Abstract
Black carp (Mylopharyngodon piceus), a large molluscivorous cyprinid native to eastern Asia, has become established in the Mississippi River basin in North America. The vulnerability of most North American snails and bivalves to black carp predation remains unknown, especially as it relates to juvenile black carp transitioning to mollusk prey. We conducted feeding experiments to assess the relative vulnerability of different mollusks to predation by age-0 and age-1 black carp. Age-0 black carp were tested with the North American native unionid Hamiota perovalis, a native pleurocerid snail Elimia livescens, and a native physid snail in the genus Physella. Age-1 black carp were tested with Elimia livescens, the North American native unionids Lampsilis cardium and Lampsilis cariosa, a native sphaeriid clam in the genus Musculium, and the non-native cyrenid clam Corbicula fluminea. Juvenile black carp readily attacked and consumed mollusks, but differences in vulnerability were evident among prey taxa exposed to age-0 and age-1 black carp. Age-0 black carp were able to consume Physella approaching the extent of their mouth gape. Age-1 black carp displayed greater feeding capabilities than age-0 black carp and easily consumed Elimia, Lampsilis, and Musculium. The only prey taxon that age-1 black carp struggled to consume was Corbicula, which had the thickest and widest shells relative to predator gape of all prey tested. Our results suggest that a wide range of small or juvenile mollusks are susceptible to predation by juvenile black carp but highlight that prey-specific characteristics, such as shell strength and shell size, may drive differential predation pressure on mollusk populations as the invaded range of black carp expands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions can have large ecosystem-level effects and failing to account for these effects hinders our understanding and management of invasion impacts (Strayer 2012). Freshwater mollusks, both bivalves and gastropods, play an integral role in the structure and function of freshwater ecosystems (Atkinson et al. 2021; Haag 2012; Haag and Williams 2014) and have long contended with threats from non-native competitors (Johnson et al. 2009; Nico et al. 2005; Riley et al. 2008). North American freshwater mollusks are among the most imperiled groups of organisms in the world, with roughly 75% of all species considered endangered, threatened, or of special concern (Johnson et al. 2013; Lopes-Lima et al. 2018; Lysne et al. 2008; Williams et al. 1993). Until recently, North American mollusks have not had to cope with a non-indigenous specialist predator (Moore et al. 2019; Nico et al. 2005). That situation has changed with the successful invasion of black carp (Mylopharyngodon piceus), a large molluscivorous fish native to Pacific drainages of northern Vietnam, eastern China, and southeastern Russia (Nico et al. 2005). Despite being introduced to the USA in the 1970s, with unreported captures as early as the 1990s and captures of wild fish reported in 2003 (Chick et al. 2003), the influence of this predator on invaded river ecosystems has not been evaluated.
Black carp are now established in the Mississippi River basin and their distribution and abundance is rapidly increasing to the extent that commercial fishers collect both adults and juveniles in the middle Mississippi River and major tributaries, including the Illinois, Missouri, and Ohio rivers (Kroboth et al. 2019). The establishment of reproducing populations within this important fluvial corridor has led some researchers to posit that black carp could be the “final nail in the coffin” for several mollusk species (Haag and Williams 2014). Nonetheless, the ecological effects of black carp on native and invasive mollusks within the Mississippi River basin are currently unknown. The few studies that have examined prey choice (Hung et al. 2013; Ledford and Kelly 2006) and feeding mechanics (He et al. 2013) of black carp were primarily designed to assess the use of black carp for biological control of snails that carry trematode parasites in fish farms, which was the principal reason for their importation to the USA (Nico and Jelks 2011; Nico et al. 2005). The only available risk assessment matched shell dimensions of native mollusks to black carp mouth size and concluded that based on size alone many native mollusks are at risk (Nico et al. 2005). As a result, little is known about the species’ potential food web impacts in the Mississippi River basin.
While we suspect that native North American mollusks are at great risk from black carp, there are many uncertainties in predicting vulnerabilities of most bivalves and snails to black carp predation. For example, the feeding capabilities and habits of the juvenile life stage of black carp have not been thoroughly assessed. Juveniles of many fish taxa can occur seasonally or periodically in high abundances and can have substantial food web impacts (Romare et al. 1999). Additionally, many non-native competitors of native mollusks, such as Corbicula spp., are also potential prey for black carp in their invaded range (Nico et al. 2005; Poulton et al. 2019). Given the diversity in shell size, shape, thickness, and ornamentation in native and non-native mollusks within the Mississippi River basin, there could be considerable variation in the susceptibility of different taxa to predation by black carp, especially at the juvenile life stage when these predators transition to hard-shelled prey (Liu et al. 1990).
We conducted a series of laboratory experiments to assess the relative vulnerability of different mollusk taxa to predation by age-0 and age-1 black carp. Our treatments included native and non-native mollusks that are predicted to be susceptible to predation and thus negatively affected by the establishment of black carp within the Mississippi River basin. We focused on juvenile black carp because little to no information exists on the trophic ecology of these predators within their invaded range at this life stage. Thus, our study represents an essential first step towards understanding the ecological effects of reproducing populations of black carp within river ecosystems of North America.
Materials and methods
Experimental predator and prey populations
Triploid black carp were acquired from a fish farm in Arkansas and maintained in a recirculating aquaculture system equipped with filtration to remove solid particulates and biofiltration to transform toxic metabolites. Temperature was held constant at 20 °C, dissolved oxygen always exceeded 8 ppm, salinity was maintained at 0.5 ppm, ammonia < 0.01 ppm, nitrite < 0.10 ppm, and alkalinity > 50 ppm (pH ~ 8.0) in the recirculating system. Prior to experiments, black carp were fed a commercially available 2-mm extruded, sinking feed (Skretting USA, Tooele, Utah, USA) ad libitum, and excess uneaten food and feces were removed daily. Mean (standard error; SE) total length of age-0 black carp used in experiments was 86.6 (2.1) mm, and mean total length (SE) of age-1 black carp used in experiments was 205.6 (5.2) mm. Mouth gape for each black carp was measured as external mouth width (external distance across the head, with the mouth closed), from the outside of one maxillary bone to the outside of the other using the same methods as Nico et al. (2005) and Shelton et al. (1995).
We tested different prey taxa with the two age groups of black carp as a result of differences in the numbers of individual mollusk taxa that could be collected or acquired at sizes small enough to be prey for age-0 or age-1 black carp (Table 1). Age-0 black carp were tested with the North American native unionid Hamiota perovalis (hereafter, Hamiota), the native pleurocerid snail Elimia livescens (hereafter, Elimia), and a native physid snail in the genus Physella. Age-1 black carp were tested with the North American native unionids Lampsilis cardium and L. cariosa (hereafter, Lampsilis), Elimia, a native sphaeriid clam in the genus Musculium, and the non-native cyrenid clam Corbicula fluminea (hereafter, Corbicula). Mollusk prey were either collected from local waterbodies or acquired from state or federal hatcheries. Corbicula were collected from Jonathan Creek (Kaskaskia River, Mississippi River drainage), Moultrie County, Illinois (39.58957, − 88.55595). Musculium were collected from ponds (Wabash River, Ohio River drainage) at the Sam Parr Biological Station, Kinmundy, Illinois (38.70993, − 88.74421). Elimia were collected from Copper Slough (Kaskaskia River), Champaign County, Illinois (40.09714, − 88.30726). Physella were collected from outdoor fishless tanks near the Kaskaskia Biological Station, Sullivan, Illinois (39.55117, − 88.57910). Lampsilis were acquired from the Virginia Fisheries & Aquatic Wildlife Center, Charles City, Virginia, USA, as well as from the U.S. Fish and Wildlife Services’ Genoa National Fish Hatchery, Genoa, Wisconsin. Hamiota were acquired from Missouri State University, Springfield, Missouri. All mollusks were held individually by taxon in separate 75.7-L holding aquariums equipped with filtration to remove solid particulates and biofiltration to transform toxic metabolites. Substrate in the holding aquariums was a mixture of rock, sand, and gravel and a 2-W powerhead was placed at the substrate within each holding aquarium to provide flow. Temperature was held constant at 20 °C, dissolved oxygen always exceeded 8 ppm, ammonia < 0.01 ppm, nitrite < 0.10 ppm, and alkalinity > 100 ppm (pH ~ 8.0) in the aquariums. Bivalves were fed a mixture of commercially available diets which consisted of 70 mL Spirulina spp. (Natural Foods Inc., particle size approximately 110 µm, Toledo, Ohio, USA), and 5 mL microalgae mix (Shellfish diet 1800, particle size 4–20 µm, Reed Mariculture Inc., Campbell, California, USA) mixed in 1 L of filtered tap water. Snails were allowed to graze on algae in the holding aquarium.
Prey vulnerability experiments
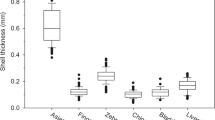
We examined the vulnerability of the different mollusk prey to predation by age-0 and age-1 black carp using treatments designed to assess the ability of black carp to feed on a specific prey taxon and not a mixed assemblage of prey with different vulnerabilities. Inherent differences in shell morphology among prey precluded standardizing prey treatments by shell dimensions. Measurements of shell dimensions (i.e., length, height, and thickness) were taken using a digital caliper (nearest 0.01 mm; Table 1). For snail taxa, shell thickness was measured at the lip (Gouveia et al. 2011). For bivalves, shell thickness was measured at the midpoint of each valve with individuals not used in experiments (n = 50 for each taxon). Mean (SE) of the longest shell axis (i.e., shell height for snails or shell length for bivalves) of prey exposed to age-0 black carp was 5.80 (0.09) mm and ranged from 3.6 to 9.6 mm. Mean (SE) of the largest shell axis of prey exposed to age-1 black carp was 12.74 (0.13) mm and ranged from 7.4 to 18.4 mm. Mean (SE) of the next largest axis (shell width) exposed to age-0 black carp was 3.43 (0.08) mm and ranged from 2.06 to 5.81 mm. Mean (SE) shell width of prey exposed to age-1 black carp was 9.01 (0.26) mm and ranged from 4.86 to 16.04 mm. Among prey tested with age-0 black carp, Hamiota had a thicker shell compared to Elimia and Physella (Fig. S1). Among prey tested with age-1 black carp, Corbicula had the thickest shells, Elimia shell thickness was intermediate, and Lampsilis and Musculium had the thinnest shells (Fig. S1). Lampsilis cardium and L. cariosa did not differ in size, shape, shell thickness, or burying behavior (t-test; all P > 0.05) and were used equally among trials.
Experimental trials were conducted within structureless sand environments with no flow. The sand substrate (Quikrete all-purpose sand) in all trials was approximately 5 cm deep and allowed for mollusks to fully bury. Experiments with age-0 black carp were conducted in 114-L aquariums (76 cm × 46 cm × 30 cm), whereas experiments with age-1 black carp were conducted in 284-L aquariums (123 cm × 47 cm × 53 cm). A set of pilot experiments indicated that the presence of conspecifics was necessary to elicit normal foraging behavior for black carp in the aquarium. As a result, both age classes of black carp were tested in groups of three fish per trial. Groups of black carp were transferred to experimental tanks prior to the start of experimental trials and were trained to forage in the structureless sand environment in a series of pretrials where individual food pellets were used as prey. Experimental trials were conducted in identical fashion to pretrials.
For each experimental trial, the black carp group was guided into an opaque cylindrical holding chamber placed within the center of the experimental tank. Six randomly selected individuals of a prey treatment were measured for length (bivalves) or height (snails), and width using a digital caliper (nearest 0.01 mm), weighed (nearest 0.01 g), and then placed in the aquarium on the surface of the sand substrate. Initial locations for individual prey were selected by using a random number generator to select X and Y coordinates that corresponded to 5-cm graduations on the X (length) and Y (width) axes of the aquarium. Initial coordinates that fell within the predator holding chamber were redrawn. Prey were allowed 1 h to acclimate to the aquarium environment and select for a different location in the aquarium, including burying in the sand or use of aquarium walls for snails. Pretrials indicated the 1-h acclimation period was sufficiently long enough for all bivalve prey taxa to bury completely in the sand. After the acclimation period, the locations and burying status of each prey individual was recorded. Prey were scored a “0” if fully exposed, a “1” if partially buried, and a “2” if completely buried (i.e., no part of the shell above the surface of the sediment). The predator group was then released and allowed to forage.
Age-0 black carp were allowed 1 h to forage, and age-1 black carp were allowed 2 h to forage. All trials were video recorded to document attacks (i.e., black carp picking up prey with their mouths) on individual prey. Each group (n = 12 groups for each age class) of black carp was tested with each prey treatment to exclude potential bias introduced by individual differences in predator behavior and each predator group was exposed to prey treatments in a unique and random order to control for the potential effect of treatment sequence. Groups (and thus individual black carp) were not used again after being tested with each prey treatment. Predator hunger level was standardized by fasting the black carp between trials (approximately 20 h). A standardized meal of 0.1 g of the commercial pellet diet was provided to each group after each trial regardless of the number of prey consumed. After each trial, the surviving prey were enumerated and removed from the tank. Prey were visually assessed for shell and body damage and given a qualitative score of “0” for no visible damage, “1” for minor (i.e., not lethal) shell damage, “2” for significant (i.e., lethal) damage, or “3” for complete destruction of the shell or if the individual was consumed.
Statistical analysis
Shell damage scores of 2 and 3 were combined to evaluate predation risk (i.e., the probability of being crushed or consumed) and analyzed separately for trials with age-0 black carp and age-1 black carp using repeated-measures generalized linear models (GENMOD procedure; SAS Institute, Cary, North Carolina, USA). A generalized estimating equation (GEE) model with a logit link function tested for an effect of prey treatment (i.e., taxonomic identity) on predation risk and accounted for multiple observations on the same predator group (Littell 2002). Generalized score statistics for each effect were computed for the GEE models and significance values were computed from the chi-square distribtion (GENMOD procedure). Post hoc differences in predation risk among prey treatments were compared with t-tests of pairwise comparisons between treatment least-squares means. The effect of burying behavior on predation risk was analyzed for bivalve taxa only and each bivalve taxon separately using the same model structure. Differences in burying behavior (fully buried, partially buried, or completely exposed) among bivalve taxa were also analyzed with a generalized linear model (GENMOD procedure). Variation in attack rate, calculated as strikes directed at prey per fish per hour, among different prey taxa was analyzed separately for trials with age-0 black carp and age-1 black carp using generalized mixed models (GLIMMIX procedure) with a random intercept for each predator group to account for repeated measurements. Differences in attack rates among prey treatments were compared with t-tests of pairwise comparisons between treatment least-squares means. Significance level for pairwise tests was modified with a Bonferroni adjustment to be α = 0.017 for trials conducted with age-0 black carp (3 groups) and α = 0.008 for trials conducted with age-1 black carp (4 groups).
Results
Both age classes of black carp displayed distinct searching behavior while foraging in the structureless sand environment. This behavior was generally consistent among individuals within each group and was characterized by individuals maintaining contact with the substrate, often taking and then quickly rejecting mouthfuls of the sand while searching for prey. Buried bivalves appeared to be easily removed from the sand substrate once detected, and most prey items were sampled (regardless of consumption); black carp were also observed to repeatedly sample stones within the substrate that resembled mollusk prey. Prey items were encountered haphazardly as a result of this foraging behavior and visual targeting of prey was rare. One exception was during trials with snail prey, when black carp would attack snails using the aquarium walls. Contrary to findings by Shelton et al. (1995), age-0 and age-1 black carp attacked and consumed snails at the water’s surface. During trials where all prey items were consumed, predator groups would often continue to search the substrate for the duration of the trial. Thus, changes in prey density did not appear to affect foraging behavior.
Burying behavior differed among bivalve taxa (Χ2 = 38.97, d.f. = 3, P < 0.0001). Corbicula were completely buried more often than any other taxon (all P < 0.007). Hamiota buried more often than both Lampsilis and Musculium, which did not differ in burying behavior (Z = − 0.56, P = 0.58) and were often fully exposed (mean 65% of all trials) or only partially buried (mean 25% of all trials). Burying behavior influenced vulnerability in Hamiota (Χ2 = 10.36, d.f. = 2, P = 0.005), Lampsilis (Χ2 = 8.04, d.f. = 2, P = 0.02), Musculium (Χ2 = 8.34, d.f. = 2, P = 0.02), and Corbicula (Χ2 = 8.04, d.f. = 2, P = 0.02). All bivalve prey were less vulnerable when buried completely (all P < 0.002), but no difference in vulnerability was detected between prey fully exposed or partially buried (all P > 0.3).
Attack rates on prey differed among prey taxa tested with age-0 black carp (F2,21 = 13.09, P = 0.0002) and age-1 black carp (F3,32 = 5.90, P = 0.003). Age-0 black carp attacked Elimia more often than Hamiota (t = − 5.12, P < 0.0001), which were rarely attacked (Fig. 1). Attack rates on Physella were intermediate compared to other taxa, but after correcting for multiple comparisons were not significantly different from Elimia (t = 2.50, P = 0.02) or Hamiota (t = − 2.50, P = 0.02) (Fig. 1). In trials conducted with age-1 black carp, Elimia and Corbicula were attacked more often than Musculium and Lampsilis (all P < 0.008; Fig. 2) but were not different from each other (Elimia vs. Corbicula: t = − 0.48, P = 0.64).
Prey taxa differed in their predation risk from age-0 black carp (Χ2 = 11.02, d.f. = 2, P = 0.004) and age-1 black carp (Χ2 = 9.94, d.f. = 3, P = 0.019). For age-0 black carp, Physella was more vulnerable than both Elimia (Z = − 4.38, P < 0.0001) and Hamiota (Z = − 6.74, P < 0.0001) (Fig. 3), despite consumed Physella shell widths approaching the maximum of age-0 predators’ mouth gape (Table 2). No difference in predation risk between Hamiota and Elimia was detected (Z = − 0.74, P = 0.46; Fig. 3). Similar to trials with age-0 carp, age-1 black carp were able to consume larger individuals of thinner shelled species (Table 2). All prey taxa except Corbicula, which had the thickest and widest shells (Table 1, Fig. S1), had similar levels of vulnerability to predation by age-1 black carp (all P ≥ 0.22). Corbicula had a lower probability of being crushed or consumed than all other taxa exposed to age-1 black carp (all P < 0.001; Fig. 4).
Discussion
Juvenile black carp readily attacked and consumed shelled prey, but differences in vulnerability to being crushed or consumed were evident among prey taxa exposed to age-0 and age-1 black carp. Physella were the most consumed prey by age-0 black carp, while thicker shelled Elimia and Hamiota were rarely consumed or damaged. Age-1 black carp in our experiment displayed a wider range of feeding capabilities and easily consumed Elimia along with Lampsilis and Musculium. The only prey taxon that age-1 black carp struggled to crush and consume was Corbicula, which had the largest shell widths relative to predator gape and shells approximately 500% thicker than other prey used in the feeding trials. Our experiment supports that a wide range of small or juvenile mollusks are likely susceptible to predation by juvenile black carp (Nico et al. 2005), and that age-0 black carp as small as 72 mm are capable of crushing and consuming shelled prey.
The highest attack rates were estimated during trials with prey taxa that were least vulnerable to predation. Black carp in both age classes were persistent in attacking individuals that were never consumed and, in the case of Corbicula, rarely damaged. One exception to this pattern was with Hamiota, which was neither attacked nor consumed at a high rate by age-0 black carp, possibly due to lower detection as Hamiota were smaller than other prey taxa and almost always buried during the experiment. When attack rates are combined with the probability of being crushed or consumed, these data provide insight into the capture efficiency or foraging performance of juvenile black carp on each prey taxon. For example, Lampsilis and Musculium were, on average, only attacked one to two times per individual, but those attacks usually resulted in consumption or destruction of the individual’s shell. In contrast, Elimia (in trials with age-0 black carp) and Corbicula (in trials with age-1 black carp) were attacked over ten and five times this rate, respectively, and had high probabilities of survival. Prey-specific differences in vulnerability have also been documented for the prey of other durophagous fishes (Meyer 1989; Wainwright 1987, 1988). For example, Wainwright (1987) found that both gape and crushing strength were constraining factors for molluscivorous Labridae, and that the relative importance of each factor depended on prey-specific physical properties, such as shell strength and shell size.
As a result of constraints related to jaw and pharyngeal mechanics, mouth gape may be a poor predictor of feeding performance for juvenile black carp. The prey-specific differences in vulnerability to juvenile black carp in our experiments lead us to conclude that, like other durophagous fish (e.g., Wainwright 1988), black carp are not solely gape-limited predators. During their ontogenetic transition to mollusk prey, young black carp are also constrained by crushing strength, and thus the development of their pharyngeal teeth (He et al. 2013; Hua and Huanliang 1988) and musculature associated with the pharyngeal apparatus (Gidmark et al. 2013, 2015). Gidmark et al. (2013) demonstrated that force–length relationships derived from musculature of the black carp jaw determine the amount of force able to be applied to prey of different sizes, with prey sizes intermediate for a given pharyngeal gape most vulnerable to crushing, especially as hardness or shell strength increases. For prey with weak and thin shells, however, consumption is likely more restricted by pharyngeal gape rather than strength of the pharyngeal apparatus (Wainwright 1987). Our results support this model as age-0 and age-1 black carp were able to crush and consume smaller, thinner-shelled prey (i.e., Physella, Lampsilis, and Musculium) at nearly the extent of their mouth gape, but larger, thicker-shelled prey, such as Elimia and Corbicula, were only consumed at higher ratios of mouth gape to shell width. Although we did not measure pharyngeal gape, our experiment suggests that the ability to overcome prey smaller than mouth gape, but larger than pharyngeal gape (e.g., Nico et al. 2005; Shelton et al. 1995), should only be expected for prey with the thinnest and weakest shells. There is also speculation as to whether black carp can consume mollusks with elongate shell dimensions (i.e., longer than high or wide) or when one shell dimension exceeds mouth gape (Nico et al. 2005). Age-1 black carp in our experiment were clearly able to overcome this limitation by manipulating the orientation of prey relative to their pharyngeal apparatus, allowing them to consume Lampsilis with shell lengths as large as 1.4 times their mouth gape. Thus, black carp are likely able to exploit mollusks with elongate or odd shaped shells that are larger than predicted by conservative models of vulnerability (Nico et al. 2005).
Our study and previous feeding experiments (Ben-Ami and Heller 2001; Shelton et al. 1995) also suggest that black carp foraging behavior presents a threat to mollusk populations in their invaded range because, regardless of the taxa directly consumed, a large number of mollusks could be sampled and subsequently injured by foraging black carp. This may be an overlooked aspect of their invasion in North America, where limited research focus has been on determining what black carp eat (Poulton et al. 2019) and less on determining the ecosystem effects of their seemingly indiscriminate foraging behavior. As predicted by Nico et al. (2005), sand substrates presented few problems to foraging black carp in our experiment. Fully buried Corbicula were often detected, dug up, and then repeatedly attacked by foraging age-1 black carp. Only 10% of Lampsilis and Musculium buried completely, and most were only partially buried or exposed, a behavior representative of most native bivalves with short siphons (Cummings and Graf 2010). Thus, encounter rates of foraging black carp with potential prey may be high within sand-dominated river systems, leading to increased rates of shell injury for more vulnerable taxa. In our experiment, 43% of prey exposed to age-0 black carp and 44% of prey exposed to age-1 black carp had some form of shell damage. Although we focused on the juvenile life stage, we posit that similar rates of injury could occur with larger, adult black carp foraging on larger mollusks of varying shell thickness. Thus, we predict that sublethal predation of mollusks would be common in natural systems invaded by black carp and contribute to decreased growth and fecundity (e.g., Lindsay 2010; Meyer and Byers 2005; Vermeij 1982), along with increased mortality rates in bivalves and snail populations through secondary predation (Chattopadhyay and Baumiller 2010; Vermeij 1983b) and increased susceptibility to parasites and disease (Gangloff et al. 2008). As with direct predation, the effects of sublethal predation are influenced by species specific defenses (Elner and Raffaelli 1980; Vermeij 1983a). However, the expansion of black carp populations within the Mississippi River basin may drive substantial ecosystem effects through sublethal predation, especially considering native mollusks have not experienced direct predation by an analogous fish predator (Nico et al. 2005).
As biological invasions continue unabated (Mack et al. 2000; Mainka and Howard 2010), understanding their effects is essential for conserving and restoring native fauna in invaded ecosystems (Simberloff et al. 2013; Strayer et al. 2006). Our results highlight variability in the relative vulnerability of different mollusks to predation by juvenile black carp. This variability can be expected to occur throughout this invasive predator’s ontogeny and suggests that prey-specific physical properties, such as shell strength and shell size, may drive differential predation pressure on mollusk populations as the invaded range of black carp expands. For example, the limitation of age-1 black carp to feed on Corbicula is overcome as black carp increase in size, as evidenced by Corbicula found in the diets of larger juvenile and adult black carp (Nico et al. 2005; Poulton et al. 2019). Thus, the potential for cascading food web effects of black carp within invaded ecosystems (e.g., Brönmark and Weisner 1996; Nico et al. 2005) will depend in large part on the distribution of different mollusk taxa, such as unionids of the upper Mississippi River basin (Tiemann et al. 2015), and their overlap with different sizes and ages of black carp. Future studies are needed to understand how differential predation pressure by black carp will affect important ecosystem services, such as filtration and nutrient cycling, provided by mollusk assemblages within the expanding range of black carp in North America.
Availability of data and material
Data made available upon request.
References
Atkinson CL, Halvorson HM, Kuehn KA et al (2021) Filter-feeders have differential bottom-up impacts on green and brown food webs. Oecologia 195:187–198
Ben-Ami F, Heller J (2001) Biological control of aquatic pest snails by the black carp Mylopharyngodon piceus. Biol Control 22:131–138
Brönmark C, Weisner SE (1996) Decoupling of cascading trophic interactions in a freshwater, benthic food chain. Oecologia 108:534–541
Chattopadhyay D, Baumiller TK (2010) Effect of durophagy on drilling predation: a case study of Cenozoic molluscs from North America. Hist Biol 22:367–379
Chick JH, Maher RJ, Burr BM et al (2003) First black carp captured in US. Science 300:1876–1878
Cummings KS, Graf DL (2010) Mollusca: bivalvia. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates. Elsevier, Amsterdam, pp 309–384
Elner RW, Raffaelli D (1980) Interactions between two marine snails, Littorina rudis Maton and Littorina nigrolineata Gray, a predator, Carcinus maenas (L.), and a parasite, Microphallus similis Jägerskiold. J Exp Mar Biol Ecol 43:151–160
Gangloff MM, Lenertz KK, Feminella JW (2008) Parasitic mite and trematode abundance are associated with reduced reproductive output and physiological condition of freshwater mussels. Hydrobiologia 610:25
Gidmark NJ, Konow N, LoPresti E et al (2013) Bite force is limited by the force–length relationship of skeletal muscle in black carp Mylopharyngodon Piceus. Biol Lett 9:20121181
Gidmark NJ, Taylor C, LoPresti E et al (2015) Functional morphology of durophagy in black carp, Mylopharyngodon piceus. J Morphol 276:1422–1432
Gouveia AR, Pearce-Kelly P, Quicke DL et al (2011) Effects of different calcium concentrations supplemented on the diet of Partula gibba on their morphometric growth parameters, weight and reproduction success. Malacologia 54:139–146
Haag WR (2012) North American freshwater mussels: natural history, ecology, and conservation. Cambridge University Press
Haag WR, Williams JD (2014) Biodiversity on the brink: an assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 735:45–60
He C, Zhou W, Wang H et al (2013) Mechanics of pharyngeal teeth of black carp (Mylopharyngodon piceus) crushing mollusk shells. Adv Eng Mat 15:684–690
Hua ZBLWL, Huanliang L (1988) The histological study on the germination and development of the pharyngeal tooth and callous pad of the black carp (Mylopharygodon Piceus). J Dalian Fish Univ
Hung NM, Duc NV, Stauffer JR et al (2013) Use of black carp (Mylopharyngodon piceus) in biological control of intermediate host snails of fish-borne zoonotic trematodes in nursery ponds in the Red River Delta. Vietnam Parasite Vector 6:142
Johnson PD, Bogan AE, Brown KM et al (2013) Conservation status of freshwater gastropods of Canada and the United States. Fisheries 38:247–282
Johnson PT, Olden JD, Solomon CT et al (2009) Interactions among invaders: community and ecosystem effects of multiple invasive species in an experimental aquatic system. Oecologia 159:161–170
Kroboth P, Cox C, Chapman DC et al (2019) Black carp in North America: a description of range, habitats, time of year, and methods of reported captures. N Am J Fish Manage 39:1046–1055
Ledford JJ, Kelly AM (2006) A comparison of black carp, redear sunfish, and blue catfish as biological controls of snail populations. N Am J Aquacult 68:339–347
Lindsay SM (2010) Frequency of injury and the ecology of regeneration in marine benthic invertebrates. Integr Comp Biol 50:479–493
Littell RC, Stroup WW, Freund R (2002) SAS for linear models. Wiley, Hoboken
Liu H, Li H, Zhai B et al (1990) Post-larval development of the masticating apparatus of black carp Mylopharyngodon piceus (Richardson). Act Hydrob Sinica 14:310–320
Lopes-Lima M, Burlakova LE, Karatayev AY et al (2018) Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia 810:1–14
Lysne SJ, Perez KE, Brown KM et al (2008) A review of freshwater gastropod conservation: challenges and opportunities. J N Am Benthol Soc 27:463–470
Mack RN, Simberloff D, Lonsdale WM et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Mainka SA, Howard GW (2010) Climate change and invasive species: double jeopardy. Integr Zool 5:102–111
Meyer A (1989) Cost of morphological specialization: feeding performance of the two morphs in the trophically polymorphic cichlid fish, Cichlasoma citrinellum. Oecologia 80:431–436
Meyer JJ, Byers JE (2005) As good as dead? Sublethal predation facilitates lethal predation on an intertidal clam. Ecol Lett 8:160–166
Moore TP, Collier KJ, Duggan IC (2019) Interactions between Unionida and non-native species: a global meta-analysis. Aquat Conserv 29:1438–1451
Nico LG, Jelks HL (2011) The black carp in North America: an update. Invasive Asian carps in North America. Bethesda, Maryland: American Fisheries Society, pp 89–104
Nico LG, Williams JD, Jelks HL (2005) Black carp: biological synopsis and risk assessment of an introduced fish. American Fisheries Society
Poulton BC, Kroboth P, George A et al (2019) First examination of diet items consumed by wild-caught black carp (Mylopharyngodon piceus) in the US. Am Midl Nat 182:89–108
Riley LA, Dybdahl MF, Hall RO Jr (2008) Invasive species impact: asymmetric interactions between invasive and endemic freshwater snails. J N Am Benthol Soc 27:509–520
Romare P, Bergman E, Hansson L-A (1999) The impact of larval and juvenile fish on zooplankton and algal dynamics. Limnol Oceanogr 44:1655–1666
Shelton W, Soliman A, Rothbard S (1995) Experimental observations on feeding biology of black carp (Mylopharyngodon piceus). Isr J Aquacult-Bamid 47:59–67
Simberloff D, Martin J-L, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Strayer DL (2012) Eight questions about invasions and ecosystem functioning. Ecol Lett 15:1199–1210
Strayer DL, Eviner VT, Jeschke JM et al (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651
Tiemann JS, McMurray SE, Sietman B, Kitchel L, Gritters S, Lewis, R (2015) Freshwater mussels of the upper Mississippi River. 3rd edition. Upper Mississippi Conservation Committee, pp 68
Vermeij GJ (1982) Unsuccessful predation and evolution. Am Nat 120:701–720
Vermeij GJ (1983a) Shell-breaking predation through time. Biotic interactions in recent and fossil benthic communities. Springer, pp 649–669
Vermeij GJ (1983b) Traces and trends of predation, with special reference to bivalved animals. Palaeontology 26:455–465
Wainwright PC (1987) Biomechanical limits to ecological performance: Mollusc-crushing by the Caribbean hogfish, Lachnolaimus maximus (Labridae). J Zool 213:283–297
Wainwright PC (1988) Morphology and ecology: functional basis of feeding constraints in Caribbean labrid fishes. Ecology 69:635–645
Williams JD, Warren ML Jr, Cummings KS et al (1993) Conservation status of freshwater mussels of the United States and Canada. Fisheries 18:6–22
Acknowledgements
We thank hatchery staff at the Virginia Fisheries & Aquatic Wildlife Center, Genoa National Fish Hatchery, and the M.C. Barnhart Lab at Missouri State for providing mussels used in this study. We thank Kyle Broadway and Randy Kramer at the Kaskaskia Biological Station and Mike Nannini and Evan Dlugos at the Sam Parr Biological Station for help with collecting mollusk prey. We also thank Carly Fenstermacher and Hayden Roberts for help measuring fish and mollusks in the laboratory. Additionally, we thank three anonymous reviewers for constructive comments that helped improve our manuscript. All animals used in this study were reared according to animal care and use guidelines established by the University of Illinois (Institutional Animal Care and Use Protocol 20068). This study was supported by the Philip W. Smith memorial fund administered through the School of Integrative Biology at the University of Illinois.
Funding
This study was supported by the Philip W. Smith memorial fund administered through the School of Integrative Biology at the University of Illinois.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Porreca, A.P., Butler, S.E., Tiemann, J.S. et al. Differential vulnerability of native and non-native mollusks to predation by juvenile black carp. Biol Invasions 24, 495–504 (2022). https://doi.org/10.1007/s10530-021-02658-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02658-6