2005; Stinson and Seidler 2014). However, light availability alone did not appear to be the most significant environmental factor driving the results in the present study (Tables 2, 3, 4 and Figs. 2, 3, 4, 5, 6, 7). Periodic disturbance within the intermediate microhabitats may explain in part why the intermediate populations are outperforming the forest understory populations (Figs. 3, 4, 5, 6, 7), even though they have similar low-light conditions (Table 2; Nuzzo 1999; Eschtruth and Battles 2009). Another possibility is that leaf litter accumulation or composition in the forest understory could be restricting seedling recruitment and reducing population density, impeding invasion (Bartuszevige et al. 2007; Taylor et al. 2015). Moist soils, however, may be signs of frequent seed washout leading to early life stage limitations on population size (Table 6; Figs. 2, 6). Thus, moist soil in conjunction with warm soil during the spring in the intermediate microhabitat (Table 2; Fig. 2) may also lead to increased risk of disease compared to the edge microhabitat (Cipollini and Enright 2009; Ciola and Cipollini 2011), preventing intermediate populations from edge population equivalent performance.
As expected, biomass was highest for plants in the edge microhabitat, which had the most available light (Table 2; Figs. 3 and 5; Stinson and Seidler 2014). Unexpectedly, root: shoot ratios did not differ among the microhabitats, and reproductive: vegetative ratios were similar for plants in the edge and intermediate microhabitats (Table 3; Fig. 3). Significantly cooler soil temperatures in the spring and summer in addition to reduced light in the forest understory, could create inhospitable growth conditions (Table 2). Another possibility is that the environmental conditions cause a delay in phenology, ultimately reducing A. petiolata’s phenological niche separation with native species, which could reduce overall reproductive output and growth (Meekins and McCarthy 2000; Engelhardt and Anderson 2011). Meekins and McCarthy (2000) showed that nutrient addition to areas of low-density invasion increased population growth; however since the microhabitats did not differ in nutrient availability (C, N, C: N), we speculate that factors such as nutrient composition are affected by larger spatial-scale processes and do not play a significant role in A. petiolata invasion into the forest understory on this smaller spatial scale (Table 1).
Decadal dynamics across microhabitats
Long-term monitoring of invasive species across heterogeneous growth microhabitats is logistically difficult and rarely accomplished in ecological studies, but characterizing invasion over relevant spatial and temporal contexts is vital for understanding invasion processes, impacts on native ecosystems, population dynamics, and best management strategies (Blossey 1999; Menges 2000; Evans et al. 2012; Evans et al. 2016). Structured life stages and density dependence on short-term time scales likely contribute to complex factors in A. petiolata populations which could confound long-term dynamics and management, though this effect may differ based on habitat quality (Smith et al. 2003; Pardini et al. 2009; Evans et al. 2016). In our study, early life stage (seedling, rosette) densities were stable over decadal timescales in intermediate and edge microhabitats, but declined in the forest understory microhabitat. We observed a decline in the number of mature reproductive adults across all microhabitat types, but populations were not impacted equally, such densities were depressed more in the intermediate microhabitat (Table 4; Fig. 6). In addition to a decline in the number of reproductive plants, both the number of seeds produced and provisioning to individual seeds declined over time across all microhabitats (Table 4; Fig. 5). This supports the hypothesis that A. petiolata invasions may become less aggressive over time due to evolutionary constraints (Lankau et al. 2009), but we found that overall long-term plant performance varied considerably by microhabitat. Unmeasured abiotic factors related to climate change may also have affected A. petiolata population dynamics. For example, reduced precipitation and snowfall trends documented in New England in the last half of the 1900s (Huntington et al. 2004) could be causing overwintering A. petiolata rosettes to have increased sun exposure during overwintering. Increased sun exposure has been shown to cause irradiation damage and increased mortality thereby reducing adult plant densities (Fig. 6; Smith and Reynolds 2014). It is also possible that these environmental trends could increase herbivory during the winter and early spring (e.g., Yates and Murphy 2008), leading to declines in overall plant survival and densities in some microhabitats.
At the beginning of the study all three microhabitats supported populations which were self-sustaining and indicated future growth. At the end of the study, two of the growth microhabitat populations (edge and intermediate) were still predicted to grow (λ > 1). However, both the edge and forest understory populations showed declines in λ at the end of the study, with the forest populations predicted to decline over time (λ < 1). Since the edge and forest λ declines closely track, it is likely that the forest populations depend heavily on propagules from the edge. Specifically, A. petiolata populations have been present at the Harvard Forest for several decades, so we did not expect—nor did we see—low densities and high λ at the beginning of the study with reversed patterns at the end of the study, as would have been expected under newly founded populations (Evans et al. 2016). It is possible that the decrease in λ between sampling periods within the edge microhabitat may be due to harsh, transient environmental conditions such as low rainfall in 2016, which were asymmetrically impacting the plants in the highest light microhabitat (Table 2; Fig. 7), especially since we were not able to calculate multiple years of life-cycle transitions during sampling 2.
Overall, our results show that population performances have remained fairly stable, with the only significant decreases in the forest understory populations. While we do not report yearly variation or dynamics here, our results do not suggest future population declines of mature A. petiolata populations on a decadal time scale within 2 of the 3 microhabitats (Fig. 7; Nuzzo 1999). In fact, λ may be underestimated due to the contributions of ungerminated seeds in the seed bank to local population growth rates. Although the seeds in the seed bank were not accounted for in our population growth rate matrices, the majority of ungerminated A. petiolata seeds (> 80%) in the seed bank can remain viable for several years (Redwood et al. 2018) and are an important factor in A. petiolata’s ability to invade and persist in an area (Eschtruth and Battles 2009). Since this study was conducted in situ without manipulation, we did not control propagule spread between microhabitats, which could impact λ. Further, since germination rates were only calculated once at the beginning of the study, it is possible that long-term germination dynamics within and among microhabitats could affect population growth.
Future extensions and caveats
Biotic factors such as presence of earthworms and/or ungulates were not measured in this study but have been shown to impact A. petiolata growth and abundance (e.g., Knight et al. 2009; Kalisz et al. 2014; Dávalos et al. 2015). Whether and how these effects impact A. petiolata populations across all microhabitats is unclear and could be a fruitful avenue of research to help determine underlying biotic drivers of invasion across heterogeneous conditions. Further, the population dynamics, growth, and ability for A. petiolata to invade forests is likely dependent on other variables which occur at different spatial scales and/or are regionally dependent (Burls and McClaugherty 2008; Urbanowicz et al. 2018). Land use history, residence time, disturbance frequency, and physical landscape characteristics, such as elevation, would likely affect invasion success and population dynamics within and across regions.
We also note that our study design and sampling regime allowed us to capture variation at a finer temporal and spatial scale than what is shown for many of our response variables. A portion of additional temporal and population-level variation of our data is shown at a finer scale in supplementary files 2, 3, 4. Investigating the finer-scale variation was not the focus of this study, but has been shown to be important for population expansion and local adaptation in other species (e.g. Moore and Stanton 2014). Investigating finer scale temporal and microsite variation could be a useful focus for future research on A. petiolata.
Management implications
While we did not explicitly ask questions related to management strategies, understanding population dynamics related to density-dependence and population growth can contribute to successful mitigation of A. petiolata (Pardini et al. 2009; Evans et al. 2016). Designing effective management strategies is especially important since A. petiolata disrupts associations between native woodland species and AMF, thereby decreasing native species’ growth and fitness (Stinson et al. 2006; Callaway et al. 2004; Cantor et al. 2011). Without active and tailored management strategies, A. petiolata can alter above and below-ground biodiversity and ecosystem function (Meekins and McCarthy 1999; Stinson et al. 2006, 2007; Anthony et al. 2017), likely for years (Lankau 2011; Lankau et al. 2014). However, if populations show signs of long-term decline or are not self-sustaining, eradication may not be the best strategy in light of limited resources available to most land managers (Lankau et al. 2009). In our study, plants were densest and had the highest reproductive capacity in the edge and intermediate populations (Figs. 3, 4, 5, 6) and two of the three microhabitats had populations which did not show signs of future self-limitation (Fig. 7). Mitigation and eradication efforts focused on the edge and intermediate populations would therefore likely lead to reduced propagule pressure on not only those populations, but existing and new forest understory populations, since the edge and possibly intermediate populations may be acting as propagule sources for incursion into the forest understory (Figs. 5, 7). In addition, focusing mitigation efforts on the edge populations may have the most impact on reducing defensive phytochemical impacts on native biota (Smith 2015). Our results have important broad implications for management, and future studies directly assessing the multi-year response of A. petiolata to mitigation efforts would provide more clarity on the direct impacts of this species source-sink dynamics to management efforts.
Conclusions
Our results show that edge, forest understory, and intermediate microhabitats support plants with differential trait expression, biomass allocation, and population demographics. Over a decadal time scale, populations in the forest edge and intermediate microhabitats did not show signs of future population declines (λ > 1). Population growth rate declines in the forest understory populations could indicate environmental conditions that are not suitable for supporting self-sustaining populations, though light availability alone did not appear be the most important environmental indicator of robust populations. The edge and intermediate microhabitats likely sustain source populations that provide propagules into the forest understory microhabitat, since the edge and intermediate populations consistently showed population growth (λ > 1) across time and had plants with higher total reproductive biomass and fruit number. Our results suggest that management strategies in areas where A. petiolata is growing in heterogeneous environments should be carefully tailored to mitigate understory incursion, perhaps through focused eradications of source populations in edge microhabitat.
Data availability
The datasets generated and analyzed during the current study will be publicly available through The Harvard Forest Data Archive. No R code is novel; all R packages/functions were unmodified and are cited within the methods section of this manuscript.
References
Anthony MA, Frey SD, Stinson KA (2017) Fungal community homogenization, shift in dominant trophic guild, and appearance of novel taxa with biotic invasion. Ecosphere 8:e01951
Baack EJ, Emery NC, Stanton ML (2006) Ecological factors limiting the distribution of Gilia tricolor in a California grassland mosaic. Ecol 87:2736–2745
Bachelot B (2016) Sky: Canopy Openness Analyzer Package. R package version 1.0. https://www.CRAN.R-project.org/package=Sky
Barto EK, Powell JR, Cipollini D (2010) How novel are the chemical weapons of garlic mustard in North American forest understories? Biol Invasions 12:3465–3471
Bartuszevige AM, Hrenko RL, Gorchov DL (2007) Effects of leaf litter on establishment, growth and survival of invasive plant seedlings in a deciduous forest. Am Midl Nat 158:472–477
Bell G, Lechowicz MJ (1991) The ecology and genetics of fitness in forest plants. I. Environmental heterogeneity measured by explant trials. J Ecol 79:663–685
Bennie J, Hodgson JA, Lawson CR, Holloway CTR, Roy DB, Brereton T, Thomas CD, Wilson RJ (2013) Range expansion through fragmented landscapes under a variable climate. Ecol Lett 16:921–929
Biswas SR, Wagner HH (2015) Spatial structure in invasive Alliaria petiolata reflects restricted seed dispersal. Biol Invasions 17:3211–3223
Blossey B (1999) Before, during and after: the need for long-term monitoring in invasive plant species management. Biol Invasions 1:301–311
Burke MW, Grime JP (1996) An experimental study of plant community invasibility. Ecol 77:776–790
Burls K, McClaugherty C (2008) Landscape position influences the distribution of garlic mustard, an invasive species. Northeast Nat 15:541–556
Callaway RM, Thelen GC, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427:731–733
Cantor A, Hale A, Aaron J, Traw MB, Kalisz S (2011) Low allelochemical concentrations detected in garlic mustard-invaded forest soils inhibit fungal growth and AMF spore germination. Biol Invasions 13:3015–3025
Caswell H (2001) Matrix population models: construction, analysis, and interpretation [2nd Ed]. Sinauer Associates, Inc. Sunderland, MA, USA
Cavers PB, Heagy ML, Kokron RF (1979) The biology of Canadian weeds: 35. Alliaria petiolata (M. Bieb.) Cavara and Grande. Can J Plant Sci 59:217–229
Christen D, Matlack G (2006) The role of roadsides in plant invasions: a demographic approach. Conserv Biol 20:385–391
Ciola V, Cipollini D (2011) Distribution and host range of a powdery mildew fungus infecting garlic mustard, Alliaria petiolata, in southwestern Ohio. Am Midl Nat 166:40–52
Cipollini D, Enright S (2009) A powdery mildew fungus levels the playing field for garlic mustard (Alliaria petiolata) and a North American native plant. Invasive Plant Sci Manag 2:253–259
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037
Contosta AR, Frey SD, Cooper AB (2011) Seasonal dynamics of soil respiration and N mineralization in chronically warmed and fertilized soils. Ecosphere 2:1–21
Cruden RW, McClain AM, Shrivastava GP (1996) Pollination biology and breeding system of Alliaria petiolata (Brassicaceae). Bull Torrey Bot Club 123:273–280
Dávalos A, Nuzzo V, Blossey B (2015) Single and interactive effects of deer and earthworms on non-native plants. For Ecol Manag 351:28–35
Durka W, Bossdorf O, Prati D, Auge H (2005) Molecular evidence for multiple introductions of garlic mustard (Alliaria petiolata, Brassicaceae) to North America. Mol Ecol 14:1697–1706
Engelhardt MJ, Anderson RC (2011) Phenological niche separation from native species increases reproductive success of an invasive species: Alliaria petiolata (Brassicaceae)-garlic mustard. J Torrey Bot Soc 138:418–433
Eschtruth AK, Battles JJ (2009) Assessing the relative importance of disturbance, herbivory, diversity, and propagule pressure in exotic plant invasion. Ecol Monogr 79:265–280
Evans JA, Davis AS, Raghu S, Ragavendran A, Landis DA, Schemske DW (2012) The importance of space, time, and stochasticity to the demography and management of Alliaria petiolata. Ecol Appl 22:1497–1511
Evans JA, Lankau RA, Davis AS, Raghu A, Landis DA (2016) Funct Ecol 30:1053–1061
Grime JP, Hodgson JG, Hunt R (1988) Comparative plant ecology: a functional approach to common British species. Unwin-Hyman, London
Hodgson JA, Moilanen A, Wintle BA, Thomas CD (2011) Habitat area, quality and connectivity: striking the balance for efficient conservation. J Appl Ecol 48:148–152
Holt RD, Barfield M (2011) Theoretical perspectives on the statics and dynamics of species’ borders in patchy environments. Am Nat 178:S6–S25
Huntington TG, Hodgkins GA, Keim BD, Dudley RW (2004) Changes in the proportion of precipitation occurring as snow in New England (1949–2000). J Clim 17:2626–2636
Jenkins JC, Motzkin G, Ward K (2008) The Harvard Forest flora. An inventory, analysis and ecological history. Harvard Forest Paper 28. Harvard Forest, Harvard University, Petersham, Massachusetts, USA
Kalisz S, Spigler RB, Horvitz CC (2014) In a long-term experimental demography study, excluding ungulates reversed invader’s explosive population growth rate and restored natives. Proc Natl Acad Sci 111:4501–4506
Kawecki TJ (2008) Adaptation to marginal habitats. Annu Rev Ecol Evol Syst 39:321–342
Kirkpatrick M, Barton NH (1997) Evolution of a species’ range. Am Nat 150:1–23
Knight TM, Dunn JL, Smith LA, Davis J, Kalisz S (2009) Deer facilitate invasive plant success in a Pennsylvania forest understory. Nat Areas J 29:110–117
Koehncke A, Telschow A, Kondoh M (2013) Invasibility as an emergent property of native metapopulation structure. Oikos 122:332–340
Lankau RA (2011) Resistance and recovery of soil microbial communities in the face of Alliaria petiolata invasions. New Phytol 189:536–548
Lankau RA, Nuzzo V, Spyreas G, Davis AS (2009) Evolutionary limits ameliorate the negative impact of an invasive plant. Proc Natl Acad Sci 106:15362–15367
Lankau RA, Bauer JT, Anderson MR, Anderson RC (2014) Long-term legacies and partial recovery of mycorrhizal communities after invasive plant removal. Biol Invasions 16:1979–1990
Loebach CA, Anderson RC (2018) Measuring short distance dispersal of Alliaria petiolata and determining potential long-distance dispersal mechanisms. Peer J 6:e4477
Meekins JF, McCarthy BC (1999) Competitive ability of Alliaria petiolata (garlic mustard, Brassicaceae), an invasive, nonindigenous forest herb. Int J Plant Sci 160:743–752
Meekins JF, McCarthy BC (2000) Response of the biennial forest herb Alliaria petiolata to variation in population density, nutrient addition and light availability. J Ecol 88:447–463
Meekins JF, McCarthy BC (2001) Effect of environmental variation on the invasive success of a nonindigenous forest herb. Ecol Appl 11:1336–1348
Menges ES (2000) Population viability analyses in plants: challenges and opportunities. Trends Ecol Evol 15:51–56
Merow C, Bois ST, Allen JM, Xie Y, Silander JA (2017) Climate change both facilitates and inhibits invasive plant ranges in New England. Proc Natl Acad Sci 114:E3276–E3284
Moore KA, Stanton ML (2014) Propagule limitation, disparate habitat quality, and variation in phenotypic selection at a local species range boundary. PLoS ONE 9:e89404
Mortensen DA, Rauschert ESJ, Nord AN, Jones BP (2009) Forest roads facilitate the spread of invasive plants. Invasive Plant Sci Manag 2:191–199
Myers CV, Anderson RC, Byers DL (2005) Influence of shading on the growth and leaf photosynthesis of the invasive non-indigenous plant garlic mustard [Alliaria petiolata (M. Bieb) Cavara and Grande] grown under simulated late-winter to mid-spring conditions. J Torrey Bot Soc 132:1–10
Nuzzo V (1999) Invasion pattern of the herb garlic mustard (Alliaria petiolata) in high quality forests. Biol Invasions 1:169–179
Nuzzo V (2000) Element Stewardship Alliaria petiolata (Alliaria officinalis) Garlic Mustard. The Nature Conservancy. Arlington, VA
Pardini EA, Drake JM, Chase JM, Knight TM (2009) Complex population dynamics and control of the invasive biennial Alliaria petiolata (garlic mustard). Ecol Appl 19:387–397
Redwood ME, Matlack GR, Huebner CD (2018) Seed longevity and dormancy state suggest management strategies for garlic mustard (Alliaria petiolata) and Japanese stiltgrass (Microstegium vimineum) in deciduous forest sites. Weed Sci 66:1–9
Renwick JAA (2002) The chemical world of crucivores: lures, treats and traps. Entomol Exp Appl 104:35–42
Rodgers VL, Stinson KA, Finzi AC (2008) Ready or not garlic mustard is moving in: Alliaria petiolata as a member of Eastern North American forests. Bio Sci 58:426–436
R Studio Team (2016) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA, http://www.rstudio.com/
R Studio Team (2015) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA https://www.rstudio.com/
R Studio Team (2017) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA https://www.rstudio.com/
Smith LM (2015) Garlic mustard (Alliaria petiolata) glucosinolate content varies across a natural light gradient. J Chem Ecol 41:486–492
Smith LM, Reynolds HL (2014) Light, allelopathy, and post-mortem invasive impact on native forest understory species. Biol Invasions 16:1131–1144
Smith GR, Dingfelder HA, Vaala DA (2003) Effect of plant size and density on garlic mustard reproduction. Northeast Nat 10:269–276
Stinson KA, Seidler TG (2014) Physiological constraints on the spread of Alliaria petiolata populations in Massachusetts. Ecosphere 5:1–13
Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Klironomos JN (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol 4:e140
Stinson K, Kaufman S, Durbin L, Lowenstein F (2007) Impacts of garlic mustard invasion on a forest understory community. Northeast Nat 14:73–88
Stinson KA, Carley L, Hancock LMS, Donohue K (2019) Effects of maternal source and progeny microhabitat on natural selection and population dynamics in Alliaria petiolata. Am J Bot 106:821–832
Stratton DA (1994) Genotype-by-environment interactions for fitness of Erigeron annuus show fine-scale selective heterogeneity. Evol 48:1607–1618
Stubben CJ, Milligan BG (2007) Estimating and analyzing demographic models using the popbio Package in R. J Stat Softw 22:11
Taylor LAV, Hasenkopf EA, Cruzan MB (2015) Barriers to invasive infilling by Brachypodium sylvaticum in Pacific Northwest forests. Biol Invasions 17:2247–2260
Thomson DM (2007) Do source–sink dynamics promote the spread of an invasive grass into a novel habitat? Ecol 88:3126–3134
Urbanowicz C, Pasquarella VJ, Stinson KA (2018) Differences in landscape drivers of garlic mustard invasion within and across ecoregions. Biol Invasions 1–10
Wallace JM, Prather TS (2013) Comparative demography of an exotic herbaceous annual among plant communities in invaded canyon grassland: inferences for habitat suitability and population spread. Biol Invasions 15:2783–2797
Warren RJ, Bahn V, Bradford MA (2012) The interaction between propagule pressure, habitat suitability and density-dependent reproduction in species invasion. Oikos 121:874–881
Whitlock MC, Schluter D (2014) The Analysis of Biological Data, 2nd ed. Macmillan Learning, New York, New York, USA
Yates CN, Murphy SD (2008) Observations of herbivore attack on garlic mustard (Alliaria petiolata) in Southwestern Ontario, Canada. Biol Invasions 10:757–760
Acknowledgements
We would like to thank Dr. Kathleen Donohue for her contributions to the initial conception of this project. We would also like to thank the many people who provided project assistance, including Dr. Jennifer Albertine, Sydney-Alyce Bourget, Amanda Carreau, Dr. Dustin Haines, Michelle Jackson, Peter Muka, Adam Trautwig, and Dr. Julia Wheeler.
Funding
This work was supported by a National Science Foundation Long Term Ecological Research (LTER) Award #1237491 to Harvard University, Harvard Forest, Petersham, Massachusetts, USA. Author L.M.S.H received financial support from The National Science Foundation Graduate Research Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hancock, L.M.S., Stinson, K.A. Trait variation and long-term population dynamics of the invasive Alliaria petiolata (garlic mustard) across three microhabitats. Biol Invasions 25, 1473–1488 (2023). https://doi.org/10.1007/s10530-022-02990-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02990-5