Abstract
This paper describes a non-traditional fisheries management concept and an indicator-based framework to encourage and guide management of invasive lionfish (Pterois spp.) fisheries in the temperate and tropical western Atlantic. We introduce the concept of optimum lionfish yield (OLY)—an extension of the concept of ecologically sustainable yield—which considers local ecological health in the establishment of fishery management targets. In contrast to traditional fishery targets, OLY is a target exceeding maximum sustainable yield (MSY) that still provides relatively high sustainable yield, but further contributes to population suppression beyond what is achievable through targets at or below MSY. Thus, OLY seeks to balance management trade-offs from both natural resource and invasive species management perspectives. In this study, we developed an age-structured population model and applied the concept of OLY to quantify targets to initiate management of a nationally-managed lionfish fishery in Belize. Socioeconomic and ecological data were used as indicators to formulate OLY values. The model indicates that lionfish in Belize are biologically robust to fishing pressure, which corroborates previous findings. Fishing lionfish at rates above MSY levels is expected to substantially reduce population abundance, much more so than fishing at rates below MSY levels, while having relatively minimal impacts on yield. Population suppression can be further enhanced by reducing size at selection, but this is expected to be done at a significant cost to landings. Together, these data support continued establishment of (managed) commercial lionfish fisheries throughout the invaded range to provide an alternative sustainable fishery resource and serve as a means of national- and international-level control. While the concept and framework described here is introduced for management of invasive lionfish, it could be applied to management of other invasive species, both aquatic and terrestrial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous studies indicate invasive lionfish (Pterois spp.) negatively affect reef communities in the temperate and tropical western Atlantic through predation on, and competition with, native species (Albins and Hixon 2008; Green et al. 2012; Albins 2015; Ballew et al. 2016; Chagaris et al. 2017). To regulate population densities and mitigate ecological impacts, researchers and coastal managers have established lionfish control and management programs that focus on direct removals (e.g., Johnston et al. 2015). These programs, as well as community-based efforts such as recreational fishing tournaments, have been successful at reducing lionfish densities and minimizing impacts on local reefs (Frazer et al. 2012; Dahl et al. 2016; Green et al. 2017). Lionfish are, however, established in most marine habitats from North Carolina, USA to Brazil, and densities can exceed 300 fish/hectare (Côté et al. 2013). Consequently, wide-scale eradication no longer seems plausible without major technological advances for capture and a significant increase in the scale and magnitude of removal efforts.
To increase the scale and magnitude of removal efforts, researchers and managers have been promoting commercialization of lionfish over the last decade, particularly as seafood. Not only are lionfish plentiful, they are safe to consume and a nutritious source of protein (Tremain and O’Donnell 2014; Hardison et al. 2018; Morris et al. 2011a). Lionfish are now being harvested recreationally and commercially throughout much of the invaded region, but are not being managed as a fishery resource. Similar to other natural resource markets, as demand increases so too does the number of people who exploit the resource and, therefore, the number of individuals who may become (more) reliant on the resource. For this reason, lionfish fisheries would benefit from science-based management (Merrick 2018). Through proper management, invasive lionfish could serve as an alternative fishery resource, which could help create more diversified markets and potentially reduce fishing pressure on traditional native fishery species. Lionfish fisheries could also indirectly improve stocks of fishery species impacted by lionfish.
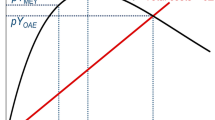
The objective of traditional fisheries management is to maintain high sustainable yield without causing overfishing. This is often accomplished by setting management targets at or below a population’s maximum sustainable yield (MSY) (Larkin 1977; Mace 2001) (Fig. 1). Maximum sustainable yield is defined as the maximum yield (or, harvest) that a population can sustain without having adverse effects on its ability to replenish itself. In United States fisheries management, for example, MSY and its associated fishing rate FMSY, are typically treated as upper limits with target levels set somewhat lower (Prager and Shertzer 2010). There exists, however, a paradox with the management of invasive lionfish fisheries. From a natural resource management perspective, lionfish fisheries should be managed to maintain high sustainable yield at levels that meet socioeconomic demand; however, from an invasive species management perspective, the fishery should also be managed to suppress populations to levels that mitigate ecological impacts. In the context of traditional fisheries management, these two objectives are not sought in tandem.
This paper describes a non-traditional fisheries management concept and an indicator-based framework to encourage and guide management of invasive lionfish fisheries throughout the western Atlantic. We propose the concept of optimum lionfish yield (OLY), which seeks to balance the competing objectives of high sustainable yield and population suppression, and describe one potential approach for formulating OLY values using the lionfish fishery in Belize as a case study. In contrast to traditional fisheries management targets, OLY is a target exceeding MSY that still provides relatively high sustainable yield, but further contributes to population suppression beyond what is achievable through targets at or below MSY (Fig. 1). Thus, OLY balances management trade-offs from both natural resource and invasive species management perspectives. Optimum lionfish yield is an extension of the concept of ecologically sustainable yield (ESY)—a target yield that a community or ecosystem can sustain without shifting to an undesirable ecological state (Zabel et al. 2003). While OLY targets lie above MSY and ESY targets lie below MSY, both concepts suggest that traditional fishery management practices that set single-species targets based solely on MSY can be insufficient. OLY and ESY suggest that managers establish fishery targets that also consider overall ecological health.
Since 2011, the Belize Fisheries Department and its partners have worked to establish a nationally managed lionfish fishery as a means of national-level control and to diversify local markets (Chapman et al. 2019; Searle et al. 2012). Commercial lionfish markets have been developed (e.g., as seafood and for jewelry) and the number of fishers targeting lionfish has grown, but management of the fishery has not yet begun (Chapman et al. 2019). One of the essential steps in establishing a well-managed fishery is understanding the population’s response to fishing pressure, which is typically achieved through application of population and stock assessment models. Thus, we developed an age-structured population model and applied the concept of OLY to quantify benchmark targets to initiate management of the lionfish fishery in Belize. Available socioeconomic and ecological data for lionfish in Belize were used as indictors to quantify and then validate that the proposed values of OLY satisfied the two general objectives of lionfish fishery management—high sustainable yield that meets socioeconomic demand and population suppression that mitigates ecological impacts. Data from the fishery are not currently available to model temporal dynamics of the population, but do exist to examine equilibrium behavior in response to fishing pressure and size at selection—two variables that fishery managers can regulate. Although no real population is ever in true equilibrium, reference points derived under this assumption are useful as long-term targets even if met with non-equilibrium dynamics in practice (Goodyear 1993; Mace 2001). Model results are discussed within the context of tradeoffs between sustainable yield and population suppression.
Methods
General overview of approach
An age-structured population model was developed to quantify benchmark management targets, including values of OLY and its associated fishing rate FOLY, to initiate management of a nationally-managed lionfish fishery in Belize. The model was used to evaluate trade-offs between equilibrium landings and abundance of lionfish in response to fishing pressure and size at selection. Available socioeconomic and ecological data for lionfish in Belize were used as indictors to quantify and then validate that the proposed values of OLY satisfied the two general objectives of lionfish fishery management. Functional forms and parameter values used in the population model were derived from Belize survey data when possible, but also drew on published data from nearby Little Cayman Island (Edwards et al. 2014; Gardner et al. 2015). Like Belize, lionfish were first observed at Little Cayman Island in 2008, and were considered established in 2009 (Schofield 2010). Data used for parameter values in this study were obtained through various surveys conducted in both locations in the period between 2011 and 2015. Age-specific parameter values are summarized in Table 1. The overall approach described here serves as a model framework for formulating lionfish fishery management targets throughout the western Atlantic.
Model description
Lionfish abundance at age (Na) was computed as
where Za is total instantaneous mortality at age a. The model included ages 1–7 years, with the oldest age treated as an accumulator class. The ages modeled were chosen based on the time since introduction to Belize and the initiation of this study (2008–2015). A plus group (7+) was used to account for older fish that may exist in the population, since we assume maximum age is 20 years (see below). The abundance of recruits (N1, age-1 fish) was computed using the steepness formulation of the Beverton-Holt spawner-recruit function (Beverton and Holt 1957)
where R0 is the asymptotic recruitment of age-1 fish, h defines the steepness of the curve, S is population fecundity (total egg production), and Φ0 is the number of spawners (eggs) per recruit in an unfished population. Population fecundity (S) was computed as
where 0.5 is the proportion of females in the population, ma is maturity at age a, and fa is fecundity at age a. Given a fishing mortality rate (F), total landings by number at age a (λa) was derived using the Baranov catch equation (Baranov 1918)
where Fa is the fishing mortality rate at age a, computed as the product of F and selectivity at age a (sa). Total landings in weight (\({\Upsilon}_{F}\)), as a function of F, was then computed as
where Wa is weight in kilograms at age a.
Length at age (La, total length in mm) was modeled using the von Bertalanffy growth equation (von Bertalanffy 1957)
where L∞ is asymptotic total length, K is the growth coefficient, and a0 is the theoretical age at which length is zero. The growth parameters provided by Edwards et al. (2014) for both sexes combined were used: L∞= 349, K = 0.42, and a0 = − 1.01. The relationship between total length (mm) and weight (W, in g) was described by fitting a power function to data from Belize (n = 352, sexes combined),
The resulting parameter estimates were \(\widehat{\mathrm{\alpha }}=0.000007\) and \(\widehat{\upbeta }=3.11\) (Fig. 2).
Maturity at age (ma) followed the logistic function from Gardner et al. (2015)
where La is total length at age, L50 = 190 mm is length at 50% maturity, and σ = 13.1 is the parameter characterizing the slope of the fitted curve. Sex ratio was determined from 375 lionfish captured throughout Belize from 2011 to 2015. Forty-nine percent (n = 184) were females and 51% (n = 191) were males. This proportion of females was not distinguishable from 0.5 (exact binomial test; p = 0.76) and thus the sex ratio in the model was assumed to be 50:50. A 50:50 sex ratio is consistent with lionfish population sex structure reported elsewhere (e.g., Edwards et al. 2014; Morris 2009). Annual fecundity at age (fa) was computed using the batch fecundity model from Gardner et al. (2015)
where B = 152 is the mean number of batches per female per year and parameters b1 = 308.67 and b2 = 58,265 define the number of eggs per batch as a function of fish total length. The range of values used in this study are consistent with estimates of lionfish fecundity from other locations (e.g., Morris 2009; Fogg et al. 2017).
Age-independent natural mortality (M) was computed using the mortality estimator recommended by the meta-analysis in Then et al. (2015)
where tmax is maximum age. Maximum age of lionfish in the wild is unknown. The longest observed lifespan of lionfish in captivity is 30 years (Potts et al. 2010). The oldest recorded lionfish in the western Atlantic was 8 years and was captured off of North Carolina (Potts et al. 2010). Given the lack of studies on maximum age of lionfish in the wild, their maximum observed age in captivity, and the presence of predation defense mechanisms, we assumed tmax = 20 years and, therefore, M = 0.3. The M value used here is typical of a moderately short-lived reef fish and is consistent with the natural mortality values used in other lionfish population modeling studies (e.g., Barbour et al. 2011; Edwards et al. 2014; Johnston and Purkis 2015; Morris et al. 2011b).
Selectivity at age (sa) was computed based on a normal distribution of sizes around the mean length at age, computed from Eq. 6 (SD = 28.2; Johnson and Swenarton 2016), with parameter Lvuln = 250 mm defining a threshold of vulnerability to harvest. That is, for each age, sa is the probability that length exceeds the vulnerability threshold, computed from the normal cumulative distribution function with mean La and SD = 28.2 mm. The value of Lvuln = 250 mm was derived from catch and fishing data obtained through semi-structured fisher and restauranteur interviews conducted throughout Belize in 2015 and 2016 (Chapman et al. 2019). Without data to estimate steepness (h), we assumed a value of 0.75. This value is consistent with that of other reef fish populations that have similar life histories as lionfish, including rockfish and scorpionfish (Scorpaenidae) (Forrest et al. 2010; Shertzer and Conn 2012; Thorson 2020).
Data from Belize on total lionfish abundance (Ntot) and total catch (Ctot) in numbers were used to estimate R0 and the current fishing mortality rate (F). Total initial abundance was estimated using geospatial data on marine habitat sizes across Belize (provided by the Belize Coastal Zone Management Authority and Institute) and habitat-specific lionfish density estimates derived from Belize-wide surveys conducted in 2015 (Chapman et al. 2019). The habitat-specific densities were scaled up to total habitat-specific abundances using the area of each habitat type. Total area of the main barrier reef, back reef areas, and atolls was estimated to be 60,704 hectares, which scaled total abundance to Ntot= 733,257 lionfish. Based on information obtained through the semi-structured interviews mentioned above, total (or current) catch in 2015 was estimated to be Ctot= 89,902 lionfish (Chapman et al. 2019). Given Ntot, natural mortality, selectivity, and the relative abundance at age implied by Eq. 1, we solved the Baranov catch equation in terms of numbers (i.e., Eq. 5 without the weight term) for the value of F that provided Ctot = 89,902 lionfish. We refer to this value, F = 0.32, as the current F. This procedure was then used to back-calculate equilibrium recruitment (Req)
Given total mortality at age (Za) and Ntot= \(\sum_{a}{N}_{a}=\mathrm{733,257}\), the recursive relationship of Eq. (11) provides \(\widehat{{R}_{eq}}=\mathrm{266,000}.\) This value was then used to compute \(\widehat{{R}_{0}}=\mathrm{289,900}\) as a function of equilibrium recruitment
where ΦF is the number of spawners per recruit of a population fished at rate F. For any F, ΦF is computed as
where ψa is the number of fish per recruit at age a computed using Eq. (11) with N1 = 1. The number of spawners per recruit of an unfished population (Φ0) is computed similarly, but with F = 0.
Model application
The population model was developed and implemented using R Statistical Software (R Core Team 2017) and applied to evaluate and identify initial lionfish fishery management targets for Belize, including values of OLY and FOLY. More specifically, the model was used to quantify equilibrium landings and abundance of lionfish across a range of fishing mortality rates from FLOW = 0.0 (no fishing effort) to FHIGH= 5.0 (the maximum rate modeled). Fishing rates of particular interest were FCURRENT (the fishing rate in 2015), FMSY, and FOLY. The model was also used to explore how lionfish size at selection (Lvuln) affects landings and abundance. For this analysis, fishing mortality was fixed at F = FOLY and selectivity was adjusted as described above, but with Lvuln set to different values across the range of 200–300 mm.
Formulation of OLY values
OLY and FOLY values will vary among management areas throughout the western Atlantic due to differences in local lionfish biology, socioeconomics, resources, data availability, and lionfish fishery management objectives (i.e., desire for greater yield vs population suppression or vice versa). This paper describes one possible indicator-based approach for formulating OLY targets. Available socioeconomic and ecological data for lionfish in Belize were used as indicators to quantify and then cross-check or validate that the proposed values of OLY and FOLY would satisfy the two general objectives of lionfish fishery management—high sustainable yield that meets socioeconomic demand and population suppression that mitigates ecological impacts. More specifically, OLY values were quantified and validated based on the estimated MSY, current yield levels (i.e., yield in 2015; Chapman et al. 2019), fisher and restauranteur satisfaction of current yield (Chapman et al. 2019), and Belize-specific lionfish ecological threshold density estimates (Chapman et al. 2019).
Sensitivity analysis
Sensitivity (Zi) of model results to parameter values were computed using local perturbation analysis (Ellner and Guckenheimer 2006)
where Y is the model output of interest and Y(pi) is the value of Y as a function of the ith parameter pi. A positive value of Zi shows that an increase in parameter pi leads to an increase in Y, while a negative value shows the opposite effect. A value of \(\left|{Z}_{i}\right|\ge 1.0\) indicates that a 10% change in parameter pi results in a > 10% change in output Y. The larger the \(\left|{Z}_{i}\right|\), the greater the sensitivity. We examined sensitivity of MSY, FMSY, and NMSY, the expected total abundance when fishing at FMSY. Model results were considered sensitive to perimeter values when \(\left|{Z}_{i}\right|\ge 1.0\). Sensitivities were also used to identify lionfish fishery research needs in Belize.
Results and discussion
Overall, the model indicates that harvest of lionfish effectively reduces population abundance (Fig. 3). Equilibrium abundance at FLOW = 0 was estimated at 1.5 million lionfish, while abundance at FHIGH = 5.0 was 220,000 lionfish. The model suggests that the current fishing effort in Belize has already reduced lionfish abundance by 34%, but increasing effort to FMSY = 0.67 could reduce abundance by an additional 21% (Fig. 3). The model also suggests that the population in Belize can withstand high rates of fishing without collapse, as indicated by the relatively high abundance of lionfish predicated at FHIGH—an improbable fishing mortality rate (Fig. 3). Other lionfish population modeling studies have reported similar findings. Morris et al. (2011b) predicted monthly exploitation of 27% of the adult population in the temperate and tropical western Atlantic would result in zero net growth; Barbour et al. (2011) predicted annual exploitation rates between 35 and 65% of the total population in North Carolina would be required to cause recruitment overfishing; Edwards et al. (2014) predicted annual exploitation rates between 15 and 35% of the total population at Little Cayman Island would be required to cause recruitment overfishing; and Chagaris et al. (2017) predicted fishing mortality rates greater than F = 1.0 are required to cause population declines on the West Florida Shelf. The results from these studies, which vary in terms of model design, data inputs, and geographic and spatial scales, all indicate a key population characteristic—invasive lionfish are biologically robust to fishing pressure. This population characteristic is likely due to lionfish biology and ecology (Côté et al. 2013) and is an important finding in terms of both lionfish control and establishing managed lionfish fisheries. This finding highlights the (1) biological sustainability of lionfish as a fishery resource and (2) indicates that the level of fishing effort required to overfish lionfish is substantial and unlikely to be achieved, especially without commercial-scale fishing practices. These data support continued establishment of (managed) commercial lionfish fisheries throughout the invaded range.
The model indicates that fishing lionfish at rates above FMSY is expected to contribute substantially to population suppression, much more so than fishing at rates below FMSY, while having relatively minimal impacts on yield (Fig. 3). Fishing at FHIGH is predicted to further reduce lionfish abundance by an additional 63%, while only reducing landings by 42% relative to fishing at FMSY (Fig. 3). While any harvest of lionfish beyond MSY can be considered beneficial for local reef ecology, the model indicates that trade-offs exist where increased population suppression is done at a cost to landings (Fig. 3). OLY seeks to balance these trade-offs to meet socioeconomic demand, while suppressing populations to levels that mitigate ecological impacts. OLY values in this study were formulated using available socioeconomic and ecological data for lionfish in Belize. These data were used as indicators to quantify and then validate that the proposed values of OLY and FOLY would satisfy the two general objectives of lionfish fishery management. While distribution challenges exists in Belize, Chapman et al. (2019) reported that Belizean fishers and restaurateurs were, in general, satisfied with catch levels in 2015 due to relatively sufficient supply to meet demand and the fact that lionfish are predominately caught and sold opportunistically. For these reasons, and because the current catch level was only 10% below the estimated MSY, yield in 2015 was used as a benchmark for quantifying OLY and FOLY. OLY, and subsequently FOLY, was quantified as the yield above MSY that produced equivalent yield as the current catch level. The proposed value of FOLY = 1.51 for lionfish in Belize is predicted to provide the same yield as the current fishing levels, thus generally satisfying current socioeconomic demand in Belize, while reducing abundance by an additional 42% relative to current levels (Fig. 3).
Belize-specific lionfish ecological threshold densities reported in Chapman et al. (2019) were then used to cross-check that the proposed OLY targets would sufficiently reduce lionfish populations to a level that is expected to mitigate their ecological impacts. Threshold densities were estimated for the five major Belizean marine protected areas (MPAs) in 2015 following the approach in Green et al. (2014). The approach in Green et al. (2012) estimates location-specific lionfish densities at which their ecological impacts are predicted to be mitigated. Threshold densities are quantified based on local sea surface temperature, reef fish densities, lionfish prey consumption rates, and lionfish prey production rates. Estimated lionfish threshold densities across the Belizean MPAs ranged from 10 to 40 fish/hectare (Chapman et al. 2019). The predicated abundance of lionfish at FOLY, converted to density, is 7 fish/hectare. Thus, the proposed OLY is expected to substantially reduce the ecological impacts of lionfish in Belize. OLY in this study was quantified and validated based on available ecological and socioeconomic indicators. When ecological and socioeconomic data are not available to formulate OLY targets, setting OLY targets based on a percent yield below MSY is a good initial approach. Based on the trade-offs identified in this study (Fig. 3), OLY targets based on percent yields closer to MSY are expected to favor higher yields while percent yields further from MSY are expected to favor population suppression.
Encouraging and/or achieving fishing effort beyond MSY levels will likely be a challenge for lionfish fishery managers. From the perspective of single-species fisheries management, fishing at rates beyond FMSY is economically counterproductive because more fishing effort is needed to obtain the same yield. However, from a broader management perspective, fishers, managers, researchers, and the public can view the effort beyond FMSY as effort devoted to marine conservation and control of an invasive species. This additional effort can be viewed and marketed as an investment in native species that are negatively affected by lionfish and/or are overfished. One approach to achieve target FOLY values is to set the target commercial F = FMSY, then make up the additional effort needed through recreational fishing. Regularly scheduled and well-advertised recreational lionfish derbies and tournaments, which almost always incorporate some form of marine conservation messaging, have been highly successful at reducing lionfish densities and impacts on local reefs throughout the invaded range (e.g., Green et al. 2017). Making up this effort deficit through recreational fishing is much more likely than through commercial fishing given that recreational fishing priorities are based more on angler satisfaction than on economic efficiency. Monitoring fishing effort and determining fishing mortality rates from these events would not be difficult; not only would these efforts aid in achieving increased fishing mortality, they can also enhance awareness and education of marine conservation issues.
In addition to fishing mortality and landings, size at selection is a variable fishery managers often regulate. Typically, the objective is to allow juveniles to reach maturity and spawn before becoming susceptible to the fishery. Like fishing mortality (Fig. 3), the model indicates that a trade-off exists in which increased harvest of smaller lionfish can significantly reduce abundance, and theoretically reduce the potential for ecological impact, but comes with a significant cost to landings (Fig. 4). Equilibrium landings and abundance at the current size selection threshold of 250 mm were estimated at 28.3 t (1000 kg) and 422,000 lionfish (Fig. 4). A shift in size selection to smaller lionfish (i.e., 200 mm fish) indicated a 68% reduction in landings as well as a 72% reduction in abundance, whereas an increase in size selection to larger lionfish (i.e., 300 mm fish) indicated a 20% increase in landings and a 56% increase in abundance (Fig. 4). This result is consistent with model predictions in Barbour et al. (2011), Morris et al. (2011b), and Edwards et al. (2014), all of which indicated that the removal of smaller (juvenile) lionfish may have the strongest effect on population abundance. As with other fish species, this characteristic is attributed to lionfish reproductive biology, particularly their age and size at maturity and their fecundity at age and size. Lionfish become mature at age 1 or ~ 100 mm (Edwards et al. 2014), and annual egg production per female generally increases with size and age (Morris 2009). As such, increased harvest of smaller lionfish reduces total annual egg production. While this population characteristic is common among fishery species, it is an important characteristic for both lionfish control and lionfish fishery management. For general lionfish control, it supports the need to target smaller lionfish to enhance population suppression. For lionfish fishery management, it indicates that size selection thresholds could help managers balance the trade-offs between sustainable yield and population suppression.
Overall, the model results were relatively sensitive to the growth and fecundity parameters (Table 2), which is consistent with other lionfish population modeling studies (e.g., Barbour et al. 2011; Edwards et al. 2014). While growth and fecundity estimates for lionfish from nearby Little Cayman Island were used in this study, robust age, growth, and fecundity data for lionfish in Belize could improve model predictions and overall management of the fishery, particularly estimates of FMSY. The largest sensitivity described the response of MSY to changes in the length–weight parameter β (\(\left|{Z}_{i}\right|\)= 19.9, Table 2), indicating the importance of precise β estimates. This level of sensitivity is due to the exponential relationship between length and weight and, therefore, the potential for β to strongly influence estimates of yield in weight. However, given the well-defined relationship between length and weight described in this study (Fig. 2), we do not view this as a critical research need in Belize. In general, model results were not sensitive to natural mortality, suggesting the model provided by Then et al. (2015) is adequate for describing natural mortality of lionfish, at least until lionfish-specific estimates become available. Data derived from several surveys conducted to develop Belize’s National Lionfish Management Strategy (Chapman et al. 2019) were used in this study. These included data on current landings, total lionfish abundance, fisher and restauranteur satisfaction of current landings, and lionfish ecological threshold densities. While these data are informative and satisfactory for model development and initiation of management, establishment of systematic and regular fishery-dependent and fishery-independent monitoring is imperative for successful long-term lionfish fishery management in Belize and elsewhere.
Conclusions
This paper describes the application and extension of fishery management concepts to the management of an invasive species. This paper introduces the concept of optimum lionfish yield, which seeks the balance management trade-offs from both natural resource and invasive species management perspectives. We applied this concept to quantify initial lionfish fishery management targets in Belize. This case study highlights an alternative approach to invasive species management and is an illustrative example of a sentiment summarized by Oficialdegui et al. (2020): “Legal instruments regulating the commercial use of non-native invasive species need to overcome simplistic approaches (full exploitation or complete ban) and involve more flexible and adaptive strategies because there is no one-size-fits-all solution.” Through proper management, invasive lionfish can serve as a biologically robust alternative fishery resource, which could help create more diversified markets and potentially reduce stress on traditional native fishery species. While the concept and framework described here is introduced for management of invasive lionfish, it could be applied to management of other invasive species, both aquatic and terrestrial.
References
Albins MA (2015) Invasive Pacific lionfish Pterois volitans reduce abundance and species richness of native Bahamian coral-reef fishes. Mar Ecol Prog Ser 522:231–243
Albins MA, Hixon MA (2008) Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Mar Ecol Prog Ser 367:233–238
Ballew NG, Bacheler NM, Kellison GT, Schueller AM (2016) Invasive lionfish reduce native fish abundance on a regional scale. Sci Rep 6:32169
Barbour AB, Allen MS, Frazer TK, Sherman KD (2011) Evaluating the potential efficacy of invasive lionfish (Pterois volitans) removals. PLoS ONE 6(5):e19666
Baranov FI (1918) On the question of the biological basis of fisheries. Izvestiya 1:81–128
Beverton RJH, Holt SJ (1957) On the dynamics of exploited fish populations. Fish. Invest. Minist. Agric. Fish. Food G.B. Ser. II 19.533 p.
Chagaris D, Binion-Rock S, Bogdanoff AK, Dahl K, Granneman J, Harris H, Mohan J, Rudd MB, Swenarton MK, Ahrens R, Patterson WF, Morris JA, Allen M (2017) An ecosystem based approach to evaluating impacts and management of invasive lionfish. Fisheries 42(8):421–431
Chapman JK, L Anderson, ML Fruitema, J Solomon, S Green, AK Bogdanoff, J Sabattis, Bueso D (2019) Belize National Lionfish Management Strategy, 2019–2023. Blue Ventures Conservation, London, UK, 102 p.
Côté IM, Green SJ, Hixon MA (2013) Predatory fish invaders: insights from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biol Cons 164:50–61
Dahl KA, Patterson WF, Snyder RA (2016) Experimental assessment of lionfish removals to mitigate reef fish community shifts on northern Gulf of Mexico artificial reefs. Mar Ecol Prog Ser 558:207–221
Edwards MA, Frazer TK, Jacoby CA (2014) Age and growth of invasive lionfish (Pterois spp.) in the Caribbean Sea, with implications for management. Bull Mar Sci 90:953–966
Ellner SP, Guckenheimer J (2006) Dynamic models in biology. Princeton University Press, New Jersey, 352 pp.
Fogg AG, Brown-Peterson NJ, Peterson MS (2017) Reproductive life history characteristics of invasive red lionfish (Pterois volitans) in the northern Gulf of Mexico. Bull Mar Sci 93(3):791–813
Forrest RE, McAllister MK, Dorn MW, Martell SJD, Stanley RD (2010) Hierarchical Bayesian estimation of recruitment parameters and reference points for Pacific rockfishes (Sebastes spp.) under alternative assumptions about the stock-recruit function. Can J Fish Aquat Sci 67(10):1611–1634.
Frazer TK, Jacoby CA, Edwards MA, Barry SC, Manfrino CM (2012) Coping with the lionfish invasion: can targeted removals yield beneficial effects? Rev Fish Sci 20:185–191
Gardner PG, Fraser TK, Jaconby CA, Yanong RPE (2015) Reproductive biology of invasive lionfish (Pterois spp.). Front Mar Sci 2:7.
Goodyear CP (1993) Spawning stock biomass per recruit in fisheries management: foundation and current use. In: Smith SJ, Hunt JJ, Rivard R (eds) Risk evaluation and biological reference points for fisheries management. Canadian Special Publication of Fisheries and Aquatic Sciences, pp. 67–81.
Green SJ, Akins JL, Maljkovi A, Côté IM (2012) Invasive Lionfish drive Atlantic Coral Reef Fish declines. PLoS ONE 7(3):e32596
Green SJ, Dulvy NK, Brooks ALM, Cooper AB, Akins JL, Miller S, Côté IM (2014) Linking removal targets to the ecological effects of invaders: a predictive model and field test. Ecol Appl 24(6):1311–1322
Green SJ, Underwood EM, Akins JL (2017) Mobilizing volunteers to sustain local suppression of a global marine invasion. Conserv Lett 10:726–735
Hardison DR, Holland WC, Darius HT, Chinain M, Tester PA, Shea D, Bogdanoff AK, Morris JA, Quintana HAF, Loeffler CR, Buddo D, Litaker RW (2018) Investigation of ciguatoxins in invasive lionfish from the greater Caribbean region: implications for fishery development. PLoS ONE 13(6):e0198358
Johnston MA, Gittings SR, Morris JA Jr (2015) NOAA National Marine Sanctuaries Lionfish Response Plan (2015–2018): responding, controlling, and adapting to an active marine invasion. Marine Sanctuaries Conservation Series ONMS-15–01. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries, Silver Spring, MD. 55 pp.
Johnston MW, Purkis SJ (2015) A coordinated and sustained international strategy is required to turn the tide on the Atlantic lionfish invasion. Mar Ecol Prog Ser 533:219–235
Johnson EG, Swenarton MK (2016) Age, growth and population structure of invasive lionfish (Pterois volitans/miles) in northeast Florida using a length-based, age-structured population model. PeerJ 4:e2730
Larkin PA (1977) An Epitaph for the concept of maximum sustained yield. Trans Am Fish Soc 106(1):1–11
Mace P (2001) A new role for MSY in single-species and ecosystem approaches to fisheries stock assessment and management. Fish Fish 2:2–32
Merrick R (2018) Mechanisms for science to shape US living marine resource conservation policy. ICES J Mar Sci 75:2319–2324
Morris JA (2009) The biology and ecology of invasive indo-pacific lionfish. PhD Thesis, North Carolina State University, Raleigh, NC, 168 pp.
Morris JA, Thomas A, Rhyne AL, Breen N, Akins L, Nash B (2011a) Nutritional properties of the invasive lionfish: a delicious and nutritious approach for controlling the invasion. Aquac Aquar Conserv Legis 5:99–102.
Morris JA, Shertzer KW, Rice JA (2011b) A stage-based matrix population model of invasive lionfish with implications for control. Biol Invas 13:7–12.
Oficialdegui FJ, Delibes-Mateos M, Green AJ, Sanchez MI, Boyero L, Clavero M (2020) Rigid laws and invasive species management. Conserv Biol 34:1047–1050
Potts JC, Berrane D, Morris JA (2010) Age and growth of lionfish from the western north Atlantic. Proc Gulf Caribbean Fish Institute 63:314
Prager MH, Shertzer KW (2010) Deriving acceptable biological catch from the overfishing limit: Implications for assessment models. North Am J Fish Manag 30:289–294
R Core Team (2017) R: A language and environment for statistical computing. https://www.R-project.org/
Schofield PJ (2010) Update on geographic spread of invasive lionfishes (Pterois volitans [Linnaeus, 1758] and P. miles [Bennett, 1828]) in the Western North Atlantic Ocean, Caribbean Sea and Gulf of Mexico. Aquat Invasions 5:S117–S122
Searle L, Chacon N, Bach L (2012) The Belize Lionfish management plan: an overview of the invasion, mitigation activities and recommendations. ECOMAR Technical Publication No 1. 80 p.
Shertzer KW, Conn PB (2012) Spawner-recruit relationships of demersal marine fishes: prior distributions of steepness. Bull Mar Sci 88:39–50
Then AY, Hoenig JM, Hall NG, Hewitt DA (2015) Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J Mar Sci 72(1):82–92
Thorson JT (2020) Predicting recruitment density dependence and intrinsic growth rate for all fishes worldwide using a data-integrated life-history model. Fish Fish 21:237–251
Tremain M, O’Donnell D (2014) Total mercury levels in invasive lionfish, Pterois volitans and Pterois miles (Scorpaenidae), from Florida waters. Bull Mar Sci 90(2):565–578
Von Bertalanffy L (1957) Quantitative laws in metabolism and growth. Quart Rev Biol 32:217–2331
Zabel RW, Harvey CJ, Katz SL, Good TP, Levin PS (2003) Ecologically sustainable yield: marine conservation requires a new ecosystem-based concept for fisheries management that looks beyond sustainable yield for individual fish species. Am Sci 91(2):150–157
Acknowledgments
We thank T. Kwak, P. Tester, D. Nowacek, C. Harms, L. Lee, S. Binion-Rock, S. Gittings, M. Balling, A. Chester, G. Piniak, E. García-Berthou, M. Allen, and one anonymous reviewer for their insightful comments that improved the manuscript. We thank the Belize Coastal Zone Management Authority and Institute for the geospatial data used to estimate total initial abundance, and we thank Blue Ventures Expeditions staff and volunteers that collected lionfish data in Belize. Financial support was provided by The Joseph E. and Robin C. Hightower Endowment, The Mesoamerican Reef Fund, The Summit Foundation, NOAA Fisheries International Affair’s Program, and the U.S. Fish and Wildlife Service Aquatic Nuisance Species Grant program. The scientific results and conclusions, as well as any views and opinions expressed herein, are those of the authors and do not necessarily reflect those of any government agency.
Funding
Financial support was provided by The Joseph E. and Robin C. Hightower Endowment, The Mesoamerican Reef Fund, The Summit Foundation, NOAA Fisheries International Affair’s Program, and the U.S. Fish and Wildlife Service Aquatic Nuisance Species Grant program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors have given consent for publication.
Availability of data and material
All data are available upon request.
Code availability
All code is available upon request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Additional analyses
Appendix: Additional analyses
Part 1: Exploring uncertainty in landings and abundance
As described in the main text, the 2015 field estimates of abundance and landings were 733,257 lionfish and 89,902 lionfish. Abundance and landings directly affect estimates of the current fishing mortality rate (current F) and the asymptotic recruitment of age-1 fish (R0) and, therefore, model output (see “Model Description” in the main text). Here, we explore uncertainty in our point estimates of abundance and landings on current F, R0, and model output.
To do this, we conducted Monte Carlo simulations (N = 2000 iterations in each analysis) in which each iteration repeated our analysis but with different values of (1) abundance and landings and then (2) current F and R0. First, we drew a new value of abundance and a new value of landings each from a normal distribution with mean equal to the 2015 field estimates and an assumed coefficient of variation (CV) of CV = 0.1 (Appendix Fig. 5a, b). Using these values, we computed distributions of current F (Appendix Fig. 5c) and of R0 (Appendix Fig. 5d). We then propagated uncertainty in current F and R0 into the estimated management quantities. Similar to above, we conducted Monte Carlo simulations (N = 2000 iterations) in which each iteration repeated our analysis but with different values of current F and R0 drawn from their distributions produced above (i.e., Appendix Fig. 5c, d). This produced distributions of current landings (Lcurrent), MSY, and OLY (Appendix Fig. 6a), as well as in the levels of abundance associated with those values (Appendix Fig. 6b). The general conclusion presented in the main text remains the same as that inferred from the corresponding point estimates—fishing at OLY provides current levels of landings (by design) while suppressing the abundance to substantially lower levels than current.
Assumed distributions of initial lionfish abundance (a) and landings (b) used to compute the current fishing rate (current F) (c) and the asymptotic recruitment of age-1 fish (R0) (d). Vertical lines indicate the 2015 field estimates of abundance and landings (a, b) and the point estimates of current F and R0 derived from those field estimates (c, d)
Distributions of results derived from assumed distributions of field estimates of abundance and landings. Panel A shows distributions of landings corresponding to Lcurrent (blue), OLY (purple), and MSY (green). Note that Lcurrent is not apparent because it overlaps entirely with OLY (by design). Panel B shows the levels of abundance that correspond to the landings in Panel A
We additionally propagated uncertainty in current F and R0 into estimates of equilibrium landings and abundance as a function of fishing rate (Appendix Fig. 7). We caution that this analysis does not produce true confidence bands as it is predicated on our assumed value of CV = 0.1. It does, however, indicate the conditional degree of uncertainty in results stemming from the field estimates of abundance and landings.
Equilibrium landings (a) and abundance (b) of lionfish in Belize across a range of fishing mortality rates: FMIN = 0 (filled square), FCURRENT = 0.32 (filled circle), FMSY = 0.67 (filled triangle), FOLY = 1.51 (filled diamond), and FHIGH = 5.0. Intervals shown represent the 2.5th and 97.5th percentiles from N = 2000 Monte Carlo simulations with variability in the 2015 field estimates of lionfish abundance and landings
Part 2: Exploring Uncertainty in the standard deviation of size-at-age
As part of our analyses in the main text, we examined the effects of size-at-selection on equilibrium abundance and landings (see Fig. 4 in main text). The size-at-selection, along with growth characteristics including the standard deviation of size-at-age, determined the pattern of selectivity as the proportion of fish-at-age that were vulnerable to fishing. In Appendix Fig. 8, we show how size-at-selection and standard deviation of size-at-age affect the resulting selectivity curves. In general, the curves are far more sensitive to the size-at-selection (our pivot) than to the standard deviation.
Selectivity as a function of the size-at-selection (Lvuln) and the standard deviation (SD) of size-at-age. Our base values were Lvuln = 250 mm and SD = 28.2, and values used to create Fig. 4 (main text) varied Lvuln over the range 200 mm to 300 mm, with SD = 28.2 in all cases. For this figure, we additionally varied SD ± 25% of the base value
Rights and permissions
About this article
Cite this article
Bogdanoff, A.K., Shertzer, K.W., Layman, C.A. et al. Optimum lionfish yield: a non-traditional management concept for invasive lionfish (Pterois spp.) fisheries. Biol Invasions 23, 795–810 (2021). https://doi.org/10.1007/s10530-020-02398-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02398-z