Abstract
Herbivory by non-native species can create strong direct and indirect effects on plant and arthropods communities that can potentially cross ecosystem boundaries. Yet, the cross-ecosystems impacts of non-native species are poorly understood. We took advantage of ongoing invasions by non-native ungulates in Patagonia, Argentina, to examine their cross-ecosystem impacts on water parameters, littoral vegetation and aquatic macroinvertebrate assemblages in wetlands. We found a gradient of invasion by non-native ungulates from intact (non-invaded) to highly invaded wetlands. These highly invaded wetlands had ~ 24% less vegetation cover, which was 72% shorter in height than vegetation in intact wetlands. As a result, the abundance of predatory macroinvertebrates such as Odonata (dragonflies) was reduced by ~ 90%; while Diptera were ~ 170% more abundant, and Oligochaeta were recorded mostly at invaded sites. In contrast, we did not find evidence that non-native ungulates altered water parameters. Understanding the indirect consequences of invasive non-native species is crucial for quantifying the real impacts of global change. Our results show strong cross-ecosystem impacts of non-native ungulates on macroinvertebrate wetland communities, highlighting the importance of indirect interactions beyond ecosystem boundaries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding how the homogenization of biodiversity at multiple scales and levels of organization will influence community and ecosystem functioning has become a major focus of modern ecology. When trying to understand how individuals move or energy flows through an ecosystem, ecologists have often focused on smaller components (e.g., the soil, a stream) (Lindeman 1942) of larger ecosystems (such as a forest) and have tended to ignore the inherent relationships between these ‘subsystems’. However, aquatic and terrestrial ecosystems are closely connected through the exchange of individuals and resources (Nakano and Murakami 2001; Marczak et al. 2007). Recently, it has been recognized that interactions between different “subsystems” should be considered to understand how ecosystems respond as a whole (Loreau et al. 2003; Wardle et al. 2004). For example, Maron et al. (2006) quantified how the introduction of foxes in the Aleutian Islands changed native plant communities by reducing the abundance of seabirds, which indirectly disrupted nutrient subsidies transported by the birds to the islands. Despite its key importance, indirect interactions—the effects of one species on another mediated by a third (Strauss 1991; Wootton 1994), are often ignored in studies of non-native species impacts (White et al. 2006) and little is known about their potential cross-ecosystems effects (but see Baxter et al. 2004; Benjamin et al. 2011; Jackson et al. 2016). Additionally, the great majority of studies of indirect effects have focused on the impacts of top predators (Estes et al. 2011).
Through predation, competition, and habitat destruction, non-native species can directly and indirectly affect native species by suppressing their population size and ultimately generating local extinctions (Mack et al. 2000; Simberloff et al. 2013). These impacts can create strong direct and cascading indirect effects. For example, intense herbivory by non-native ungulates can directly reduce aboveground biomass and alter nutrient cycles, and indirectly influence interactions with other herbivores, pollinators and seed dispersers (Williamson and Griffiths 1996; Mack and D’Antonio 1998; Vázquez and Simberloff 2004; Rodriguez-Cabal et al. 2013, 2019). Also, large ungulates can potentially affect input and resource flow across terrestrial-aquatic ecosystems via nutrient loading (Subalusky et al. 2017), removal of littoral vegetation, modifying river courses (Beschta and Ripple 2006) or by trampling (Barrios-Garcia and Ballari 2012). Despite their widespread introduction into different environments and regions of the world, non-native ungulates pose serious threats to conservation globally (Spear and Chown 2009) and studies of their impacts on lentic aquatic ecosystems are very scarce (but see Howell et al. 2019).
In this study, we evaluated the cross-ecosystem indirect impacts of three coexisting non-native ungulates on wetlands in Patagonia (Argentina). Wetlands are globally recognized as hotspots of biodiversity, both in terms of species composition and biological traits (Mitsch and Gosselink 2007) and play an important role in the provisioning of ecosystem services (Clarkson et al. 2013). However, wetlands are globally threatened by different factors such as pollution, changes in land use and non-native species (Mitsch and Gosselink 2007). The goal of this study was to evaluate the possible cross-ecosystem impacts of non-native ungulates on wetlands of Patagonia. Specifically, we asked whether non-native ungulates alter: (a) water parameters, (b) littoral vegetation and (c) aquatic macroinvertebrate assemblages.
Materials and methods
Study area and site selection
This study was conducted in Nahuel Huapi National Park (NHNP) (705,000 ha), located in northwestern Patagonia, Argentina. Climate is humid and cold, with an average of 0.6 °C in winter and 13.4 °C in summer (Garreaud 2009). The native forest vegetation belongs to the Subantartic biogeographical region (Cabrera and Willink 1973), dominated by the evergreen southern beech (Nothofagus dombeyi), with a dense understory of the native shrub maqui (Aristotelia chilensis) and bamboo (Chusquea culeou). Besides two native deer at very low densities, Hipocamelus bisulcus and Pudu puda (SIB 2020), there are no large herbivores in the area except for introduced ungulates, which represent the main source of disturbance in the area. Cattle (Bos taurus) and horse (Equus cabalus) were introduced by Europeans in the late eighteenth century (Novaro et al. 2000), and wild boar (Sus scrofa) in the early twentieth century for hunting purposes. Current browsing pressure by non-native ungulates is estimated to greatly exceed the historical herbivory pressure of the region (Vázquez 2002; Flueck 2010). For our study, invaded sites correspond to sites with documented presence of cattle, wild boars and horses for several years; while sites without ungulates correspond to NHNP regions where there are no historical records of the presence or evidence of introduced ungulates. All non-native species have feral populations, and currently are the most widespread ungulates in Patagonian forests (Jaksic et al. 2002), occupying 56% of the NHNP (Lauría Sorge and Romero 1999). In addition, 5% of the landscape in Patagonia is occupied by wetlands (Gaitán et al. 2011), which are used by free-ranging cattle, wild boar and horse, mainly for water drinking and high quality food resources.
We selected 16 wetlands (ranging from 0.4 to 1.8 ha) embedded within Nothofagus forests within the NHNP protected area (see Online Resource 1, Figure S1). Most wetlands in the area are seasonally inundated, but have water year-round with maximum water levels peaking during the rainy season (March–May). The wetland littoral vegetation was similar at all sites, and mainly composed of native grasses such as Carex chillanensis, Poa andina, Marsippospermum grandiflorum and Schoenoplectus californicus, and the non-native Holcus sp. (SIB 2020). Aquatic emergent vegetation was characterized by Juncus sp, Potamogeton sp and Myriophyllum sp (Perotti et al. 2005). Fish were absent in all wetlands.
Non-native ungulates abundance
At each wetland, we estimated ungulate relative abundance using camera-traps (Bushnell Trophy Cam HD Agressor). Cameras were active 24 h a day for 42 days: 21 days during January (mid Austral summer 2019) and 21 days in May (mid Austral autumn 2019), in order to capture most variability in the seasonal use of the wetlands by ungulates. Cameras were placed near animal trails and to cover most of the littoral area of wetlands (Harmsen et al. 2010). Ungulates roam freely throughout the year among these sites, so that littoral zones of some invaded wetlands are continuously degraded. Since sampled environments were all similar and target species were all large ungulates, we assumed that the probability of detection in our design was constant (Sollmann et al. 2013). We set the cameras to be triggered by motion to record 30 s videos, with 2 min interval between triggers. Capture events were considered independent (individual records) if recorded more than 15 min apart (O’Brien 2011). Independent records of cattle, horses and wild boar during the 42 days were summed as a measure of ungulate abundance (proxy measure of impact) at each site.
Cross-ecosystem impacts
Sampling was carried out during the austral summer of 2019, all variables measured at all sites between 16 and 27 of January. We measured a set of standard water parameters (pH, water temperature, dissolved oxygen and electrical conductivity) at a single point within each wetland (preferably where the water level was maximum) using a WA—2017SD Lutron multi-parameter sensor. We estimated vegetation cover in a 5 m wide strip around the coastal area of each site, by randomly placing five 0.5 × 0.5 m quadrats grilled in 25 cells. In each of the 25 cells, we registered vegetation height (cm) and the number of occupied cells to calculate mean vegetation cover (percentage). We collected macroinvertebrates by doing one minute sweeps using a D-framed net (250-μm mesh) (Cheal et al. 1993) in three randomly chosen points in the littoral area of each wetland and so as to sample water column, vegetation and surface sediments. Samples were collected in late January, when invertebrate diversity is typically highest (MacSween et al. 2019; Swartz et al. 2019). We preserved the samples in 70% ethanol for later taxonomical identification (Merritt and Cummins 2006). We identified all taxa to family level to calculate abundance and family richness at each site, except for worms that were classified into subclasses (Hirudinea and Oligochaeta).
Statistical analysis
We used separate generalized linear models (GLMs) with non-native ungulate abundance as a continuous explanatory variable to test their impacts on each abiotic (conductivity, pH, water temperature and dissolved oxygen) and biotic (vegetation cover and height, macroinvertebrates richness and abundance) response variables. To avoid multicollinearity, environmental variables were analyzed based on their Pearson correlation coefficients and controlling the variance inflation factors (Dormann et al. 2013). For macroinvertebrate richness and abundance, data from each site was pooled and analyzed using GLMs with Poisson and Negative binomial error distributions respectively (package MASS, Venables and Ripley 2002). To evaluate the amount of total variation explained by each model we used analysis of deviance (pseudo R2, package MuMIn; Barton 2012). Adequacy of macroinvertebrate sampling was assessed by building species accumulation curves (Online Resource 2). Macroinvertebrates known to be environmental indicators were aggregated into orders and analyzed as response variables (Merritt and Cummins 2006). Also, we studied whether ungulates influenced the macroinvertebrate assemblage composition using ‘adonis’ function (999 permutations, Bray–Curtis distance) (Oksanen et al. 2017). We visualized the results using non-metric multidimensional scaling (NMDS) with ‘metaMDS’ function of the vegan package (Bray–Curtis dissimilarity). Finally, we performed confirmatory path analysis (SEM, structural equation modeling) (Lefcheck 2016) to test causal linkages between non-native ungulates, vegetation and aquatic macroinvertebrates (package piecewiseSEM, Lefcheck 2016). All analyses were conducted in R (R Core Team 2019).
Results and discussion
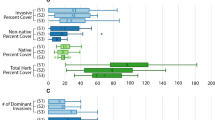
Non-native ungulate abundance varied among our study sites, from intact wetlands (i.e. uninvaded) to highly invaded wetlands (Fig. 1). Three wetlands were uninvaded, since no signs of non-native ungulates were found (rooting by wild-boars or dung from cattle and horses), and zero camera-trap records (“Intact”). Four wetlands were highly invaded with non-native ungulate records over 40 individuals, and all showing multiple species (cattle + horse + wild boar, or cattle + wild boar). Eight sites showed intermediate abundances, in which only two sites showed multiple ungulates (cattle + horses). Overall, most abundant ungulate was cattle, followed by horses (Fig. 1). Wetland area and non-native ungulate abundance relationship was non-significant (see Online Resource 3, Figure S3). No visual signs or video records of native ungulates were found at any of the sites. One site was excluded from analyses given that only 2 macroinvertebrate individuals were collected. We found no evidence that non-native ungulate abundance influenced water conductivity, pH, water temperature or dissolved oxygen (P > 0.05; Online Resource 3, Table S3), although we also observed ungulates entering the water. Previous studies have found that wetlands subject to grazing or farming regimes can show weak to strong changes in different parameters related to water quality (Scrimgeour and Kendall 2003; Steinman et al. 2003; Campbell et al. 2009; Epele and Miserendino 2015). For example, Steinman et al. (2003) reported few significant effects of cattle stocking on water-column nutrient concentration, temperature, conductivity, pH, and dissolved oxygen in Florida wetlands; and Epele and Miserendino (2015) found strong changes in conductivity, pH, salinity, and total dissolved solids in wetlands in southern Patagonia. Our study sites are embedded in the same environmental matrix (forest) and within a protected area, and therefore under no intensive farming history or other disturbance beyond the presence of non-native ungulates. This, coupled with the dynamic nature of wetlands might buffer the effect of non-native ungulate on water quality. In contrast, both littoral plant cover and height were negatively affected by ungulate abundance (Fig. 2). Highly invaded wetlands had ~ 24% less vegetation cover (r2 = 0.39, P = 0.007) and vegetation height was 72% shorter (r2 = 0.41, P = 0.05) compared to intact wetlands (see Online Resource 3, Table S3). This result match extensive evidence showing that browsing and trampling by non-native ungulates can negatively affect vegetation structure and cover in wetlands (Paine 2000; Ausden et al. 2005; Beever et al. 2008; Doupé et al. 2010; Barrios-Garcia and Ballari 2012; Boyd et al. 2017; Vandegehuchte et al. 2017).
SEM results showing vegetation-mediated effects (indirect effects) of non-native ungulates on macroinvertebrates (solid arrows: significant relationships, dashed arrows: non-significant paths). Panels show GLM results for a littoral vegetation cover, b littoral vegetation height, c Odonata abundance, d Oligochaeta abundance and e Diptera abundance to non-native ungulate abundance at wetlands (see “Methods”) (pies represent the proportion of each ungulate of the total records at each site). Values on top of arrows are standardized estimates, *P < 0.05, **P < 0.01, ***P < 0.001. r2 values indicate proportion of variation explained)
Through their foraging behavior, large terrestrial herbivores can connect terrestrial and aquatic ecosystems with various effects on aquatic biota (Beschta and Ripple 2006; Schieltz and Rubenstein 2016; MacSween et al. 2019). We found that non-native ungulates reduced predatory Odonata (dragonflies) larvae abundance by ~ 90% (r2 = 0.32, P = 0.007, see Online Resource 3, Table S3), while disturbance tolerant groups such as Diptera and Oligochaeta responded positively to non-native ungulate impacts. Specifically, highly invaded wetlands supported ~ 170% more Diptera than non invaded sites (r2 = 0.38, P = 0.003, see Online Resource 3, Table S3), and Oligochaeta were registered mostly at invaded sites (r2 = 0.42, P > 0.001, see Online Resource 3, Table S3). In general, larval or adult odonate community can be an accurate indicator of overall health status of aquatic environments (e.g., d’Amico et al. 2004; Kutcher and Bried 2014). Odonata insects link aquatic and terrestrial environments, having aquatic predatory larvae and aerial predatory adults stages along their life cycle. It is known that adults follow visual cues to detect breeding habitats, and that shorter and scarcer littoral vegetation can be perceived as lower habitat quality, affecting Odonata oviposition and reproduction (Lee Foote and Hornung 2005; Raebel et al. 2012). Furthermore, because dragonflies and particularly damselflies rely on aquatic vegetation for oviposition (Corbet 1980), declines observed in larval odonate richness have been linked to trampling and removal of vegetation from the littoral zone that can interrupt odonate emergence (Lee Foote and Hornung 2005). SEM analysis showed indirect negative effect of non-native ungulates on larval Odonates, mediated by a reduction in vegetation height (Fisher’s C = 6.975, df = 12, P = 0.871; Fig. 2). Great declines of apex predators such as Odonates in wetlands (particularly where fish are absent) can have important implications in ecosystem function through cascading effects. For example, Knight et al. (2005) showed how differential consumptive effects of fish on larval dragonflies, triggered a trophic cascade that facilitated terrestrial plant reproduction. Future studies should aim to identify if such reciprocal cross-ecosystem effects could be taking place in our study sites.
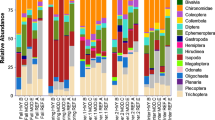
Additionally, we found that highly invaded wetlands supported more Diptera (mostly chironomids) and Oligochaeta than intact wetlands. These groups of macroinvertebrate are well known to be disturbance-tolerant taxa (Merritt and Cummins 2006) mostly including benthic feeders and detritivores. Although SEM analysis did not show a significant relationship between predatory Odonata and their potential prey, Diptera and Oligochaeta, a possible explanation for this finding that would need further research, could be that a great reduction in predatory Odonata enhances abundance of Diptera and Oligochaeta, by reduced (direct or indirect) predation pressure. Odonate larvae are voracious predators of several aquatic macroinvertebrates, including chironomids larvae (Merrill and Johnson 1984; Fincke et al. 1997) and Oligochaeta, and can strongly influence prey density (Fincke et al. 1997; Turner and Chislock 2007; Mortensen and Richardson 2008). Another possible explanation is that in invaded wetlands with low vegetation cover, the nutrient input from the littoral area could be higher due to runoff, defecating and trampling by ungulates, providing more organic sediments that promote development of such taxa (Campbell et al. 2009; Epele and Miserendino 2015; Hill et al. 2017; Swartz et al. 2019). Although macroinvertebrate family richness and abundance did not show a statistically significant relation to non-native ungulate abundance (P > 0.05, see Online Resource 3), macroinvertebrate assemblage composition did differ among our wetlands (adonis F = 2.35, P = 0.01, Online Resource 4). Macroinvertebrate family composition was more similar within intact sites, than with sites with non-native ungulates.
Aquatic and terrestrial ecosystems are closely connected, and as a result, changes in one of them are susceptible to cross boundaries and alter the structure and functions of the adjacent ecosystem (Polis et al. 1997; Loreau et al. 2003; Baxter et al. 2004), making terrestrial-aquatic linkages of key importance in non-native species studies. Our results show that globally widespread non-native ungulates such as cattle, horses and wild boar can have negative impacts beyond the ecosystem in which they occur, reducing vegetation structure and cover, which drastically reduces aquatic apex predators abundance and enhances disturbance-tolerant taxa in wetlands. Moreover, the fact that water parameters showed no response to non-native ungulates abundance suggest that the changes we observed in aquatic macroinvertebrates are explained by non-native ungulates. In NW Patagonia, livestock were introduced prior to the creation of the National Park (1934), with no consistent management practices enforced to the present. Although we are limited in our ability to provide management recommendations given the lack of data on stocking rotation schedules, as well as on feral population numbers, fencing or setting rotation schemes have shown to help maintain native biodiversity and provide ecosystem services for landowners (Scrimgeour and Kendall 2003; Ausden et al. 2005; King et al. 2017). For wild boar, hunting schemes in Argentina have also shown efficacy in reducing its population and therefore impacts on native vegetation (Gürtler et al. 2017). Despite its paramount importance, this subject is still a challenge, given the conflicting views of landowners or policy makers. Landowners can be averse to implement management practices of non-native species without evidence of their impact, and even more if those non-native species have an economic value to them (Jaric et al. 2020), such as livestock and wild boar. Wetlands are critical environments for freshwater biodiversity and provide important ecosystem services to humans. Future studies should pay special attention to the indirect impacts caused by non-native species, taking into account possible strong cross-ecosystem effects as we have showed here.
References
Ausden M, Hall M, Pearson P, Strudwick T (2005) The effects of cattle grazing on tall-herb fen vegetation and molluscs. Biol Conserv 122:317–326
Barrios-Garcia MN, Ballari SA (2012) Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol Invasions 14:2283–2300
Barton K (2012) Package ‘MuMIn’. Model selection and model averaging based on information criteria. R package version 1.15.6. https://CRAN.R-project.org/package=MuMIn
Baxter CV, Fausch KD, Murakami M, Chapman PL (2004) Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 85:2656–2663
Beever EA, Tausch RJ, Thogmartin WE (2008) Multi-scale responses of vegetation to removal of horse grazing from Great Basin (USA) mountain ranges. Plant Ecol 196:163–184
Benjamin JR, Fausch KD, Baxter CV (2011) Species replacement by a nonnative salmonid alters ecosystem function by reducing prey subsidies that support riparian spiders. Oecologia 167:503–512
Beschta RL, Ripple WJ (2006) River channel dynamics following extirpation of wolves in northwestern Yellowstone National Park, USA. Earth Surf Process Landf 31:1525–1539
Boyd CS, Davies KW, Collins GH (2017) Impacts of feral horse use on herbaceous riparian vegetation within a sagebrush steppe ecosystem. Rangel Ecol Manag 70:411–417
Cabrera AL, Willink A (1973) Biogeografía de América latina. In: Americanos E (ed) Programa regional de desarrollo científico y tecnológico. Departamento de asuntos científicos, Secretario General de la Organización de los Estados Americanos, Washington
Campbell BD, Haro RJ, Richardson WB (2009) Effects of agricultural land use on chironomid communities: comparisons among natural wetlands and farm ponds. Wetlands 29:1070–1080
Cheal F, Davis JA, Growns JE, Bradle JS, Whittles FH (1993) The influence of sampling method on the classification of wetland macroinvertebrate communities. Hydrobiologia 257:47–56
Clarkson BR, Ausseil AG, Gerbeaux P (2013) Wetland ecosystem services. In: Dymond JR (ed) Ecosystem services in New Zealand: conditions and trends. Manaaki Whenua Press, Lincoln, pp 192–202
Corbet PS (1980) Biology of Odonata. Annu Rev Entomol 25:189–217
d’Amico F, Darblade S, Avignon S, Blanc-Manel S, Ormerod SJ (2004) Odonates as indicators of shallow lake restoration by liming: comparing adult and larval responses. Restor Ecol 12:439–446
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, Mcclean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lauten-Bach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:027–046
Doupé RG, Mitchell J, Knott MJ, Davis AM, Lymbery AJ (2010) Efficacy of exclusion fencing to protect ephemeral floodplain lagoon habitats from feral pigs (Sus scrofa). Wetl Ecol Manag 18:69–78
Epele LB, Miserendino ML (2015) Environmental quality and aquatic invertebrate metrics relationships at Patagonian wetlands subjected to livestock grazing pressures. PLoS One 10:e0137873
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ et al (2011) Trophic downgrading of planet Earth. Science 333:301–306
Fincke OM, Yanoviak SP, Hanschu RD (1997) Predation by odonates depresses mosquito abundance in water-filled tree holes in Panama. Oecologia 112:244–253
Flueck WT (2010) Exotic deer in southern Latin America: what do we know about impacts on native deer and on ecosystems? Biol Invasions 12:1909–1922
Gaitán JJ, López CR, Bran DE (2011) Vegetation composition and its relationship with the environment in mallines of north Patagonia, Argentina. Wetl Ecol Manag 19:121–130
Garreaud R (2009) The Andes climate and weather. Adv Geosci 22:3
Gürtler RE, Izquierdo VM, Gil G, Cavicchia M, Maranta A (2017) Coping with wild boar in a conservation area: impacts of a 10-year management control program in north-eastern Argentina. Biol Invasions 19:11–24
Harmsen BJ, Foster RJ, Silver S, Ostro L, Doncaster CP (2010) Differential use of trails by forest mammals and the implications for camera-trap studies: a case study from Belize. Biotropica 42:126–133
Hill MJ, Biggs J, Thornhill I, Briers RA, Gledhill DG, White JC et al (2017) Urban ponds as an aquatic biodiversity resource in modified landscapes. Glob Change Biol 23:986–999
Howell HJ, Mothes CC, Clements SL, Catania SV, Rothermel BB, Searcy CA (2019) Amphibian responses to livestock use of wetlands: new empirical data and a global review. Ecol Appl 29:01976
Jackson MC, Woodford DJ, Bellingan TA, Weyl OLF, Potgieter MJ, Rivers-Moore NA et al (2016) Trophic overlap between fish and riparian spiders: potential impacts of an invasive fish on terrestrial consumers. Ecol Evol 6:1745–1752
Jaksic FM, Iriarte JA, Jiménez JE, Martínez DR (2002) Invaders without frontiers: cross-border invasions of exotic mammals. Biol Invasions 4:157–173
Jarić I, Courchamp F, Correia RA, Crowley SL, Essl F, Fischer A, González-Moreno P et al (2020) The role of species charisma in biological invasions. Front Ecol Environ. https://doi.org/10.1002/fee.2195
King LE, Lala F, Nzumu H, Mwambingu E, Douglas-Hamiliton I (2017) Beehive fences as a multidimensional conflict-mitigation tool for farmers coexisting with elephants. Conserv Biol 31:743–752
Knight TM, McCoy MW, Chase JM, McCoy KA, Holt RD (2005) Trophic cascades across ecosystems. Nature 437:880
Kutcher TE, Bried JT (2014) Adult Odonata conservatism as an indicator of freshwater wetland condition. Ecol Indic 38:31–39
Lauría Sorge RM, Romero CA (1999) La ganadería doméstica de los pobladores con permiso de ocupación y pastaje en tierras fiscales del Parque Nacional Nahuel Huapi. Informe, Administración de Parques Nacionales, Parque Nacional Nahuel Huapi, Bariloche
Lee Foote A, Hornung CL (2005) Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecol Entomol 30:273–283
Lefcheck JS (2016) piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579
Lindeman RL (1942) The trophic-dynamic aspect of ecology. Ecology 23:399–417
Loreau M, Mouquet N, Holt RD (2003) Meta-ecosystems: a theoretical framework for a spatial ecosystem ecology. Ecol Lett 6:673–679
Mack MC, D’Antonio CM (1998) Impacts of biological invasions on disturbance regimes. Trends Ecol Evol 13:195–198
Mack RN, Simberloff D, Mark Lonsdale W, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
MacSween J, Leroux SJ, Oakes KD (2019) Cross-ecosystem effects of a large terrestrial herbivore on stream ecosystem functioning. Oikos 128:135–145
Marczak LB, Thompson RM, Richardson JS (2007) Meta-analysis: trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 88:140–148
Maron JL, Estes JA, Croll DA, Danner EM, Elmendorf SC, Buckelew SL (2006) An introduced predator alters Aleutian Island plant communities by thwarting nutrient subsidies. Ecol Monogr 76:3–24
Merrill RJ, Johnson DM (1984) Dietary niche overlap and mutual predation among coexisting larval Anisoptera. Odonatologica 13:387–406
Merritt RW, Cummins KW (2006) Trophic relationships of macroinvertebrates. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology, 2nd edn. Elsevier, Amsterdam, pp 585–609
Mitsch WJ, Gosselink JG (2007) Wetlands. Wiley, New York
Mortensen L, Richardson JM (2008) Effects of chemical cues on foraging in damselfly larvae, Enallagma antennatum. J Insect Behav 21:285–295
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci 98:166–170
Novaro AJ, Funes MC, Walker RS (2000) Ecological extinction of native prey of a carnivore assemblage in Argentine Patagonia. Biol Conserv 92:25–33
O’Brien TG (2011) Abundance, density and relative abundance: a conceptual framework. In: Camera traps in animal ecology. Springer, Tokyo, pp 71–96
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MH (2017) Vegan: community ecology package. R package version 2. 4–2. https://cran.r-project.org, https://github.com/vegandevs/vegan
Paine RT (2000) Phycology for the mammalogist: marine rocky shores and mammal-dominated communities show different are the structuring processes? J Mammal 81:637–648
Perotti MG, Diéguez MC, Jara FG (2005) Estado del conocimiento de humedales del norte patagónico (Argentina): aspectos relevantes e importancia para la conservación de la biodiversidad regional. Rev Chil Hist Nat 78:723–737
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316
R (2019) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Raebel EM, Merckx T, Feber RE, Riordan P, Macdonald DW, Thompson DJ (2012) Identifying high-quality pond habitats for Odonata in lowland England: implications for agri-environment schemes. Insect Conserv Divers 5:422–432
Rodriguez-Cabal MA, Barrios-Garcia MN, Amico GC, Aizen MA, Sanders NJ (2013) Node-by- node disassembly of a mutualistic interaction web driven by species introductions. Proc Natl Acad Sci 110:16503–16507
Rodriguez-Cabal MA, Barrios-Garcia MN, Greyson-Gaito CJ, Slinn HL, Tapella MP, Vitali A, Crutsinger GM (2019) Non-native ungulates indirectly impact foliar arthropods but not soil function. Biol Invasions 21:3077–3084
Schieltz JM, Rubenstein DI (2016) Evidence based review: positive versus negative effects of livestock grazing on wildlife. What do we really know? Environ Res Lett 11:113003
Scrimgeour GJ, Kendall S (2003) Effects of livestock grazing on benthic invertebrates from a native grassland ecosystem. Freshw Biol 48:347–362
SIB (2020) Sistema de Información de Biodiversidad, Administración de Parques Nacionales. Argentina. https://www.sib.gov.ar. Accessed 27 Mar 2020
Simberloff D, Martin J-L, Genovesi P, Maris V, Wardle DA, Aronson J et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Sollmann R, Mohamed A, Samejima H, Wilting A (2013) Risky business or simple solution—relative abundance indices from camera-trapping. Biol Conserv 159:405–412
Spear D, Chown SL (2009) Non-indigenous ungulates as a threat to biodiversity. J Zool 279:1–17
Steinman AD, Conklin J, Bohlen PJ, Uzarski DG (2003) Influence of cattle grazing and pasture land use on macroinvertebrate communities in freshwater wetlands. Wetlands 23:877–889
Strauss SY (1991) Indirect effects in community ecology: their definition, study and importance. Trends Ecol Evol 6:206–210
Subalusky AL, Dutton CL, Rosi EJ, Post DM (2017) Annual mass drownings of the Serengeti wildebeest migration influence nutrient cycling and storage in the Mara River. Proc Natl Acad Sci 114:7647–7652
Swartz LK, Hossack BR, Muths E, Newell RL, Lowe WH (2019) Aquatic macroinvertebrate community responses to wetland mitigation in the Greater Yellowstone Ecosystem. Freshw Biol 64:942–953
Turner AM, Chislock MF (2007) Dragonfly predators influence biomass and density of pond snails. Oecologia 153:407–415
Vandegehuchte ML, Schütz M, De Schaetzen F, Risch AC (2017) Mammal-induced trophic cascades in invertebrate food webs are modulated by grazing intensity in subalpine grassland. J Anim Ecol 86:1434–1446
Vázquez DP (2002) Multiple effects of introduced mammalian herbivores in a temperate forest. Biol Invasions 4:175–191
Vázquez DP, Simberloff D (2004) Indirect effects of an introduced ungulate on pollination and plant reproduction. Ecol Monogr 74:281–308
Venables WN, Ripley BD (2002) Spatial statistics. In: Ripley BD (ed) Modern applied statistics with S. Springer, New York, pp 419–434
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, Van Der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
White EM, Wilson JC, Clarke AR (2006) Biotic indirect effects: a neglected concept in invasion biology. Divers Distrib 12:443–455
Williamson M, Griffiths B (1996) Biological invasions. Springer, Berlin
Wootton JT (1994) The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Evol Syst 25:443–466
Acknowledgements
We thank the Conservation Department of Nahuel Huapi National Park for providing camera-traps, to Ing. Hugo Galván and Lic. Verónica Gómez for their assistance regarding livestock practices in the National Park, and Delegación Regional Patagonia (APN) for issuing the permits for conducting this study. We specially thank Genevieve Conley (Northern Arizona University, USA) and park rangers Antuel Sánchez and Damián Abdalla for their assistance in the field. John Maron provided advice that greatly improved this manuscript.
Funding
This work was done during a postdoctoral research position at Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Contributions
LM, MNB-G, SAB and MAR-C conceived and designed the study. LM carried out the fieldwork, data processing and analysis. All the authors wrote the article.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Informed consent
All persons entitled to authorship have been so named. All authors have approved its submission for publication in Biological Invasions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Motta, L., Barrios-Garcia, M.N., Ballari, S.A. et al. Cross-ecosystem impacts of non-native ungulates on wetland communities. Biol Invasions 22, 3283–3291 (2020). https://doi.org/10.1007/s10530-020-02323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02323-4