Abstract
We report a 6-year study of an irruptive invasion of emerald ash borer (EAB) into a 36-year-old comparison of 60 green ash (Fraxinus pennsylvanica Marsh.) and 5 white ash (F. americana L.) populations. As the infestation progressed, annual measures of EAB injury (density of adult emergence holes on the trunk, crown condition, and mortality) were significantly influenced by genetic effects (population and in some instances family within population), site quality (field blocks), and neighborhood (contagion effects over distances of 5–13 m). At the last measurement, 99% of green ash trees and 87% of white ash were dead, and most of the remaining few trees had badly deteriorating crowns. Although final destruction was nearly complete, the rapidity with which trees were injured and killed was moderated by genetic and site effects and influenced by proximity to infested trees. These facts suggest that some genotypes, especially on favorable sites, will disproportionately survive under future equilibrium conditions with lower densities of ash and EAB in the landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its accidental introduction into the U.S. sometime before 2002, the emerald ash borer (EAB) (Agrilus planipennis) has spread rapidly from its point of discovery in southeastern Michigan to surrounding regions of the U.S. and Canada (Haack et al. 2002; http://www.emeraldashborer.info/timeline/by_county/index.html). The insect is a buprestid whose larvae feed on the cambium, phloem, and xylem of ash (Fraxinus spp.) before exiting the bark as adults (Cappaert et al. 2005). Within the current American distribution of the insect, forested sites have experienced as much as 100% mortality of ash trees at immense economic and ecological cost (Klooster et al. 2014, 2018; Knight et al. 2013; Herms and McCullough 2014). Green ash (F. pennsylvanica), white ash (F. americana), black ash (F. nigra), blue ash (F. quadrangulata), and pumpkin ash (F. profunda) are known to be susceptible, and all five species have been designated as “critically endangered” because of the threat posed by EAB (https://www.iucnredlist.org). Its impact on green and white ash is particularly significant because of their abundance, wide distributions, and economic importance (MacFarlane and Meyer 2005).
A small number of trees survive initial colonization by EAB, and an even smaller number retain healthy crowns (Knight et al. 2012; Kappler et al. 2018). Early results from testing clonally propagated ramets of these “lingering” trees showed that survival, development, and weight gain of EAB larvae are impaired in some clones compared to susceptible control trees of the same species (Koch et al. 2015). In addition, the foliage of some clones is less preferred by adults for feeding compared to control trees. If, as suggested by these findings, EAB resistance could be increased through breeding and selection, it is imperative to learn more about the structure of genetic variation in response to EAB (Romero-Severson and Koch 2017).
Common-garden tests of progenies from different parents (progeny tests) and of offspring from geographically separated and autochthonous populations (provenance tests) enable statistical inferences about the genetic control of traits and how they will respond to selection (Mather and Jinks 1971; Wright 1976; Morgenstern 1996). The premise of such tests is that members of the experimental units (populations or families) will share genetic similarities because of common ancestry and may differ from trees with other pedigrees in character traits of interest. Two combined provenance/progeny tests of white and green ash species were assembled in the 1970s and replicated in a total of 37 plantations in the eastern United States (Bey et al. 1976; Steiner 1983). The largest and most complete of these experiments that remained in the 2000s was a green ash test in central Pennsylvania and is the subject of this paper.
The existence of this comparison of cognate groups of ash trees at the leading edge of EAB’s spread afforded the opportunity to study patterns of decline and mortality as the colonization grew to epidemic proportions. Our principal objective was to test for variation in population and family response to attack by the insect, the existence of which would be prima facie evidence for heritable genetic variation in susceptibility. We also wanted to determine whether there existed site-related effects on tree response and whether there were neighborhood effects on the year-by-year spread and growth of the EAB population within the plantation. We report 6 years of data, encompassing the transition from negligible EAB mortality to almost complete destruction of the 2.8-ha plantation.
Methods
Our study is based on a common garden test of 60 green ash and 5 white ash populations planted as 2-year-old seedlings in 1978 in College Township, Pennsylvania (40.811°N, 77.845°W). Each population collection began with seed sampled from female parents separated from one another by a minimum of 90 m but all within an area of no more than 26 km2. All 5 white ash populations and 57 of the 60 green ash populations were judged at the time of seed collection to be autochthonous to their location, i.e., descendants of trees that presumably had lived in the vicinity for generations (Steiner et al. 1988).
The plantation was organized into 8 replicate blocks with each population represented in a block by a randomly located, linear plot of 4 trees on a 3.7-m spacing. Thirty-six of the green ash populations were represented by the offspring of exactly 4 female parents, and the identity of these open-pollinated families was maintained with one sibling planted in each of the 8 plots for that population. This design afforded the opportunity to statistically test for family-within-population effects. Family identities were not retained for trees representing the other 29 populations of green and white ash.
The site of the plantation is a well-drained, upland field that was formerly in pasture, the soil a silty clay loam underlain by fragmented limestone. The topography is sloping, and blocks were laid out approximately along slope contours, with blocks 1–3 on a concave slope with relatively deep soil, blocks 4–7 on a convex slope with limestone bedrock fragments at or near the surface in many places, and block 8 at or near the hilltop on moderately deep soil. The plantation was kept free of invading woody vegetation by regular mowing.
The site lies within an agricultural/rural-residential landscape with locally native white ash and cultivated green ash present in woodlots and plantings a few hundred meters distant in most directions. EAB was reported 33 km southeast of the plantation site in 2009, and subsequent monitoring by the Pennsylvania Department of Agriculture detected EAB 19 km east of the plantation in June 2010. By June 2012, when the trees were 36 years old, it was obvious that EAB was present in the plantation because adult insects were observed and holes in the bark created by emerging adults were visible on some trees. We estimate that infestation could have occurred as early as 4 years prior to 2012.
In 2012 we measured each tree for diameter at breast height (1.37 m) (DBH) and rated the crown condition of each tree on a 5-point scale from 1 (no evidence of dieback or thinning within the crown) to 5 (entire crown dead above the lowest branchFootnote 1) (Smith 2006; Knight et al. 2014). Crown condition has been shown to correlate well with the percentage of the tree trunk covered by EAB larval galleries and with a measure of leaf water stress associated with vascular system injury (Flower et al. 2013). We excluded from measurement trees that were declining from other causes in 2012 and had no evidence of EAB attack. This left 1762 green and white ash trees that were alive and apparently healthy prior to the arrival of EAB. At that time, the plantation had a basal area of 16.0 m2/ha and an estimated 70% of maximum stocking but with virtually complete canopy coverage (Gingrich 1967).
As a measure of the quantity of successful EAB attacks on each tree, we counted the number of EAB emergence holes on the trunk between the heights of 1.25 and 1.75 m. This is a direct count of the number of adult beetles that emerged after feeding as larvae within the sampled portion of the tree. Counts of lower trunk emergence holes and ratings of crown condition were continued in each subsequent year through 2017, when almost all trees in the plantation had been killed by EAB. In all years, including 2012, these observations were made beginning at the end of the first week in June or later, after peak emergence of the insect but not necessarily after all had emerged.
For analysis and presentation, the incidence of EAB emergence holes was usually expressed as the cumulative density of holes, per m2 trunk surface area, created during the current and previous years. We call this variable “emergence hole density.” The term “new” emergence hole density is used in the few instances where we refer to emergence holes created only since the previous year’s measurements. Emergence hole density was assumed to remain fixed after two successive annual measurements of crown condition = 5. By 2017, a few trees (1.5%) had been killed by EAB but had no emergence holes within the sampled length of trunk. Most of these were small trees whose zero counts could be attributed, in part at least, to the proportionately smaller bark area that we sampled on small trees. This would have introduced a size-related bias because zero counts would not scale in proportion to trunk area as the unit of analysis was changed from the tree (or 4-tree plot) to m2 trunk surface. To avoid this, we assumed a count of “1” on all EAB-killed trees without holes on the sampled section of trunk. This resulted in a range of density counts for these trees that was at or near the low end of all measured values, and the counts became higher as the trees were smaller and the likelihood of an incidental miss (by insect or observer) was greater.
Emergence hole densities were analyzed with generalized linear models to determine whether species, population, block, or plot mean DBH caused significant departures from a random (Poisson) distribution of responses. Data elements were mean densities for the 1–4 remaining trees in each population plot. Separate analyses were performed for green and white ash, and for each year from 2012 through 2017, because we expected that different patterns would emerge through time as colonization progressed from patchy to widespread to aftermath conditions. Similar comparisons were made between species in each year. Raw numbers were corrected for over-dispersion from an underlying Poisson distribution using Pearson Chi square as a scaling factor, and counts were standardized to a unit-trunk-area basis by using the logarithm of trunk area as a model “offset.” The significance of predictors was determined using likelihood ratio F-tests. In addition to population-level analyses, we tested for the existence of mother-tree effects in the 36 populations with known family identities by fitting 2017 hole densities for individual trees to a model with population, block, and diameter as fixed effects and with, or without, family-in-population as a random effect (likelihood ratio test for family effect).
Crown condition was analyzed by fitting condition classes to a multinomial distribution as a function of species, population, block, and DBH. This was done for each year and for green and white ash together (to compare species) and separately (to compare populations within species), and the significance of predictors was determined using F-tests.
Mortality was analyzed as a binary function of species, population, block, and DBH in logistic regression models applied to each year’s data in turn, as above. Firth’s penalized maximum likelihood estimation (Firth 1993) was used to obtain bias-adjusted population estimates in the presence of quasi-separation of data points at either end of the observation period caused by very low or very high mortality probabilities. Wald Chi square tests were used to determine predictor significance. Similar analyses were used to test for family effects within green ash populations.
Mortality across years was examined by survival time analysis using the year of death for individual trees as data elements. We fitted a shared frailty model of clustered data using block as the clustering variable and right censoring to account for trees that were alive and had unknown future longevity at the end of the study. The significance of population, DBH, and block effects was evaluated using the Wald Chi square test. The analysis produced hazard ratio estimates for each population, with higher ratios indicated shorter duration of survival starting from the year 2012.
To examine spatial patterns of EAB spread within the plantation, we created spatial variograms (Isaaks and Srivastava 1989) to test for spatial autocorrelation effects in response variables and determine the distances over which those effects were manifested, if present. Individual-tree values for new emergence hole density and crown condition were used as response variables, and species, population, and block effects were ignored. Analyses were performed separately for each year (2012–2017). Trees were assigned coordinates based on their column and row locations. Because the plantation is divided into two slightly separated sections whose rows do not align, a separate analysis was conducted for each portion. The significance of spatial autocorrelation within the plantation was tested with Geary’s contiguity ratio statistic.
Variograms were used to visualize the distances over which spatial autocorrelation was important. Semivariance is a measure of the dissimilarity between a focal tree and all trees just within a radial distance of n + 0.5 “lag” units, which in our study were the 3.7-m intervals between tree positions. Thus, lag 1 comparisons were made with the 8 adjacent trees (if all were present) just within a 5.5-m radius, lag 2 with the 12 trees forming a perimeter just within a 9.2-m radius, etc. Spatial autocorrelation causes an increase in semivariance with lag distance until a plateau is reached at a distance corresponding to the spatial limit of clumping. We calculated semivariances separately for each section of the plantation and then averaged the two values at each lag distance to obtain plantation-wide variograms.
Because populations were planted in linear, 4-tree plots, every tree had related neighbors, even sometimes siblings, within the same row but unrelated neighbors (except by rare chance) in adjacent rows. To test whether spatial autocorrelations could be fully explained by similarities in response among related neighbors, we tested for associations between each tree and its 2 immediate and unrelated neighbors in adjacent rows. We used Poisson regression to model new emergence hole density on individual trees as a function of four variables from the previous year’s data: number (0, 1, 2) of unrelated neighbors that had evidence of EAB attack (emergence holes or declining crowns), number (0, 1, 2) of unrelated neighbors that were killed by EAB, number of new holes counted on unrelated neighbors, and total number of holes counted on unrelated neighbors. For this analysis we used only those trees in the interior of the plantation that had two living and unrelated neighbors at the start of the EAB colonization (n = 1187).
As described, all statistical analyses were conducted using generalized linear mixed models appropriate for the variable and coded using SAS (SAS Institute 2010). Models were fit using the GENMOD procedure for emergence hole density, LOGISITIC for yearly mortality, and GLIMMIX for crown condition and other models with both fixed (DBH) and random (population and block) effects. PHREG was used to fit the shared frailty model. VARIOGRAM was used to create the spatial variogram. In every procedure, the significance of predictors was determined using Type III tests to avoid biases arising from the order of predictors in model statements. Unless otherwise indicated, predictors were considered significant based on the appropriate test with a 95% confidence level (5% chance of Type I error), and the best-fit models were chosen as those with the most explanatory value in which all predictors were statistically significant. To remove confounding bias, we report only modeled effect means after fitting other significant, independent variables.
Results
Effects of year, site, tree size, and neighborhood
Over the 5-year course of the study, EAB-attributable mortality increased from 2 to 98% as the proportion of trees with fully healthy crowns decreased from 78 to 0.2% (Table 3). As described below, the effects of independent variables on EAB injury were strongest in the middle years of the study, i.e., after the incidence of attack became high enough to reveal predisposing factors against background variation, but before infestation became so pervasive that weak effects were masked. These years are emphasized in the following presentation. The effects of predisposing factors on mortality (population, block, DBH) were particularly strong in 2014, when approximately half of all trees were dead from EAB yet 10% still showed no evidence of dieback.
Statistically significant block effects were found for one or more measures of injury in every year and for every measure in 2014 and 2015 (Table 1). Analysis of variance components showed that the block fraction of total variance in emergence hole density and mortality was greatest in 2014, after which block effects diminished. Block differences in EAB injury generally followed a progression of earlier and more severe attack occurring in those blocks where tree growth was slower and the soil shallower (Fig. 1). Block was also a statistically significant (P < 0.0001) effect in the multi-year survival time analysis.
Relationships between block mean DBH of green ash trees versus a block mean emergence hole density and b block-level percentage of EAB-killed trees for the year 2014, when block effects were strongest. Plotted points are modeled block means after adjustment for the independent effects of population and DBH. Bars represent ± 1 standard error of block estimates
Tree diameter influenced injury levels independently of block and population effects (Table 1). Larger diameters were significantly associated with lower hole densities, lower mortality, and healthier crowns. This depended upon the year, and the relationship was particularly strong in 2014. For mortality, the direction of the relationship switched in 2016 and 2017, when a slightly disproportionate percentage of the smallest diameter classes were among the few trees that remained alive.
The presence of spatial clumping for new emergence hole density in 2013–2017 and crown condition in 2012–2015 was indicated by statistically significant increases in semivariance estimates as distance between trees became greater. Variograms are shown in Fig. 2 for the year 2013, when semivariance estimates had the narrowest confidence limits. Neighborhoods with elevated autocorrelation among trees (lower semivariances) appeared to occur at two spatial scales: one within radial distances of < 13 m, depending on the variable, and a second, less distinct one whose outer boundary was > 35 m. The larger neighborhood is approximately the scale of a field block, and autocorrelation at that scale probably reflects coarse-scale site effects also detected as block effects. The smaller neighborhood necessarily included a minority of trees belonging to the same population, which probably strengthened autocorrelation because of genetic similarities in susceptibility. However, we found significant relationships between EAB hole densities on trees and several measures of injury on adjacent (3.7 m) but unrelated neighbors (Table 2). These relationships were strongest in 2013 and diminished thereafter as the EAB population grew, and they demonstrate that mere proximity to infested trees was affecting the spatial occurrence of EAB.
Average semivariances between focal trees and comparison trees at various “lag” distances (expressed here in meters) shown for a density of new emergence holes in 2013 and b crown condition in 2013. Error bars indicate the upper and lower 95% confidence limits for semivariance estimates. Confidence limits were narrowest for this year (2013), but variograms for other years were similar
Species differences in injury by EAB
White ash had significantly less EAB injury than green ash by every measure and in almost every year (Table 3). For most measures and most years, these differences were highly significant (P < 0.0001). In 2017, 13.0% of white ash trees and 0.9% of green ash trees remained alive.
Effects of genotype on injury by EAB
Green ash populations differed statistically in emergence hole density (cumulative) in most years, mortality in the years 2013–2015, and crown condition in 2012–2015 (Table 1; Fig. 3). Green ash populations also differed significantly (P < 0.0001) in hazard ratio based on shared frailty analysis. The 5 white ash populations also differed significantly in every injury variable in the last year or two of measurement (Table 1).
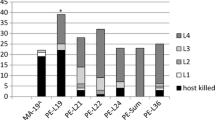
Proportional distribution of crown condition classes in 2014 for the ten populations of green ash least injured by EAB (black circles) and the ten most injured (gray circles). Lines connect the means for each group. All members of each group are significantly different in multinomial response from all members of the other. Plotted points are modeled population means after adjustment for the independent effects of block and DBH
For green ash, the population share of total variance in emergence hole density peaked in 2017, and population variance in mortality peaked in 2014. Hole density in 2017 represents a summative, multi-year index of adult EAB emergence in the lower trunk because holes accumulated while the tree remained alive but ceased with death. Green ash populations had similar mortality levels in 2017, when overall mortality was > 99%, but population hole densities (essentially at-death measures) varied from 24 to 73/m2 (P < 0.0001).
In contrast, increasingly variable mortality among populations culminated in 2014, when half of all trees were dead. Population differences grew smaller in subsequent years as the insect became increasingly pervasive. Thus, 2014 mortality is an index of the rapidity with which populations succumbed to the growing EAB infestation. It was highly correlated (r2 = 0.82) with mortality hazard ratio, also an across-the-years measure of population persistence. Population mean mortality in 2014 was uncorrelated with 2017 emergence hole density (r2 = 0.03).
For the 57 green ash populations believed to be native to where the seed was collected, hole density in 2017 tended to be lowest in those populations from southern and eastern locations (Fig. 4). In contrast, 2014 mortality tended to be lowest in populations from the middle latitudes of the American Midwest, from Ohio to the Dakotas, although this generalization had more exceptions than that for emergence density (Fig. 5).
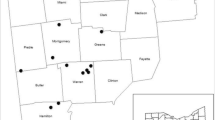
Geographic distribution of autochthonous populations of green ash and their relative density of emergence holes in 2017 (low, intermediate, or high) based on means adjusted for the independent effects of block and DBH. Each population in the low- and high-density group, respectively, is significantly (P < 0.05) different from all those in the other. Shaded area represents the putative pre-Columbian distribution of the species (Little 1971)
Geographic distribution of autochthonous populations of green ash and their relative mortality in 2014 (low, intermediate, or high) based on means adjusted for the independent effects of block and DBH. Each population in both the low- and high-mortality group, respectively, is significantly (P < 0.05) different from all those in the other. Shaded area represents the putative pre-Columbian distribution of the species (Little 1971)
For the 36 populations in which family identities were kept on individual trees, family-within-population variation was statistically significant (P = 0.02) for emergence hole density in 2017. We found no persuasive evidence of within-population, mother-tree effects on mortality.
Discussion
When first measured in 2012, 8% of green and white ash trees had emergence holes on the lower trunk and 2% had been killed by EAB. Because some EAB larvae are semivoltine, especially at low population densities (Cappaert et al. 2005; Wei et al. 2007), it is likely that EAB initially entered the plantation no later than 2010, and it may have entered as early as 2008 or 2009 judging by the presence of mortality in 2012 (Siegert et al. 2010; Kovacs et al. 2010). From this we estimate that a minimum of 7–9 years of colonization were required for green ash mortality to reach 99%. The progression of mortality in white ash was significantly slower, and over half (18) of the 33 surviving trees in 2017 were white ash despite their minority representation in the plantation. Others have shown that white ash is slower to die, and more likely to survive, when both species occur together in irruptive infestations of EAB (Cappaert et al. 2005; Anulewicz et al. 2007; Tanis and McCullough 2015). We expect that many of the 2017 survivors will still succumb to EAB because most (28) were in crown condition classes that typically do not recover (Knight et al. 2012).
The spatial distribution of EAB-killed trees was loosely aggregated around several locations in the plantation when first measured in 2012, suggesting that there were multiple entries of the insect into the plantation. This spatial effect was no longer apparent in the following year, but until 2017 our analyses continued to reveal a clustering of injury levels at a radial scale of about 5 m. Unless one posits soil-related factors at a very fine scale, it is most likely that neighborhood effects arose from short-distance dispersal of gravid females from their larval hosts. In “aftermath” forests, Kappler et al. (2018) found that EAB-attacked trees had more conspecific neighbors within 6 m than did healthy survivors, and they interpreted this as a neighborhood effect. Although EAB adults are known to oviposit on trees hundreds of meters from their larval hosts (Mercader et al. 2009; Siegert et al. 2010), this does not preclude the existence of contagion-like neighborhood effects at the scale of a few meters, especially if emerging adults are induced to remain sedentary by the nearby abundance of the ash foliage for feeding.
The design of the study permitted us to test the effect of blocked, site-related effects on EAB impact, and block effects were significant for every measured variable. EAB had the greatest initial impact where conditions for tree growth were least favorable, and this may be consistent with observations that rates of EAB landing, feeding, oviposition, and/or larval development are higher on trees that are stressed (Cappaert et al. 2005; Chen and Poland 2009; McCullough et al. 2009a, b; Tluczek et al. 2011; Jennings et al. 2014). Our results provide experimental proof that site differences over short distances (ca. 20–40 m) can affect tree response to EAB.
Until plantation-wide mortality grew beyond about 80%, larger trees had significantly less mortality, lower emergence hole densities on the lower trunk, and healthier crowns than did smaller trees. Knight et al. (2012) similarly observed that ash trees in dominant and codominant crown positions survived longer than smaller trees. We suggest several plausible and compatible reasons for the longer survival of larger trees. First, there is the obvious fact that more larval feeding is required to girdle and kill larger trees, so they should survive longer unless large stems are preferred by EAB females for oviposition. Second, it appears that larger stems are not only non-preferred as oviposition sites but even avoided during the early stages of EAB colonization (Haack et al. 2002; Cappaert et al. 2005; Timms et al. 2006; Ryall et al. 2011). Finally, it is possible that the larger trees in our plantation were better able to tolerate EAB injury or employ defenses because they had more exposure to full sunlight and were likely under less physiological stress than smaller neighbors.
The two variables that discriminated most strongly among populations were 2017 emergence hole densities and 2014 mortality. The former is our best index of the population of insects that successfully grew to adulthood from among the cohort of larvae that caused tree death. As explained above, the latter can be regarded as an index of how fast populations succumbed to the 7- to 9-year EAB invasion. Although both variables are directly (though not necessarily completely) related to EAB-induced injury levels, they bore no relationship to one another at the level of population means (r2 = 0.03), and they had dissimilar patterns of genetic variation on a geographic scale (Figs. 4, 5). Interestingly, the populations with low 2014 mortality appear to fall within a “northwestern” group of autochthonous green ash populations based on analysis of genetic markers, and those with low emergence hole densities appear to correspond with a “southern” genetic group (Noakes et al. 2019). The fact that the two measures of response to EAB infestation sorted differently by pedigree indicates that they are at least partially controlled by different underlying genetic factors.
As a measure of resistance, 2014 mortality could reflect population differences in avoidance (attractiveness as sites for oviposition), narrow-sense resistance (suitability as hosts for successful larval development), or tolerance (ability to remain healthy despite successful larval development). At-death (2017) emergence hole density is not so easily interpreted in terms of resistance mechanisms because the connection between tree condition and successful adult EAB emergence can be influenced by factors that are not under the control of the tree. Especially important among these is the fact that larvae may cause injury but still fail to emerge as adults because of predation (Lindell et al. 2008) or because of competition among larvae (a density-dependent function of oviposition rates) leading to starvation or cannibalism (Duan et al. 2013). Resistance mechanisms have little or no obvious connection with these phenomena at the individual tree level. However, population differences in emergence hole densities are strong evidence of a connection with underlying genetic resistance factors such as, for example, traits that discourage high rates of oviposition or physiological conditions that predispose trees to remain healthy at high larval densities. The dissociation between emergence hole densities and mortality rates at the population level reinforces the finding of Koch et al. (2015) that multiple resistance mechanisms are involved in the response of ash trees to EAB.
We believe our most significant finding is the presence of well-defined differences among green ash populations during the years of peak variability in every measure and derived measure of EAB injury: emergence hole density, crown condition, mortality, and multi-year mortality hazard ratio. In later years, as injury and mortality to white ash increased, the five white ash populations also differed significantly in emergence hole density, crown condition, and mortality. Additionally, we found evidence of within-population differences in emergence hole densities among open-pollinated families of green ash. Differences among populations and open-pollinated families can be attributed to heritable, additive genetic effects that can be captured (“fixed”) through selection and breeding without resorting to specific male–female matings (Namkoong 1979).
Koch et al. (2015) have shown that after the initial irruption of EAB some of the few green ash trees that survive have measurable and sometimes distinct defenses against EAB. Although most of the trees that remained alive in our plantation in 2017 may still succumb, the fact that they survived this far through such an irruptive invasion of EAB indicates the presence of heritable factors that were sufficiently strong to delay death. Resistance was distributed unequally among and within populations and is probably complex in the sense of comprising a plurality of traits that affect oviposition preference and tolerance or resistance to larval feeding. Our results show that the threshold at which tree defenses are overcome by EAB varies not only among individuals (Koch et al. 2015) but also among populations and families.
It is impossible to predict how specific populations of green or white ash will respond to initial attack by EAB because the conditions of our study were necessarily different from their home environments. However, under these conditions, the rapidity with which trees were injured and killed was moderated by population, family, local site effects, and proximity to infested trees. Despite almost 100% mortality at the end of the study, these findings suggest that the more resistant populations and families would survive under future equilibrium conditions with lower densities of ash, and therefore EAB, in the landscape. Furthermore, our results suggest that programs of selection and breeding in green and white ash could succeed in furthering the level and frequency of resistance.
Notes
For analysis and presentation, we have treated all trees with dead crowns (crown condition = 5) as dead trees. Most trees produced epicormic sprouts from dormant buds in response to injury, but these sprouts did not survive EAB unless they were at the base of the tree. Of trees that had dead crowns for > 1 year, 81% had living sprouts from dormant buds at the base of the trunk, but we regarded these as replacement trees rather than surviving originals.
References
Anulewicz AC, McCullough DG, Cappaert DL (2007) Emerald ash borer (Agrilus planipennis) density and canopy dieback in three North American ash species. Arboric Urban For 33:338–349
Bey CF, Kung FH, Daniels RA (1976) Genotypic variation in white ash nursery results. Proc Central States For Tree Improv Conf 10:141–145
Cappaert D, McCullough DG, Poland TM, Siegert NW (2005) Emerald ash borer in North America: a research and regulatory challenge. Am Entomol 51:152–165
Chen Y, Poland TM (2009) Biotic and abiotic factors affect green ash volatile production and emerald ash borer adult feeding preference. Environ Entomol 38:1756–1764
Duan JJ, Larson K, Watt T, Gould J, Lelito JP (2013) Effects of host plant and larval density on intraspecific competition in larvae of the emerald ash borer (Coleoptera: Buprestidae). Environ Entomol 42:1193–1200
Firth D (1993) Bias reduction of maximum likelihood estimates. Biometrika 80:27–38
Flower CE, Knight KS, Rebbeck J, Gonzalez-Meler MA (2013) The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus) tree decline: using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For Ecol Manag 303:143–147
Gingrich SF (1967) Measuring and evaluating stock and stand density in upland hardwood forests in the Central States. For Sci 13:38–53
Haack R, Jendek E, Liu H, Marchant KR, Petrice TR, Poland TM, Ye H (2002) The emerald ash borer: a new exotic pest in North America. Mich Entomol Soc Newsl 47(3–4):1–5
Herms DA, McCullough DG (2014) Emerald ash borer invasion of North America: history, biology, ecology, impacts and management. Annu Rev Entomol 59:13–30
Isaaks EH, Srivastava RM (1989) Applied geostatistics. Oxford University Press, New York
Jennings DE, Tayler PB, Duan JJ (2014) The mating and oviposition behavior of the invasive emerald ash borer (Agrilus planipennis), with reference to the influence of host tree condition. J Pest Sci 87:71–78
Kappler RH, Knight KS, Koch J, Root KV (2018) Neighboring tree effects and soil nutrient associations with surviving green ash (Fraxinus pennsylvanica) in an emerald ash borer (Agrilus planipennis) infested floodplain forest. Forests 9:183. https://doi.org/10.3390/f9040183
Klooster WS, Herms DA, Knight KS, Herms CP, McCullough DG, Smith A, Gandhi KJK, Cardina J (2014) Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol Invasions 16:859–873
Klooster WS, Gandhi KJK, Long LC, Perry KI, Rice KB, Herms DA (2018) Ecological impacts of emerald ash borer in forests at the epicenter of the invasion in North America. Forests 9:250. https://doi.org/10.3390/f9050250
Knight KS, Herms D, Plumb R, Sawyer E, Spalink D, Pisarczyk E, Wiggin B, Kapper R, Ziegler E, Menard K (2012) Dynamics of surviving ash (Fraxinus spp.) populations in areas long infested by emerald ash borer (Agrilus planipennis). In: Proceedings of the 4th international workshop on genetics of host-parasite interactions in forestry, USDA Forest Service General Technical Report PSW-GTR-240, pp 143–152
Knight KS, Brown JP, Long RP (2013) Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biol Invasions 15:371–383
Knight KS, Flash BP, Kappler RH, Throckmorton JA, Grafton B, Flower CE (2014) Monitoring ash (Fraxinus spp.) decline and emerald ash borer (Agrilus planipennis) symptoms in infested areas. U.S. Department of Agriculture, Forest Service, Northern Research Station, General Technical Reports NRS-139
Koch JL, Carey DW, Mason ME, Poland TM, Knight KS (2015) Intraspecific variation in Fraxinus pennsylvanica responses to emerald ash borer (Agrilus planipennis). New For 46:995–1011
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol Econ 69:569–578
Lindell CA, McCullough DC, Cappaert D, Apostolou NM, Roth MB (2008) Factors influencing woodpecker predation on emerald ash borer. Am Midl Nat 159:434–444
Little EL Jr (1971) Atlas of United States trees. Conifers and important hardwoods, vol 1. USDA Forest Service Misc. Publ. No. 1146
MacFarlane DW, Meyer SP (2005) Characteristics and distribution of potential ash tree hosts for emerald ash borer. For Ecol Manag 213:15–24
Mather K, Jinks JL (1971) Biometrical genetics. Cornell University Press, Ithaca, New York, p 382
McCullough DG, Poland TM, Anulewicz AC, Cappaert D (2009a) Emerald ash borer (Coleoptera: Buprestidae) attraction to stressed or baited ash trees. Environ Entomol 38:1668–1679
McCullough DG, Poland TM, Cappaert D (2009b) Attraction of the emerald ash borer to ash trees stressed by girdling, herbicide treatment, or wounding. Can J For Res 39:1331–1345
Mercader RJ, Siegert NW, Liebhold AM, McCullough DG (2009) Dispersal of the emerald ash borer, Agrilus planipennis, in newly-colonized sites. Agric For Entomol 11:421–424
Morgenstern EK (1996) Geographic variation in forest trees: genetic basis and application of knowledge in silviculture. University of British Columbia Press, Vancouver, p 209
Namkoong G (1979) Introduction to quantitative genetics in forestry. USDA Forest Service Technical Bulletin 1588. United States Department of Agriculture Forest Service, Washington, D.C., 342p
Noakes AG, Shirley DL, Snyder T, Stanley RK, Coggeshall M, Koch JL, Romero-Severson J (2019) Range-wide genetic analysis of green ash (Fraxinus pennsylvanica) reveals unexpected patterns of population differentiation and offspring from landscaping cultivars (submitted for publication)
Romero-Severson J, Koch JL (2017) Saving green ash. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, General Technical Reports PNW-GTR-963, pp 102–110
Ryall KL, Fidgen JG, Turgeon JJ (2011) Detectability of the emerald ash borer (Coleoptera: Buprestidae) in asymptomatic urban trees by using branch samples. Environ Entomol 40:679–688
SAS Institute (2010) The SAS system for windows, version 9.3. Cary, NC
Siegert NW, McCullough DG, Williams DW, Fraser I, Poland TM, Pierce SJ (2010) Dispersal of Agrilus planipennis (Coleoptera: Buprestidae) from discrete epicenters in two outlier sites. Environ Entomol 39:253–265
Smith A (2006) Effects of community structure on forest susceptibility and response to the emerald ash borer invasion of the Huron River watershed in southeast Michigan. Master’s of Science Thesis, Ohio State University, Columbus, OH
Steiner KC (1983) A provenance test of green ash. In: Proceedings of the 28th Northeastern Forest tree improvement conference, pp 68–75
Steiner KC, Williams MW, DeHayes DH, Hall RB, Eckert RT, Bagley WT, Lemmien WA, Karnosky DF, Carter KK, Cech FC (1988) Juvenile performance in a range-wide provenance test of Fraxinus pennsylvanica Marsh. Silvae Genet 37:104–111
Tanis SR, McCullough DG (2015) Host resistance of five Fraxinus species to Agrilus planipennis (Coleoptera: Buprestidae) and effects of Paclobutrazol and fertilization. Environ Entomol 44:287–299
The Ohio State University. M.S. thesis
Timms LL, Smith SM, de Groot P (2006) Patterns in the within-tree distribution of the emerald ash borer Agrilus planipennis, (Fairmaire) in young, green-ash plantations of south-western Ontario, Canada. Agric For Entomol 8:313–321
Tluczek AR, McCullough DG, Poland TM (2011) Influence of host stress on emerald ash borer (Coleoptera: Buprestidae) adult density, development and distribution in Fraxinus pennsylvanica trees. Environ Entomol 40:357–366
Wei X, Wu Y, Reardon R, Sun T-H, Lu M, Sun J-H (2007) Biology and damage traits of emerald ash borer (Agrilus planipennis Fairmaire) in China. Insect Sci 14:367–373
Wright JW (1976) Introduction to forest genetics. Academic Press, New York
Acknowledgements
This work was supported by the USDA National Institute of Food and Agriculture Appropriations under Project PEN04532 and Accession Number 1000326. The authors wish to acknowledge the assistance of Jennifer Berkebile, Mackenzie Kessenich, and Nicole Zembower.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Steiner, K.C., Graboski, L.E., Knight, K.S. et al. Genetic, spatial, and temporal aspects of decline and mortality in a Fraxinus provenance test following invasion by the emerald ash borer. Biol Invasions 21, 3439–3450 (2019). https://doi.org/10.1007/s10530-019-02059-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02059-w