Abstract
Widespread interest in the development of environmentally safe management actions has prompted research into the use of sensory cues to manipulate the movements of invasive species. The push–pull approach, for which attractive and repellent semiochemicals operate synergistically to guide individuals toward traps, has proven successful in insect pest management applications. We examined the effectiveness of a natural repellent (an alarm cue) and a natural attractant (a partial sex pheromone) in push-only (repel), pull-only (attract), and push–pull configurations, to guide invasive sea lamprey (Petromyzon marinus) toward and into a target trap during spawning migration into rivers. Using PIT telemetry to monitor sea lamprey movement within the river, we found that the alarm cue was capable of strongly altering sea lamprey distribution, “pushing” them toward target areas and generating rates of encounter with trap entrances sufficient to achieve trapping-for-control targets. Encounter rate with trap entrances was not improved, but performed more consistently, with the addition of the attractant in the push–pull configuration. There was evidence this could stem from a transition in internal state of motivation, from migration to reproduction. Use of the attractant alone was ineffective. No odor combination improved trap captures. We conclude that push–pull strategies will prove effective in guiding sea lamprey movements and recommend two improvements for subsequent testing in management scenarios: (1) use of a superior attractant (e.g. a sea lamprey migratory cue derived from conspecific larvae), and (2) its subsequent application to a capture methodology based on the entrainment of individuals near trap entrances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mass trapping of adults is an environmentally benign means of suppressing pest populations by removing individuals from the population prior to reproduction (El-Sayed et al. 2006; Tripathi 2014). Although conceptually straightforward, mass trapping as a strategy is rarely viable unless pests can be aggregated near and/or attracted into traps (Howse et al. 1998). Push–pull application of semiochemicals (stimulo-deterrent diversion) was conceived as a strategy to protect agricultural crops from invertebrate pests (Miller and Cowles 1990). By applying repellents to “push” while simultaneously “pulling” with attractants, it is possible to guide pests to an area and either remove or target them with pesticide applications. Despite the prevalence of semiochemical communication in vertebrates and its role in modulating behavior, there have been few proposals for the simultaneous application of attractants and repellents to control vertebrate pests, and no tests of this approach (e.g. rodents, Pickett et al. 2014; fishes, Sorensen and Johnson 2016; reptiles, Clark et al. 2017).
The Laurentian Great Lakes of North America are the largest freshwater ecosystem on the planet and support annual recreational and commercial fisheries worth several billion US dollars (Lauer 2015). Non-indigenous sea lamprey (Petromyzon marinus) became established as an invasive species of major concern in this region after they gained access sometime after 1921 (Sullivan et al. 2003). Juvenile sea lamprey parasitize a wide variety of fish species (Silva et al. 2014; Happel et al. 2017) and within three decades they contributed to the collapse of several ecologically and commercially important fish stocks and the extirpation of some locally-adapted populations (Marsden and Siefkes 2019). Annual assessments by agencies tasked with implementing a control program (US Fish and Wildlife Service, USFWS and Fisheries and Oceans Canada, DFO) suggest sea lamprey have spread throughout the Great Lakes, spawning each spring–summer within several hundred tributary streams. These same agencies apply two pesticides (3-trifluormethyl-4-nitrophenol TFM, and 2′,5-dichloro-4-nitrosalicylanilide, Bayluscide) to nursery streams to kill developing larvae prior to metamorphosis into the parasitic juvenile life stage (Smith and Tibbles 1980). Pesticide application has reduced sea lamprey population size by 90% relative to the historical peak, but sea lamprey-induced mortality in the basin remains a major concern. Further expansion of the pesticide program, the building of dams to block sea lamprey migration, and balancing management of other species in the basin (e.g. restoring habitat connectivity for desirable migratory species via dam removal) has resulted in substantial interest in developing benign approaches to sea lamprey control to achieve management targets (Marsden and Siefkes 2019).
The use of traps to capture invasive sea lamprey migrating to either feed in the lakes or reproduce in tributary streams predates the use of pesticides, beginning in the 1940s (Marsden and Siefkes 2019). The majority of current trapping effort entails the deployment of both permanent and seasonal traps in conjunction with barriers to block migration (Miehls et al. 2019). Currently, a network of approximately 37 traps are operated to assess adult abundance as the trapping approach experiences low and variable efficiency (mean = 44%; range = 8–100%, defined as the proportion of adults in a river removed by traps, Mullett and Sullivan 2017). A number of physical modifications to improve performance of barrier-integrated traps have been attempted (e.g. the addition of attractant lighting), but with limited success (Purvis et al. 1985; McLaughlin et al. 2007; Stamplecoskie et al. 2012). Control agencies (USFWS and DFO) do not consider streams without barriers viable for targeting sea lamprey using traps; nets and temporary trap-and-weir combinations suffer from trap efficiencies that are typically less than 30% (Miehls et al. 2019). Using models developed for the St Mary’s River, Haeseker et al. (2007) concluded that increasing mean trap efficiency from 40 to 70% (probability distribution = 50–84%) could provide a sufficient and cost-effective alternative control to pesticide application in this system. However, decision analysis of control program effectiveness based on theoretical performance of tactics inevitably suffers from statistical uncertainty, and Jones et al. (2015) concluded this rate may need to be greater. Despite uncertainty surrounding population demographics and potential compensatory responses, as well as trap efficacy, management decisions are nevertheless informed by such modelled data. Therefore, trap operators should aim to remove at least 70% of adults prior to reproduction to account for uncertainty (Haeseker et al. 2007; Jones et al. 2015). A series of recent empirical studies has ascertained that the currently poor performance of sea lamprey traps is due principally to low encounter rates with the entrances (Bravener and McLaughlin 2013; Holbrook et al. 2014, 2016; Dawson et al. 2016). Barrier-integrated traps outperform free-standing traps in rivers due to the presence of dams impeding upstream movement. As sea lamprey search across the face of the barrier for a passage opportunity, they encounter traps installed against the dam face situated along their preferred movement paths, and subsequently may or may not enter (Rous et al. 2017).
Applying semiochemicals directly to sea lamprey traps has long been suggested as a means of improving trap localization and entry (Teeter 1980; Twohey et al. 2003; Imre et al. 2010), based on the historical use of sexually mature males as bait in French fisheries where sea lamprey are exploited for food (Fontaine 1938). Males release a multi-component sex pheromone that includes 3-keto-petromyzonol sulfate (3kPZS; Li et al. 2002). This compound functions in part as a localized attractant on spawning grounds that facilitates nest finding by sexually mature females (Johnson et al. 2009), and has been synthesized, enabling its application in management (Siefkes and Li 2003). It improves the capture of sexually immature migrants in barrier-integrated traps by an average of 9%, though performance in the field is highly variable and context dependent (Johnson et al. 2013, 2015a, b). Baiting with 3kPZS alone does not achieve the trapping improvement necessary to replace pesticide application (Dawson et al. 2016). However, it constitutes an available “pull” in stimulo-deterrent diversion tactics designed to capture migrating sea lamprey in open river channels. A repellent odor released from the tissues of sea lamprey upon injury or death (a putative alarm cue, Wagner et al. 2011) has also shown promise in its ability to manipulate the distribution of sea lamprey undertaking the spawning migration. In laboratory trials, this alarm cue effectively repels sea lamprey from one side of an artificial stream (Bals and Wagner 2012), which is consistent with the animal’s response in a natural stream when one side of the channel is similarly activated (Hume et al. 2015). Therefore, when used in opposition to 3kPZS, the sea lamprey alarm cue may aggregate sea lamprey within open river channels by repelling them from a section of the stream—thus guiding them to a relatively small area where they may be more likely to encounter traps emitting 3kPZS (Imre et al. 2010). In essence, push–pull applications of these semiochemicals could constrain the lateral space available for sea lamprey during migration and increase exposure to 3kPZS-baited traps.
Here, we report the effect of semiochemical applications on the distribution of sea lamprey undertaking their reproductive migration in a natural spawning stream in the Great Lakes. The goal of our study was to compare the relative effectiveness of push only (alarm cue), pull only (3kPZS), and push–pull (alarm cue and 3kPZS presented on opposite sides of the stream) approaches in their ability to increase encounter rates with traps in open river channels. All previous tests of semiochemical guidance to lamprey traps have involved barrier-integrated devices. We hypothesize that upon detection of these semiochemicals, nominal movement tendencies of sea lamprey are altered because these compounds induce olfactory-mediated behavioral responses, movement away from the odor (alarm cue), and movement toward the odor (3kPZS). To test this hypothesis, we released migratory-phase sub-adult sea lamprey implanted with passive integrated transponder (PIT) tags into a tributary to Lake Michigan and monitored their upstream movement and approach toward the entrances of three traps arrayed across the river channel. We applied the semiochemical odors alone and in combination in attempts to guide the migrants toward the entrance of a pre-determined “target” trap (vs. a control application of no odor). We predicted when compared to control nights (no semiochemicals applied to traps) that (1) the application of 3kPZS to a trap will result in increased encounter rates with the entrance of that trap by pulling sea lamprey towards the source, (2) the application of the alarm cue to a trap will result in decreased encounter rates with that trap entrance by pushing sea lamprey away from the source, and (3) applying both semiochemicals in opposition (push–pull) will have an additive effect, increasing encounter rates with the target area to levels greater than either semiochemical alone.
Materials and methods
Study location
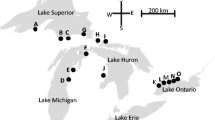
The experiment was conducted in Carp Lake River, a moderate sized stream located in the northern lower peninsula of Michigan, USA (45°44′56.9″ N, 84°49′46.9″ W) (Fig. 1). The stream flows into Lake Michigan and attracts a modest number of migrating sea lamprey each year (mean 817, range 11–3110, years 1977–2014). As a result, a low-head barrier is located 550 m upstream from the confluence with the lake to block sea lamprey access to a further 16.4 km of river. The USFWS applies the pesticide TFM in an irregular cycle to kill any larval sea lamprey present downstream of the barrier (16 times since 1965, most recently 2013, T. Sullivan, USFWS, pers. comm. 2017).
Location of study site on Carp Lake River, northern Michigan. Grey triangles (top-right section) indicate the positions of double-funnel trap-nets in the stream. Depth profile of Carp Lake River downstream of trapping area is illustrated in the bottom-right. Abbreviations: C-81 = road crossing; L, C, and R = traps positions in the left, center, and right portion of the stream, respectively
Experimental design
The USFWS provided sea lamprey from traps operating in the Cheboygan and Ocqueoc rivers, located approximately 45 and 80 km southeast of Carp Lake River, respectively. Both streams drain east to Lake Huron. A barrier-integrated trap in Carp Lake River itself also provided sea lamprey. Removal of sea lamprey from traps on all three rivers began 12 April 2016. Collected sea lamprey were then transferred to the US Geological Survey Hammond Bay Biological Station near Millersburg, Michigan, USA. Sea lamprey were housed in 1000 L tanks in same-sex groups, with each tank receiving full exchange of Lake Huron water every 2 h at temperatures ranging 5–18 °C (varying with ambient lake temperature).
Sea lamprey selected for inclusion in the experiment (n = 24 per trial, 12 males and 12 females), were removed from holding tanks, weighed (wet weight in grams, g) measured (total length in millimeters, TL) and surgically implanted with a 32 mm passive integrated transponder (PIT) tag (Oregon RFID, Portland, Oregon, USA, mass 0.8 g). A minor incision was made in the abdomen using a sterile scalpel (3 mm across), a PIT tag gently inserted into the opening parallel with the body orientation, and the wound sealed with surgical glue (3 M Vetbond Tissue Adhesive, St Paul, Minnesota, USA). Each PIT tag enabled the detection of individuals following their release into a stream when in range of a half-duplex PIT antenna. Sea lamprey were not anaesthetized prior to this procedure to avoid potential damage of the olfactory epithelium (Lewis et al. 1985). Following the procedure (< 1 min per fish), we moved tagged sea lamprey into 200 L tanks for a 24 h period of recovery. At the end of the recovery period, we transported tagged sea lamprey to Carp Lake River in a separate 200 L aerated tank containing Lake Huron water. Once at the release site, we adjusted holding tank water to within 5 °C of the stream water temperature by adding stream water in 19 L increments. Once tagged sea lamprey were suitably acclimated to stream temperatures, we transferred them into the stream using mesh bags that were submerged for several minutes until they exited the bags of their own volition. Release of experimental animals took place between 1200 and 1400 h each day in order to encourage natural shelter-seeking behavior in the stream prior to the onset of darkness and the resumption of natural upstream migration (Hume et al. 2015; Luhring et al. 2016). Michigan State University Institutional Animal Care and Use Committee approved all procedures in accordance with permit #01/14–007-00.
We released tagged sea lamprey into Carp Lake River and designated an area 150 m upstream the “trapping area” (Fig. 1). This stream reach has an average summer discharge of 0.77 m3 s−1, is 9 m wetted-width, and does not have a uniform depth profile (Fig. 1). A stream-wide antenna could detect the presence of experimental animals moving upstream following their release. Ten meters further upstream of this full stream antenna, three 1 m wide × 0.5 m tall hoop-net traps were positioned across the stream, each effectively targeting one-third of the stream bottom (left, center, and right, relative to facing upstream). Our aim, however, was not to test the efficacy of this particular trap design. Rather, the principle function of these nets was to hold an antenna fitted to the entrance to detect encounter rate with an area that could contain a well-designed trap. Traps constructed from mesh, such as these, are not effective when targeting lampreys, presumably this material precludes attachment to the surface before entering. Instead, we included these traps in the current study to hold an antenna for detecting the proximity of individuals to trap entrances, generate similar hydrological changes to stream flow, and provide physical structures for sea lamprey to interact with. We adjusted the read range on all antennas mounted to trap entrances each day and refined them to approximately one sea lamprey body length to enable accurate detection of a trap encounter. Tagged sea lamprey detections by antennas were time-stamped along with each unique PIT tag 64-bit ID. We uploaded data files from the antenna reader to a Meazura PDA (Aceeca, New Zealand) each day and downloaded files to a computer for archiving, editing, and analysis.
We conducted trials daily between 24 May and 12 July 2016, a period consistent with sea lamprey migration in the northern areas of Lakes Michigan and Huron that year. Accounting for losses due to telemetry or other equipment malfunctions, we successfully completed trials on 32/49 days. During this period, four experimental treatments were tested: no odor (control N = 8), alarm cue (left N = 4, right N = 4), 3kPZS (left N = 4, right N = 4), and alarm cue + 3kPZS presented on opposite sides (cue on left N = 4, cue on right N = 4). We randomized treatment order within eight blocks of four to reduce the influence of environmental variables on the outcome of any one treatment. Water temperature of Carp Lake River was variable, but generally increased across the study period (range = 14–22 °C), whereas discharge was generally low and consistently decreased across the same period (range = 0.07–0.84 m3 s−1). Neither temperature (Type II SS ANOVA: F3,28 = 0.4, P = 0.8) nor discharge (F3,28 = 1.0, P = 0.4) differed among the four treatments.
Semiochemical preparation and application
The extraction procedure for the alarm cue has been provided in detail previously (Bals and Wagner 2012) and will be briefly reiterated here. We used Soxhlet extraction to derive the repellent compound(s) from whole carcasses of adult male and female sea lamprey. Nine sea lamprey carcasses produced every 5.2 L of final liquid solution containing the repellent compound(s), with a mean tissue mass of 2.36 kg at the start of each extraction process. We loaded carcasses inside a thimble, which was then placed into the main chamber of one of three 2.08 m tall Soxhlet extractors. Then, we mixed a 50:50 solution of absolute ethanol and deionized water in a 12 L distillation flask seated on a heating element and raised to 75–80 °C for three cycles. Ethanol was then rotovaporated from this extract under vacuum at 35 °C and the resulting liquid stored at − 20 °C. Bridge Organics Co. (Kalamazoo, Michigan, USA) provided synthesized partial sex pheromone (3kPZS) in salt form (ammonium salt dihydrate). Professor W. Li at Michigan State University confirmed chemical purity was > 97%. We subsequently created aliquots of 10 mg−1 mL−1 in a 50/50 v/v of methanol and distilled water and stored the solution at − 80 °C.
Prior to the onset of odor application, stream discharge (m3 s−1) was estimated with a Doppler flow meter (Flo-Mate model 2000, Marsh-McBirney) using the midsection method (Gore 1996) at a point roughly equidistant between the sea lamprey release point and the trapping area. We used this discharge estimate to calculate the volume of alarm cue extract required to produce a 1 PPM concentration (by volume) when fully mixed with one-third of the stream’s discharge that day. Rhodamine WT fluorescent red dye was used to confirm where one-third of the stream channel would be activated downstream of the application points. We then added requisite volumes of alarm cue to a carboy and mixed with stream water collected upstream of the area containing traps, for a total volume of 9 L. This mixture was pumped into the stream from a point close to the stream substrate, at the entrance to a trap located at either edge of the stream, at a rate of 60 mL h−1 for 4 h (2100–0100 h) by a laboratory-grade peristaltic pump (Masterflex 7553–70, Cole Palmer) powered by a 12 V battery. The US Environmental Protection Agency (permit #75437-EUP-5) approved application of “Dead Sea Lamprey Odor” to Carp Lake River as required under Section 5 of the Federal Insecticide, Fungicide, and Rodenticide Act.
We also applied the synthesized sex pheromone 3kPZS by pumping directly into the trap using a peristaltic pump (Admiral Reef Dosing Pump, Norwich, Connecticut, USA) so that the plume emitted from the entrance between 2100 and 0100 h. We combined a synthesized pheromone aliquot with stream water until the application rate reached 10 mg h−1, which is equivalent to the concentration emitted by 12–25 spermiating males (Yun 2012; Brant 2015). The State of Michigan and US Environmental Protection Agency (permit #75437-EUP-3) approved application of 3kPZS to Carp Lake River as required under Section 5 of the Federal Insecticide, Fungicide, and Rodenticide Act.
Data treatment and analyses
Inclusion of data for analysis was constrained to tagged sea lamprey detected during the hours of cue pumping (2100–0100 h) on the first night following their release into the stream. This eliminates effects from any repeated exposures to experimental treatments on successive nights, should tagged sea lamprey remain “at large” in the stream. It also excludes tagged sea lamprey that moved through the trapping area in the absence of semiochemical treatments. Only the first detections of tagged sea lamprey on antennas were included in analyses. We calculated encounter rates with traps as the proportion of sea lamprey detected at trap entrances relative to the number detected moving upstream. We considered detections on the stream-wide antenna 150 m upstream of the release point as tagged sea lamprey moving upstream, towards the trapping area. Of the sea lamprey moving upstream, we recorded the number detected in the left, center, or right side of the stream. For comparisons across treatments, the target area was always the opposite side of the alarm cue and on the same side as 3kPZS. For the control, in which there was no target area, we divided the stream into thirds and used the average percent of sea lamprey moving upstream on the left, center, or right side as the response (0.35 ± 0.05, mean ± SD).
Preliminary analyses showed that Julian date was correlated with both stream discharge (adj. R2 = 0.87, P < 0.0001) and temperature (adj. R2 = 0.17, P = 0.01), and discharge was correlated with temperature (adj. R2 = 0.11, P = 0.04) (Fig. S1). We logit transformed the percent of sea lamprey moving through the target area each night (defined as a function of the number of available animals, Warton and Hui 2011) and checked model residuals for heteroscedasticity and for differences in variance across treatments. Preliminary comparisons of models with or without various combinations of all three environmental variables (temperature, discharge, and Julian date) indicated that the treatment-only model was the top model but shared predictive power with models incorporating a single environmental covariate (treatment + one of Julian date, temperature, or discharge). Regardless of the model or environmental covariate chosen, treatment effects were strong (P < 0.0001) and effectively identical. We used Julian date as the single covariate in our final set of candidate models because of the robust nature of the treatment effect, correlations of environmental variables with each other, and because Julian date incorporates temporal shifts in temperature and discharge as well as potential internal states of sea lamprey (Luhring et al. 2016).
We constructed five a priori candidate models to predict the percent of upstream-migrating sea lamprey moving through the target area each night. These included: treatment only, Julian date only, treatment and Julian date without an interaction, treatment and Julian date with an interaction, and an intercept only model (Table 1). Because male and female sea lamprey potentially respond differently to 3kPZS during migration, we ran an additional set of model comparisons on male-only and female-only subsets (e.g. percent of upstream-migrating males that move through target area). Candidate models were ranked according to their Akaike Information Criterion values corrected for small sample size (AICc) with AICctab in the bbmle package (Bolker 2017). Models with ∆AICc < 2.0 were determined to have substantial support (Burnham and Anderson 2002) and were analyzed with an analysis of variance (ANOVA in car package; Fox and Weisberg 2011) with type II SS (when the interaction term was absent) or type III SS (when the interaction term was present). When the treatment term was included in a model without an interaction, we used a Tukey’s HSD post hoc test to compare among treatments (glht in multcomp package; Hothorn et al. 2008). AICc model comparisons and subsequent model analyses were all conducted with R 3.4.0 (R development core team 2017).
We calculated trap capture rate as the proportion of sea lamprey removed from a trap relative to the number detected at the trap entrance. Pearson’s chi-square was used to test for any effect of treatment on capture rate in any given trap. Analysis was conducted in IBM SPSS Statistics V. 25 (IBM Corp. NY).
Results
Of 768 sea lamprey tagged and released into Carp Lake River, 91.6% (n = 704) were detected again moving upstream toward the trapping area after 2100 h on the first night of their release. Treatment did not have a significant effect on the percent of tagged sea lamprey that moved upstream (Kruskal–Wallace χ2(3) = 2.863, P = 0.413), with generally high proportions of animals detected on all nights (mean ± 2 S.E., control = 0.96 ± 0.03; 3kPZS = 0.98 ± 0.02; alarm cue = 0.98 ± 0.03; alarm cue + 3kPZS = 0.97 ± 0.02). On control nights (N = 8), the average nightly distribution of sea lamprey across the stream was slightly skewed to the right with median (mean ± S.E.) percent of 35.5% moving up the right side of the stream (43.7 ± 6.6%), 29.6% moving up the center (30.6 ± 3.7%), and 23.3% moving up the left side of the stream (25.7 ± 5.0%) (Fig. 2a). Wild sea lamprey in the stream migrated mostly between JD 148 and JD 168, with only 6 captured while migrating upstream after Julian date 170. Our trials thus encompass the responses of sea lamprey early in the migration and later.
Panel (a) shows the proportion (by treatment) of tagged sea lamprey detected at the entrance to three trap-nets deployed across the width of Carp Lake River in response to stimulo-deterrent diversion using semiochemicals. A description of the attempted behavioral manipulation of sea lamprey (as a consequence of treatment) is also shown, where the “push” was attempted using the alarm cue and “pull” attempted using 3kPZS. The dashed line indicates the nominal distribution of sea lamprey if they were equally distributed across the stream width. Left, center, and right refer to the application point of semiochemicals to the river, relative to facing upstream. Panel (b) shows a summary of the treatment effects with mean proportion of sea lamprey detected. The alarm cue was an effective push, increasing the proportion detected in target areas of the channel, whereas 3kPZS was an ineffective pull, failing to increase the proportion of sea lamprey detected “on-target.” The application of push–pull was similarly effective to push alone, but exhibited less variance
The percent of upstream-migrating sea lamprey (males and females combined) moving through the target area was best explained by models with treatment as the only predictor variable (Table 1). Although the model with treatment plus Julian date also had some support (∆AICc = 1.2), treatment was the only significant term (Julian Date F1,27 = 1.6, P = 0.22; Treatment F3,27 = 15.4, P < 0.0001). In the top model (treatment only), treatment had a strong effect on the percent of upstream-migrating sea lamprey moving through the target area (F3,28 = 14.6, P < 0.0001; Fig. 2b). Control and 3kPZS did not differ in the percent of sea lamprey moving into the target area (Tukey HSD, z = 1.6, P = 0.38), with both being close to the expected nominal distribution of 0.35 (control: 0.35 ± 0.05, 3kPZS: 0.28 ± 0.1, mean ± S.D.). The alarm cue, however, increased the percent of sea lamprey in the target zone (0.57 ± 0.2) relative to both control (z = 2.7, P = 0.04) and 3kPZS (z = 4.3, P < 0.001). Push–pull nights likewise increased the percent of sea lamprey in the target zone (0.71 ± 0.1) relative to the control (z = 4.4, P < 0.001) and 3kPZS (z = 6.1, P < 0.001). While slightly more effective on average than the alarm cue, push–pull (0.71 ± 0.1) was statistically indistinguishable from the alarm cue treatment (0.57 ± 0.2) (z = 1.8, P = 0.3). The 71% detection rate of push–pull was 36% higher than what would be expected on a given control night (35%). Variances among treatments did not significantly differ (Levene’s Test, F3,28 = 1.5, P = 0.23).
When males or females were analyzed separately the percent of the upstream-migrating population moving through the target area was best explained by a model with treatment as its sole predictor variable (Table 1). However, males diverged from females in that their movement was also well supported by a model incorporating a treatment by Julian date interaction (∆AICc = 0.4). Within this model, the interaction term (Treatment:Julian date) was significant (F3,24 = 3.8, P = 0.02), as were both Treatment (F3,24 = 4.5, P = 0.01) and Julian date (F1,24 = 4.3, P < 0.05). The interaction term was created by a temporal change in how male sea lamprey responded to treatments (Fig. S2 and S3). Early in the experiment, when trials were concurrent with natural migrations, both the alarm cue and push–pull treatments were effective at redistributing males within the stream to the target area. Both the alarm cue and push–pull appeared to decline in effectiveness later as Julian Date increased, but the alarm cue declined at a faster rate and became indistinguishable from the control and 3kPZS treatments by Julian date 177 (Fig. S2). The percent (logit transformed) of upstream-migrating female sea lamprey traveling through the target zone was best explained by two models receiving similar weights (Table 1). Both models showed a strong effect of treatment (Treatment only model: F3,28 = 11.3, P < 0.001; Treatment within the Treatment + Julian date model: F3,27 = 12.4, P < 0.001), but the model incorporating Julian date showed no effect of the covariate (F1,27 = 2.0, P = 0.2).
Capture rate by traps was low overall (n = 163, mean efficiency 23%, range = 17–31%) and not affected by treatment (Pearson’s χ2(30) = 31.12, P = 0.409; Fig. 3).
Discussion
Trapping will only prove successful in managing invasive fishes if encounter rate with the traps can be maximized (Bravener and McLaughlin 2013). Control strategies employing the application of semiochemicals have been suggested as a means to achieve high rates of trap encounter. In this study, the application of a repellent alarm cue provided a strong enough “push” to guide on average 57% of migrating sea lamprey toward the entrance of a free-standing trap (vs. 28–35% without the odor). The presence of a synthesized sex pheromone (3kPZS) as a “pull” was not effective in attracting sea lamprey when presented alone. The alarm cue alone appears as effective as push–pull during the early part of the spawning migration, either due to environmental effects (stream temperature or discharge) or because sexually-immature animals do not respond to 3kPZS. As the spawning migration progressed and the stream became slower and warmer, push–pull appeared to maintain effectiveness while the alarm cue alone did not. It is key, however, to note that this is not due to a lack of effect of the alarm cue in the push–pull configuration, rather, the combination of 3kPZS and the alarm cue works more effectively later in the migration when neither appears to have much effect on their own. The majority of the sea lamprey spawning migration occurs earlier in spring–summer (late-May through mid-June) when the alarm cue alone is sufficient to manipulate their distribution, but later run sea lamprey may require a push–pull approach to redistribute them laterally within the stream. Additionally, although encounter rates with trap entrances were consistent with modeled target levels to achieve trapping-for-control (Haeseker et al. 2007), actual rates of entry into traps were insufficient. There remains the need, therefore, to either identify a more effective pull to work in conjunction with the alarm cue early in the spawning migration (e.g. the migratory cue emitted by stream resident larvae, Wagner et al. 2006; Li et al. 2018), or identify a lamprey-specific fishing technique (e.g. approaches based on entrainment). Should both be identified, these findings should be extended to field-test scale to validate these proof-of-concept findings across a broader range of streams and annual environmental stochasticity.
For mass trapping to reduce numbers of parasitic sea lamprey in the Great Lakes to target levels, trap operators should aim to capture and remove ~ 70% of sub-adults migrating to spawning grounds annually (Haeseker et al. 2007; Jones et al. 2015). Due to uncertainties surrounding population demographics and the efficacy of traps in any given river, this annual removal rate could vary widely (0.5–0.84, Haeseker et al. 2007). Regardless, trap efficacy must increase from both historic and current levels (~ 45%, Miehls et al. 2019). Our data indicate that applying the alarm cue might permit this by generating high rates of encounter with traps in a consistent manner, even in an open stream environment (mean encounter = 57 and 71% for push and push–pull, respectively). Previous applications of the alarm cue in this same stream resulted in sea lamprey encountering a barrier-integrated trap entrance more than twice as quickly compared to control nights, and trap capture efficacy in that study was exceedingly high (91, 97, and 93% for control, push, and push–pull, respectively) (Hume et al. 2015). However, rates of entrance into stand-alone traps in the current study were poor and not influenced by treatment application (calculated as the number of sea lamprey detected at trap entrances relative to the number removed from traps). Thus, a greater than two-fold increase in the efficacy of net-traps following encounter with them would be required to meet target levels—effectively having to capture every individual encountered. It is probable that the lack of a barrier in the current study reduced the number of times individual sea lamprey repeatedly encountered a trap entrance relative to the study by Hume et al. (2015), which in turn would have reduced the likelihood of trap entry (Bravener and McLaughlin 2013). Low probability of encounter with trap entrances might explain poor sea lamprey trap performance at barriers (Rous et al. 2017), but this is not sufficient to explain our data in open-water because the alarm cue resulted in high levels of encounter. It was confirmed that the alarm cue application will not prevent upstream movement of sea lamprey in streams, but it can act as an effective and consistent influence on movement tendency during the spawning migration by altering their distribution in response to their perception of risk from predators (Hume et al. 2015; Luhring et al. 2016).
Based on the energetic costs of their spawning migration, Beamish (1979) concluded anadromous sea lamprey do not take the shortest linear path during upstream movements. One potential explanation for this phenomenon is that sea lamprey frequently move laterally within a stream to locate favorable areas as they migrate. The distribution of detections of PIT-tagged individuals in the current study (slightly skewed to one side) support this hypothesis as sub-adult sea lamprey were more likely to move through the deepest section of the stream. In addition, the repellent effect of the alarm cue was strengthened when applied to the shallowest side. Both observations are suggestive of a nominal tendency by migratory sea lamprey to track the deepest water in shallow streams, which is a behavior consistent with shoreline predator avoidance. Together with their nocturnal behavior (Binder and McDonald 2008), by avoiding shallow water where possible sea lamprey reduce risk associated with shoreline predator encounters (Sjöberg 1985, 1989; Cochran 2009), while possibly improving the likelihood of locating daytime refugia. In deep rivers, the alarm cue may fail to yield as effective a response by sea lamprey as was observed in Carp Lake River, as they are less likely to perceive risk from shoreline predators in these environments. This will require careful consideration of where semiochemicals are applied in sea lamprey management.
In the Great Lakes region, sea lamprey traps rely firstly on blocking upstream movement with a barrier. Searching for a route past the barrier, sea lamprey encounter attraction flow emitted from the entrance to the trap positioned perpendicular to the flow and presumably enter traps as the flow signals a route through the impediment (Bravener and McLaughlin 2013; Rous et al. 2017). The relatively poor performance of such traps is in sharp contrast to the successful strategies of lamprey exploitation in fisheries elsewhere in the world. Today, sea lamprey are commercially exploited in Spain, Portugal, and France, and have been for at least 1000 years (Araújo et al. 2016). Exploitation of the adult population in some rivers has been estimated at 75% (Andrade et al. 2007) and Silva et al. (2019) recently reported a 97% decrease in adult captures over a 7 km stretch of river containing sea lamprey traps. Manipulating sea lamprey movement in a predictable manner (e.g. using the alarm cue to signal areas of risk to avoid), means we could position traps to intercept them in the Great Lakes. Why then were traps so ineffective in the current study despite high encounter rates? Because the alarm cue is hypothesized to indicate areas of risk (Wagner et al. 2011), it may result in a reduced probability of sea lamprey entering a novel structure like a trap due to possible neophobia. There are consistent behavioral differences that may influence sea lamprey interactions with traps (McLean and McLaughlin 2018; Reinhardt and Hrodey 2019). McLean and McLaughlin (2018) found that sea lamprey captured in traps reduced their movement rates in the presence of a predator cue relative to sea lamprey electrofished from the same stream, and were more active in general. It is conceivable that using previously trapped sea lamprey (as used in this study) may have introduced unintended bias.
Field tests of 3kPZS have taken place on a large scale in recent years following frequent proposals that its presence would improve the capture of migratory sea lamprey in traps (Johnson et al. 2013). This semiochemical appears most effective as a pull when applied to traps in wide streams (~ 40 m) containing sea lamprey at low densities (< 1000 adults, Johnson et al. 2015a, b), which is consistent with the use of attractants in the mass trapping of invertebrates (El-Sayed et al. 2006) but inconsistent with low density conditions during the present study (654 wild adults captured; USFWS unpub. data). When applied alone, 3kPZS had no statistically significant effect on migratory sea lamprey, but when present in conjunction with the alarm cue we observed a greater proportion of sea lamprey in target areas. Although barrier-integrated trap efficacy improves on average 9% with the application of 3kPZS vs. historic performance of traps, there is wide variance in trap efficacy across years and among streams (− 10% to + 24% change), resulting in an inconsistent proportion of adults removed annually (Johnson et al. 2013). Furthermore, Johnson et al. (2015a, b) report that 3kPZS is most effective as a pull during the early days of the spawning migration when water temperatures are still rising. Sea lamprey exhibit greater activity in rising stream temperatures (Binder and McDonald 2008; Binder et al. 2010), therefore the improvements in trap performance evidenced both here, and in other studies applying 3kPZS, could equally be explained by an increase in encounter rate with trap entrances caused simply by greater rates of movement in the vicinity of barrier-integrated traps (Bravener and McLaughlin 2013; Rous et al. 2017). Our findings are consistent with 3kPZS functioning as a cue inducing upstream movement in sexually immature animals (Brant et al. 2015, 2016). However, the lack of improvements to nominal trap encounter or entrance rates in this study indicates 3kPZS acting as a pull is not sufficient to achieve trapping-for-control targets, and its role in push–pull applications may be restricted to late in the migration to influence movement of more mature individuals.
At the onset of the spawning migration sea lamprey move toward and into suitable streams indicated by the presence of conspecific larvae that emit bile acids as a by-product of feeding, but which adults respond to as a migratory pheromone (Teeter 1980; Vrieze and Sorensen 2001; Sorensen et al. 2005). Adult sea lamprey entering such tributaries respond more strongly to the odor of larvae compared to 3kPZS (Meckley et al. 2012, 2017). Under natural circumstances, both odors operate in different ways: firstly providing sexually-immature sea lamprey with information on suitable reproductive habitats (larval odor, Wagner et al. 2009) and secondly advertising the proximity of suitable mates for sexually-mature sea lamprey (3kPZS Brant et al. 2016). Both odors will, therefore, operate differently as a pull during mass-trapping operations. Larval odor is highly attractive to sexually-immature sea lamprey when present in the absence of any background conspecific cues (Wagner et al. 2009), and will consequently motivate sea lamprey to move upstream toward an area that could contain traps. It does not draw them to specific points in space based on concentration. However, as established here and elsewhere (Bals and Wagner 2012; Hume et al. 2015), the alarm cue functions as an effective and consistent push in shallow water environments that will guide migrating sea lamprey toward a comparatively small area (< 1 m wide). By providing sea lamprey with information on historical breeding success (reward) and alerting individuals to potential harm (risk), together larval odor and the alarm cue may represent a more synergistic combination when applied in a push–pull configuration as they are both strong signals of conspecific fitness. Identifying and synthesizing the compounds responsible for these behavioral responses to enable field-scale tests of this combination will be key to testing this hypothesis.
By avoiding the alarm cue in a consistent and predictable manner, and given that the species remains closely associated with the stream substrate during the spawning migration (Holbrook et al. 2014), sea lamprey in the Great Lakes should be vulnerable to management actions while constrained to narrow stream channels. This could be used to physically remove adults from the system, but the ability to aggregate sea lamprey into relatively small areas in space will also improve the accuracy of control program assessment by reducing uncertainty surrounding population sizes (Jones et al. 2009). For example, technologies such as dual-frequency identification sonar (DIDSON; McCann et al. 2018) and automated detection cameras (Negrea et al. 2014) can count the numbers of sea lamprey adults each spawning season without the need to capture them in traps. Therefore, control agencies could be more confident managing their fixed budget, for example by ensuring pesticide application occurs only in the most heavily infested streams, an action that ultimately improves program efficiency (Jones et al. 2009). Sea lamprey remain an injurious and stubborn invasive species in the Great Lakes, and it seems probable that combining multiple supplemental methodologies—such as push–pull application of semiochemicals with restraining fishing gear—will be required to realize notable improvements in population reduction.
References
Andrade NO, Quintella BR, Ferreira J, Pinela S, Póvoa I, Pedro S, Almeida PR (2007) Sea lamprey (Petromyzon marinus L.) spawning migration in the Vouga River basin (Portugal): poaching impact, preferential resting sites and spawning ground. Hydrobiologia 582:121–132
Araújo M, Silva S, Stratoudakis Y, Gonçalves M, Lopez R, Carneiro M, Martins R, Cobo F, Antunes C (2016) Sea lamprey fisheries in the Iberian Peninsula. In: Orlov A, Beamish R (eds) Jawless fishes of the world, vol 2. Cambridge Scholars Publishing, Cambridge, pp 115–148
Bals JS, Wagner CM (2012) Behavioral responses of sea lamprey (Petromyzon marinus) to a putative alarm cue derived from conspecific and heterospecific sources. Behavior 149:901–923
Beamish FWH (1979) Migration and spawning energetics of the anadromous sea lamprey, Petromyzon marinus. Environ Biol Fishes 4:3–7
Binder TR, McDonald G (2008) The role of temperature in controlling diel activity in upstream migrant sea lampreys (Petromyzon marinus). Can J Fish Aquat Sci 65:1113–1121
Binder TR, McLaughlin RL, McDonald DG (2010) Relative importance of water temperature, water level, and lunar cycle to migratory activity in spawning-phase sea lampreys in Lake Ontario. Trans Am Fish Soc 139:700–712
Bolker B (2017) Tools for general maximum likelihood estimation: package ‘bbmle’. CRAN electronic resource
Brant CO (2015) Characterization of sea lamprey pheromone components. Ph.D. Thesis, Michigan State University
Brant CO, Li K, Johnson NS, Li W (2015) A pheromone outweighs temperature in influencing migration of sea lamprey. R Soc Open Sci 2:150009
Brant CO, Johnson NS, Li K, Buchinger TJ, Li W (2016) Female sea lamprey shift orientation toward a conspecific chemical cue to escape a sensory trap. Behav Ecol 27:810–819
Bravener GA, McLaughlin RL (2013) A behavioural framework for trapping success and its application to invasive sea lamprey. Can J Fish Aquat Sci 70:1438–1446
Burham KP, Anderson DR (2002) Model selection and multimodal inference. Springer, New York
Clark L, Clark C, Siers S (2017) Brown tree snakes methods and approaches for control. In: Pitt WC, Beasley J, Witmer GW (eds) Ecology and management of terrestrial invertebrate invasive species in the United States. CRC Press, Boca Raton, pp 107–134
Cochran PA (2009) Predation on lampreys. In: Brown LR, Chase SD, Mesa MG, Beamish RJ, Moyle PB (eds) Biology, management and conservation of lampreys in North America, Bethesda, vol 72. American Fisheries Society, Bethesda, pp 139–151
Dawson HA, Jones ML, Irwin BJ, Johnson NS, Wagner CM, Szymanski MD (2016) Management strategy evaluation of pheromone-baited trapping techniques to improve management of invasive sea lamprey. Nat Resour Model 29:448–469
Development Core Team R (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
El-Sayed AM, Suckling DM, Wearing CH, Byers JA (2006) Potential of mass trapping for long-term pest management and eradication of invasive species. J Econ Entomol 99:1550–1564
Fontaine PR (1938) La lamproie marine: sa pêche et son importance économique. Bulletin de la Société D’Océanographie de France 97:1681–1687 (In French, translated by Hume JB)
Fox J, Weisberg S (2011) An R companion to applied regression. SAGE Publications, Thousand Oaks
Gore JA (1996) Discharge measurements and stream flow analysis. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology. Academic Press, San Diego, pp 53–74
Haeseker SL, Jones ML, Peterman RM, Bence JR, Dai W, Christie GC (2007) Explicit consideration of uncertainty in Great Lakes fisheries management: decision analysis of sea lamprey (Petromyzon marinus) control in the St. Marys River. Can J Fish Aquat Sci 64:1456–1468
Happel A, Rinchard J, Czesny S (2017) Variability in sea lamprey fatty acid profiles indicates a range of host species utilization in Lake Michigan. J Great Lakes Res 43:182–188
Holbrook CM, Johnson NS, Steibel JP, Twohey MB, Binder TR, Krueger CC, Jones ML (2014) Estimating reach-specific fish movement probabilities in rivers with a Bayesian state-space model: application to sea lamprey passage and capture at dams. Can J Fish Aquat Sci 71:1713–1729
Holbrook CM, Bergstedt RA, Barber J, Bravener GA, Jones ML, Krueger CC (2016) Evaluating harvest-based control of invasive fish with telemetry: performance of sea lamprey traps in the Great Lakes. Ecol Appl 26:1595–1609
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Howse PE, Stevens IDR, Jones OT (1998) Mass trapping. In: Howse PE, Stevens IDR, Jones OT (eds) Insect pheromones and their use in pest management. Springer, Dordrecht, pp 280–299
Hume JB, Meckley TD, Johnson NS, Luhring TM, Siefkes MJ, Wagner CM (2015) Application of a putative alarm cue hastens the arrival of invasive sea lamprey (Petromyzon marinus) at a trapping location. Can J Fish Aquat Sci 72:1799–1806
Imre I, Brown GE, Bergstedt RA, McDonald R (2010) Use of chemosensory cues as repellents: potential directions for population management. J Great Lakes Res 36:790–793
Johnson NS, Yun S-S, Thompson HT, Brant CO, Li W (2009) A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. PNAS 6:1021–1026
Johnson NS, Siefkes MJ, Wagner CM, Dawson HA, Wang H, SteevesTwoheyLi TBMW (2013) A synthesized mating pheromone component increases adult sea lamprey (Petromyzon marinus) trap capture in management scenarios. Can J Fish Aquat Sci 70:1101–1108
Johnson NS, Siefkes MJ, Wagner CM, Bravener G, Steeves TB, Twohey M, Li W (2015a) Factors influencing capture of invasive sea lamprey in traps baited with a synthesized sex pheromone component. J Chem Ecol 41:913–923
Johnson NS, Tix JA, Hlina BL, Wagner CM, Siefkes MJ, Wang H, Li W (2015b) A sea lamprey (Petromyzon marinus) sex pheromone mixture increases trap catch relative to a single synthesized component in specific environments. J Chem Ecol 41:311–321
Jones ML, Irwin BJ, Hansen GJA, Dawson HA, Treble AJ, Liu W, Dai W, Bence JR (2009) An operating model for the integrated pest management of Great Lakes sea lampreys. Open Fish Sci J 2:59–73
Jones ML, Brendan TO, Irwin BJ (2015) Re-examination of sea lamprey control policies for the St. Marys River: completion of an adaptive management cycle. Can J Fish Aquat Sci 72:1538–1551
Lauer TE (2015) Fishery of the Laurentian Great Lakes. In: Craig JF (ed) Freshwater fisheries ecology. Wiley-Blackwell, Chichester, pp 134–150
Lewis DH, Tarpley RJ, Marks JE, Sis RF (1985) Drug induced structural changes in the olfactory organ of the channel catfish (Ictalurus punctatus Rafinesque). J Fish Biol 26:355–358
Li W, Scott AP, Siefkes MJ, Yan H, Liu Q, Yun S-S, Gage DA (2002) Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 296:138–141
Li KE, Brant CO, Huertas M, Hessler EJ, Mezzei G, Scott AM, Hoye TR , Li W (2018) Fatty-acid derivative acts as a sea lamprey migratory pheromone. In: PNAS 1803169115
Luhring TM, Meckley TD, Johnson NS, Siefkes MJ, Hume JB, Wagner CM (2016) A semelparous fish continues upstream migration when exposed to alarm cue, but adjusts movement speed and timing. Anim Behav 121:41–51
Marsden JE, Siefkes MJ (2019) Control of invasive sea lamprey in the Great Lakes, Lake Champlain, and Finger Lakes of New York. In: Docker MF (ed) Lampreys: biology, conservation, and control, vol 2. Springer, Dordrecht, pp 411–479
McCann EL, Johnson NS, Hrodey PJ, Pangle KL (2018) Characterization of sea lamprey stream entry using dual-frequency identification sonar. Trans Am Fish Soc 147:514–524
McLaughlin RL, Hallett A, Pratt TC, O’Conner LM, McDonald DG (2007) Research to guide use of barriers, traps, and fishways to control sea lamprey. J Great Lakes Res 33:7–19
McLean AR, McLaughlin RL (2018) Consistent individual differences in sea lamprey (Petromyzon marinus) behaviour: Implications for control via trapping. J Great Lakes Res 44:482–490
Meckley TD, Wagner CM, Luehring MA (2012) Field evaluation of larval odor and mixtures of synthetic pheromone components for attracting migrating sea lampreys in rivers. J Chem Ecol 38:1062–1069
Meckley TD, Gurarie E, Miller JR, Wagner CM (2017) How fishes find the shore: evidence for orientation to bathymetry from the non-homing sea lamprey. Can J Fish Aquat Sci 74:20145–22058
Miehls S, Sullivan P, Twohey M, Barber J, McDonald R (2019) The future of barriers and trapping methods in the sea lamprey (Petromyzon marinus) control program in the Laurentian Great Lakes. Rev Fish Biol Fisheries. https://doi.org/10.1007/s11160-019-09587-7
Miller JR, Cowles RS (1990) Stimulo-deterrent diversion: a concept and its possible application to onion maggot control. J Chem Ecol 16:3197–3212
Mullett K, Sullivan P (2017) Sea lamprey control in the Great Lakes 2016. In: Annual report to the Great Lakes Fishery Commission, p 111
Negrea C, Thompson DE, Juhnke SD, Fryer DS, Loge FJ (2014) Automated detection and tracking of adult Pacific lampreys in underwater video collected at Snake and Columbia River Fishways. N Am J Fish Manag 34:111–118
Pickett JA, Barasa S, Birkett MA (2014) Vertebrate pheromones and other semiochemicals: the potential for accommodating complexity in signaling by volatile compounds for vertebrate management. Biochem Soc Trans 42:846–850
Purvis HA, Chudy CL, King EL, Dawson VK (1985) Response of spawning-phase sea lampreys (Petromyzon marinus) to a lighted trap. Great Lakes Fish Comm Tech Rep 42:15–25
Reinhardt UG, Hrodey PJ (2019) Trap happiness and catch bias in sea lamprey traps. Fishes 4:34
Rous AM, McLean AR, Barber J, Bravener G, Castro-Santos T, Holbrook CM, Imre I, Pratt TC, McLaughlin RL (2017) Spatial mismatch between sea lamprey behaviour and trap location explains low success at trapping for control. Can J Fish Aquat Sci 74:2085–2097
Siefkes MJ, Li W (2003) Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lampreys (Petromyzon marinus). J Comp Physiol A 190:193–199
Silva S, Araújo MJ, Bao M, Mucientes G, Cobo F (2014) The haemotophagous feeding stage of anadromous populations of sea lamprey Petromyzon marinus: low host selectivity and wide range of habitats. Hydrobiologia 734:187–199
Silva S, Barca S, Viera-Lanero R, Cobo F (2019) Upstream migration of the anadromous sea lamprey (Petromyzon marinus Linnaeus, 1758) in a highly impounded river: impact of low-head obstacles and fisheries. Aquat Conserv 29:389–396
Sjöberg K (1985) Foraging activity patterns in the goosander (Mergus merganser) and the red-breasted merganser (M. serrator) in relation to patterns of activity in the major prey species. Oecologia 67:35–39
Sjöberg K (1989) Time related predator-prey interactions between birds and fish in a northern Swedish river. Oecologia 80:1–10
Smith BR, Tibbles JJ (1980) Sea lamprey (Petromyzon marinus) in Lakes Huron, Michigan, and Superior: history of invasion and control, 1936–1978. Can J Fish Aquat Sci 37:1780–1801
Sorensen PW, Johnson NS (2016) Theory and application of semiochemicals in nuisance fish control. J Chem Ecol 42:698–715
Sorensen PW, Fine JM, Dvornikovs V, Jeffrey CS, Shao F, Wang J, Vrieze LA, Anderson KR, Hoye TR (2005) Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat Chem Biol 1:324–328
Stamplecoskie KM, Binder TR, Lower N, Cottenie K, McLaughlin RL, McDonald DG (2012) Response of migratory sea lampreys to artificial lighting in portable traps. N Am J Fish Manag 32:563–572
Sullivan PW, Christie GC, Cornelius FC, Fodale MF, Johnson DA, Koonce JF, Larsen GL, McDonald RB, Mullett KM, Murray CK, Ryan PA (2003) The sea lamprey in Lake Erie: a case history. J Great Lakes Res 29:615–636
Teeter J (1980) Pheromone communication in sea lampreys (Petromyzon marinus): implications for population management. Can J Fish Aquat Sci 37:2123–2132
Tripathi RS (2014) Integrated management of rodent pests. In: Abrol DP (ed) Integrated pest management: current concepts and ecological perspective. Elsevier, San Diego, pp 419–459
Twohey MB, Sorensen PW, Li W (2003) Possible applications of pheromones in an integrated sea lamprey management program. J Great Lakes Res 29:794–800
Vrieze LA, Sorensen PW (2001) Laboratory assessment of the role of larval pheromone and natural stream odor in spawning stream localization by migrating sea lamprey (Petromyzon marinus). Can J Fish Aquat Sci 58:2374–2385
Wagner CM, Jones ML, Twohey MB, Sorensen PW (2006) A field test verifies that pheromones can be useful for sea lamprey (Petromyzon marinus) control in the Great Lakes. Can J Fish Aquat Sci 63:475–479
Wagner CM, Twohey MB, Fine JM (2009) Conspecific cueing in the sea lamprey: do reproductive migration consistently follow the most intense larval odors. Anim Behav 78:593–599
Wagner CM, Stroud EM, Meckley TD (2011) A deathly odor suggests a new sustainable tool for controlling a costly invasive species. Can J Fish Aquat Sci 68:1157–1160
Warton DI, Hui FKC (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Yun S-S (2012) Comparative studies of bile acid release in the mature male lampreys. Fish Aquat Sci 15:63–67
Acknowledgements
We thank US Fish and Wildlife Service staff for technical assistance, and US Geological Survey Hammond Bay Biological Station staff for generous provision of their facilities. We also thank K. Henige, K. Bennett, and L. Hawkins for their assistance with experimental set-up and data collection. Synthesized 3kPZS was provided by the Great Lakes Fishery Commission. We also thank three anonymous reviewers for their comments, which greatly improved this manuscript. This work was funded by the US Environmental Protection Agency through the Great Lakes Restoration Initiative, Contract No. GL00E01294-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hume, J.B., Luhring, T.M. & Wagner, C.M. Push, pull, or push–pull? An alarm cue better guides sea lamprey towards capture devices than a mating pheromone during the reproductive migration. Biol Invasions 22, 2129–2142 (2020). https://doi.org/10.1007/s10530-020-02242-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02242-4