Abstract
Ecosystem invasion by non-native plants depends on plant life history characteristics that influence the species’ invasiveness, as well as environmental factors that determine site invasibility. Small, insular ecosystems are thought be especially vulnerable to invasion but evidence for this pattern has been mixed. Freshwater springs form island-like ecosystems, allowing for a test of this proposal. Here, we investigated the effects of physical environmental factors, human disturbance, and plant life history traits on the occurrence of native and non-native plant species at 55 springs across different biomes in Alberta, Canada. A total of 526 plants were identified, 12.5% of which were non-native. Among these, species richness and abundance were greater at springs within biomes subject to increased land use intensity, especially livestock grazing, as compared to springs in parks and protected areas with limited land use. Subsequently, springs with higher human impact supported greater richness (r2 = 0.13) and abundance (r2 = 0.31) of non-native species, while native species abundance declined with increasing human impact (r2 = 0.14). Common native and non-native plant taxa exhibited life history traits that confer greater tolerance to human disturbance, such as that arising from livestock production that can disperse propagules, including clonal capacity and physical and chemical herbivory defenses. Our results indicated that springs ecosystems with greater human disturbance were more vulnerable to invasion by non-native plants, and this can reduce plant biodiversity and the ecological services provided by these distinctive, insular ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecosystem invasion by non-native plant species, also called alien, exotic or introduced plants, represent a major cause of the observed declines in global biodiversity (Vilà et al. 2011). In many regions, the removal of invasive plants is virtually impossible (Genovesi 2005) and, consequently, limiting the spread of such species is often the most effective means of avoiding the negative impacts of invasion (Simberloff 2013). Identifying key factors that contribute to ecosystem invasion can facilitate the development of management strategies to limit the spread and impact of non-native plants (Inderjit et al. 2017). Several factors need to be considered when investigating ecosystem invasion, including characteristics of the site, or site invasibility, the traits of the non-native plants that increase their ability to spread through a new range, or species invasiveness (Richardson and Pyšek 2006). In part, it is the combination of site invasibility and species invasiveness that allow the new colonists to reproduce and rapidly expand their range (Grubb 1977; Grime 2006; Rosbakh et al. 2018).
Successful establishment of non-native plants in the initial stages of invasion is partially dependent on the abiotic or physical characteristics of the recipient environment (Richardson and Pyšek 2006; Pyšek et al. 2012). Attributes of the geographic location, such as the hydrogeology and surface substrates provide important influences on non-native plant colonization potential (Stevens and Ayers 2002; Pino et al. 2005). However, the contribution of abiotic factors to site invasibility depends on the characteristics of the potential invader, making generalizations difficult. The ecoclimatic niche of a non-native plant can sometimes be used to predict areas within novel regions where the species may successfully establish (Pyšek and Richardson 2010), but some invasive non-native plants can thrive in physical conditions that differ from those of their native range (Broennimann et al. 2014).
In addition to the abiotic environment, the life history traits and ecophysiology of non-native plants also affect their ability to invade ecosystems outside of their native range. Numerous studies have attempted to define a suite of traits that can be used to predict invasiveness of particular plant species (reviewed in Pyšek and Richardson 2007). These studies typically examine the traits of plants that have successfully invaded a region, and then compare those to the traits of plants native to the region to identify similarities and differences, which can then be assessed using co-existing frameworks (MacDougall et al. 2009). Invasive non-native plants often possess generalist life history traits, are capable of growing under a broad range of habitat conditions, and exhibit prolific or protected reproduction (e.g., clonality) (Stevens and Ayers 2002; Simieon and Stevens 2015; Sciance et al. 2016). Consequently, there has been limited consensus on which and to what extent groups of traits explain invasion success. Based on previous work, the most effective approach seemingly involves integrating information of specific biotic traits of non-native species and the introduction history within the environmental context of the invaded ecosystem (reviewed in Simberloff 2013).
Identifying ecosystems at greater risk of invasion by non-native plants should allow for the development of effective management programs to limit invasion. Ecosystems that are limited in area, such as small islands, may be more vulnerable to invasion as compared to larger, less isolated ecosystems (Richardson and Pyšek 2012; but see Vilà et al. 2011). In their review, Pauchard and Shea (2006) point out that ecosystems with high native biodiversity often support rich communities of non-native plants because the factors that promote high biodiversity, such as access to suitable habitat, moisture, and nutrients, also promote invasion. Given this, freshwater springs, which form relatively small, island-like ecosystems, may be at greater risk of non-native plant invasion. Springs often support sensitive, endemic species that may be less resistant to invasion, and frequently are used by humans as water sources (Stevens and Meretsky 2008; Kløve et al. 2014; Kreamer et al. 2015). The subsequent disturbance resulting from human use can alter groundwater availability, modify the surrounding geomorphology, and increase pollution, leading to decreased biodiversity and diminished ecological functionality (Stevens & Meretsky 2008). The risk of invasion by non-native plants generally increases in regions with high human disturbance (Mack and Lonsdale 2001; McKinney 2002; Stevens and Ayers 2002; Pyšek et al. 2010). Anthropogenic disturbances, such as raising livestock can facilitate non-native plant colonization by removing established native species and disturbing germination sites through soil erosion, thereby by lowering the biotic resistance of the site (Keeley et al. 2003), as well as supporting vectors for non-native propagule delivery (Mack and Lonsdale 2001). Due to intensive human usage, particularly for domestic and agricultural water supplies, springs are among the most threatened ecosystems on the globe (Stevens and Meretsky 2008; Kreamer et al. 2015). While much attention has been devoted to non-native invertebrate and fish introductions (e.g., Shepard 1993; Unmack and Minckley 2008), few studies have addressed how human disturbance affects the vulnerability of freshwater spring ecosystems to non-native plant invasions.
This study was undertaken to assess the extent and modes of invasion of freshwater springs ecosystems by non-native plants. The primary objectives were to identify factors that increase the susceptibility of springs to invasion with respect to ecosystem invasibility and the life history attributes of non-native plants within springs communities. Springs in southern Alberta, Canada provided a suitable study system since springs occur abundantly among several biomes and are used in many cases as agricultural water sources, particularly for livestock (Springer et al. 2015). We surveyed a wide array of springs across the southern Alberta landscape to examine species richness and abundance patterns among native and non-native plant species. The patterns of richness and abundance were compared to physical environmental factors and anthropogenic disturbances to assess abiotic factors affecting site invasibility. We predicted that springs with greater human disturbance intensity, parameterized using a semi-quantitative assessment of the type and extent of anthropogenic ecosystem impacts, would support greater non-native plant richness and abundance. We also surveyed the life history traits of commonly-occurring native and non-native plant species to identify traits that confer differential colonization potential and invasion success. We predicted that invasive non-native plant species possessed traits that increased their ability to rapidly colonize disturbed sites, such as those used for livestock grazing.
Methods
Field sites
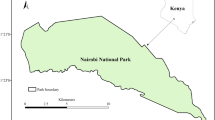
We explored environmental and vegetation conditions at 55 springs across southern Alberta that were inventoried from 2008 to 2012, as described by Springer et al. (2015). That prior report provides information about physical characteristics of the springs, including the geomorphic contexts, and groundwater and surface water chemistry, which reflect site hydrogeology. The springs locations ranged over 3° of latitude (49°1′N to 52°3′N, 440 km) and 6° of longitude (− 109°59′W to − 115°35′W, 432 km), and from 822 to 2048 msl in elevation, from prairie grassland to sub-alpine forest biomes (Fig. 1). Springs were selected using provincial resources, including maps published by the Alberta Geological Survey (Stewart 2009), hydrogeology maps (Borneuf 1983) and regional reports (e.g., Toop and de la Cruz 2002). Park managers, non-governmental organizations, and private landowners who represented regional watershed conservation groups were contacted for information on the locations, conditions, and history of the springs (Springer et al. 2015). Spring names are listed in Supplemental Table 1.
Map of springs study area in Alberta. Locations of springs are symbolized by an X with the numbers corresponding to those in Springer et al. (2015) and Supplemental Table 1. Natural region boundaries are delineated by dashed lines
Inventory protocols
Data related to physical and biological site characteristics were collected at each spring following the Level 2 Springs Ecosystem Inventory Protocol developed by the Springs Stewardship Institute (Stevens et al. 2016). These are intended to establish baseline conditions and have been used to inform monitoring and management of surveyed springs (Springer et al. 2015; Paffett et al. 2018). Many variables were included in the inventory protocol, and we present the methods that specifically related to vegetation data collection. Springer et al. (2015) provide further information on the sampling of other components, such as hydrogeology, water chemistry, and aquatic and terrestrial vertebrate and invertebrate fauna.
Physical environmental characteristics
Sites were classified according to the biome, or natural region, and defined on the basis of biome-level environmental factors, including climate, water, soil and vegetation (Pettapiece 1986; Samuelson and Rood 2004). As characterized by Downing and Pettapiece (2006), these biomes included the higher elevation Rocky Mountains, with the treeless alpine, conifer forested subalpine, and mixed woodland montane ecoregions combined. Dropping in elevation, the foothills biome supported mixed woodlands, such as with lodgepole pine (Pinus contorta) and balsam poplar (Populus balsamifera), and the slightly drier parklands were dominated by trembling aspen (Populus tremuloides). The lowest and driest biome was the prairie region, which is treeless except in riparian zones (Samuelson and Rood 2004; Downing and Pettapiece 2006) (Fig. 1).
Each spring was classified according to its sphere of discharge, which describes the type and mechanism of groundwater emergence, following the criteria outlined in Springer and Stevens (2009). The rate of groundwater discharge (m3/s) was measured where possible. Measurements of elevation (msl), latitude, and longitude were taken at or near the point of groundwater emergence. The area (m2) of each spring ecosystem was determined based on the extent of land affected by emergent groundwater (Stevens et al. 2016).
The area of each distinct geomorphic microhabitat surface in each springs ecosystem was estimated after delineation based on characteristics of the landscape, hydrology, and substrata within the site (Stevens and Meretsky 2008; Stevens et al. 2016). These geomorphic microhabitats were subsequently grouped as wet, intermediate, or dry, according to the extent of groundwater-derived surficial moisture (Fig. 2). This grouping reflected similarities of water permanence and substratum texture, with finer particles drying more slowly and providing capillary rise around and above the emergent groundwater.
Human impacts
The degree of anthropogenic disturbances was characterized at each site using the Freedom from Human Influences section in the Springs Stewardship Institute’s ecosystem assessment protocol (Stevens et al. 2016). A numeric score was assigned based on assessments of eight criteria: surface water quality; extent of flow regulation; evidence of effects from adjacent transportation corridors (e.g., roadways, railway lines); fences; construction; livestock grazing (e.g., animals at or near the spring, surface perforation from hooves, or fecal pads); recreational use (e.g., eroded trails for hiking, horses, or off-highway vehicles); and condition of adjacent lands (e.g., proximity to cultivated fields). Based on the site visit and consultation with the landowner or site manager, ecological condition scores of 0–6 were assigned for each criterion, where 0 indicated extensive impact, poor condition, and irrecoverably low site quality, and 6 indicated minimal impact and high ecological integrity and site quality. Human impact condition scores, which were scored out of 6, were averaged, divided by 6, and then subtracted from 1 to produce a human impact (HI) score for each spring. Thus, a HI score of 0 represented a relatively pristine site condition with minimal human disturbance, and 1 represented a severely degraded site condition with high human disturbance intensity.
Vegetation inventory
Springs were surveyed during the months of July or August to characterize vegetation during the interval of maximal foliar cover. All terrestrial and emergent plants growing within the total spring ecosystem area were identified to genus and species level, where possible. Plants were assigned status as native or non-native, according to the USDA Plants Database (USDA 2017) for the Great Plains or Western Mountains regions. Plants that were unidentified or identified only to the family level were excluded from the analysis due to the uncertainty of assigning nativity status. Voucher plant specimens are housed at the University of Lethbridge Herbarium (LEA), Lethbridge, Alberta.
Plant species richness values (counts of the number of taxa, primarily species) were determined for both native and non-native plants at each site and within each microhabitat type. To account for observations of plants such as several Carex sedges whose taxonomy could not be resolved beyond the genus level, plant taxa were also grouped by genus to more clearly represent the plant community composition at each spring. It is explicitly stated whether analyses were conducted on species- or genus-level groupings of taxa. Aquatic plants were surveyed but were not included in any part of the analyses due to generally poor identification of these species.
Due to differences in the area of springs and contribution of associated geomorphic microhabitats in this study, species richness values were transformed to correct for area sampled, where transformed species richness = (number of plant taxa/log10 of area) (Gotelli and Colwell 2001; Samuelson and Rood 2011; Hasselquist et al. 2015). The percent cover of each species in each microhabitat was visually estimated for six vegetation structure strata: emergent, non-vascular, ground (deciduous herbaceous or graminoid), shrub (woody, 0–4 m), mid-canopy (4–10 m), and tall canopy (> 10 m). Recognizing that a species’ total percent cover across all strata could exceed 100%, we summed cover across strata to produce a cover index value for each native or non-native species detected in each microhabitat type. We then weighted the cover index for each species at each site by taking the sum of the cover index in each microhabitat type and then multiplying this by the proportional area of the spring ecosystem. These calculations also were made on plant groupings by genus.
Analyses across environments: invasibility
For continuous environmental factors, Pearson product-moment correlations were calculated using SPSS v. 21 (IBM, Armonk, NY, USA) to test and assess linearity and autocorrelation among prospective environmental factors and dependent vegetation variables. After identifying significant correlations with individual environmental factors, multiple factors were analyzed using multiple linear regression with forward model selection through Akaike’s Information Criterion (Bozdogan 1987) with the base lm package in R (R Core Team 2016). To conform to the assumptions of these analyses, cover index values were log10-transformed.
For categorical environmental factors, one-way analyses of variance (ANOVA) were conducted, comparing native or non-native plant species richness or log10-transformed percent cover across biomes, spring discharge spheres, and microhabitat surface type moisture levels. Significant differences were identified with Tukey’s HSD post hoc test using the lm package in R (R Core Team 2016). Where assumptions of normality were not met, Kruskal–Wallis non-parametric H tests were conducted, with pairwise significant differences identified using the Kruskal–Wallis multiple comparison test using the pgirmess package in R (Giraudoux et al. 2018).
Analyses of vegetation: invasiveness
A subset of the taxa grouped by genera within the top quartile of observations (observed at ≥ 9 of 55 springs) represented the most commonly observed plants. From these, an equal number of native and non-native plant taxa were assessed for life history trait comparison. The life history traits were selected based on their potential to contribute to establishment success, and included: guild (growth form), longevity, flowering phenology, pollination strategy, fruit or seed type, seed dispersal mechanism, vegetative reproduction ability, and tolerance to disturbance. Disturbance tolerance included the ability to colonize areas of subjected to physical disturbance, such as floodplains, roadsides, slough edges, or waste ground. Due to the interest in disturbance from cattle use, including grazing, trampling, and pugging (soil perforation), traits conferring the ability to tolerate grazing and the presence of structures or compounds to deter herbivory also were documented. Information on these traits and wetland status was compiled for each of the selected common taxa, where available, from the USDA Plants database (USDA 2017) and the Alberta Conservation Information Management System (2018). The proportion of native versus non-native taxa exhibiting each life history trait was calculated, and if a taxon included species that fit more than one category of life history trait (e.g., biennial and perennial), that taxon was counted under each trait.
Analyses of environment and vegetation: invasibility and invasiveness
Non-metric multidimensional scaling (NMDS) ordinations using PC-ORD v.6 (McCune and Mefford 2011) were undertaken to investigate environmental patterns of the presence/absence and abundance of the most common native and non-native plant taxa grouped by genera (Kruskal 1964). Sørensen’s distance was used for calculation in two dimensions with a maximum of 250 iterations, a stability criterion of 0.00001, and a step length of two, with Varimax rotation applied. Unrelated environmental variables, as determined from the Pearson’s product-moment correlation analysis were used in the NMDS ordinations to distinguish which factors were most strongly associated with native versus non-native plant richness and abundance. In cases where multiple species in the same genus were surveyed at the same spring ecosystem, cover index values for the species were summed to produce one cover index value for that genus.
Results
Springs vegetation characteristics
A total of 526 plant taxa were identified across the 55 springs surveyed. Of those, 460 (87.5%) were native to the province and 66 (12.5%) were non-native. Across these springs, numbers of plant species ranged 9–90 native and 0–18 non-native species, with a maximum of 101 species (90 native, 11 non-native) and a minimum of 10 species (9, 1). The top quartile of most commonly observed plants included 74 taxa that were observed at least 9 times across the 55 springs. Of these, 63 were native and 11 were non-native (Supplemental Table 2).
Hydrology and biomes
One half of the sites were classified as hillslope springs (Springer and Stevens 2009), with emergence from a slope of 30°–60° (n = 28 of 55) and not occurring in an established channel. One quarter of the sites (13) were helocrene springs, with emergence from low-gradient wetlands, often with multiple or indistinct sources. Seven were rheocrene springs, with emergence with upslope stream channels, and four were pool-forming limnocrene springs. There were single observations of a gushet spring that emerged from a discrete source on a cliff wall, and a hanging garden with emergence that dripped from a geologic contact along a cliff wall. There was a single cave spring surveyed at the iconic Cave and Basin National Historic Site in Banff National Park. Only springs types with multiple occurrences were included in ANOVAs (Fig. 3). Neither native transformed plant species richness nor cover indices were significantly different across these discharge spheres (richness: F3,48 = 1.43, p = 0.25; cover index: F3,48 = 0.24, p = 0.87). Non-native species richness also did not differ across discharge spheres (F3,48 = 0.77, p = 0.52). For non-native cover index, there appeared to be a lower value for limnocrene springs but, with limited sampling that difference was not statistically significant (F3,48 = 0.67, p = 0.57). Thus, limited by the small sample size, there was little correspondence between the spring discharge sphere, which represents the hydromorphic context of the source water and the outflow zone.
The surface moisture conditions strongly influenced both native and non-native plant distributions (Fig. 4). Kruskal–Wallis tests indicated that native species richness was lower in wet as compared to dry or intermediate microhabitats (H = 11.4, df = 2, p = 0.003). Native plant cover also was lower in wet microhabitats (H = 23.2, df = 2, p < 0.001), but did not differ between dry or intermediate microhabitats. Non-native species richness and cover index displayed similar patterns (richness: H = 21.1, df = 2, p < 0.001; cover: H = 36.3, df = 2, p < 0.001), and were significantly reduced in the wet microhabitat compared to dry or intermediate microhabitats. Thus, similar distribution patterns existed among native and non-native plant taxa, with fewer species and reduced cover in the zones with wet, saturated substrate.
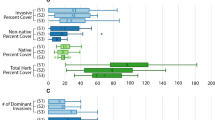
Transformed species richness (spp/log[m2]; mean ± SE) and cover index of native and non-native plants at freshwater springs across microhabitat surface moisture levels in Alberta, Canada. Letters indicate statistical significance between regions (uppercase = richness, lowercase = cover index). Note the difference in y-axis scales
Comparison of springs among the different biomes, or biophysical natural regions, revealed consistency among native species richness and cover index (richness F3,51 = 0.22, p = 0.88; cover index F3,51 = 1.31, p = 0.28; Fig. 5). In contrast, non-native vegetation characteristics varied, displaying a unimodal, inverted-U response, with higher values in the biomes situated between the prairies and Rocky Mountains, with increased non-native richness in the parklands region as compared to the Rocky Mountains, and with the spatially intermediate foothills intermediate in richness (F3,51 = 6.96, p < 0.001; Fig. 5). The non-native cover index indicated a similar pattern (F3,51 = 16.1, p < 0.001), with the significant differences occurring among the parklands, the Rocky Mountains, and the foothills regions.
Transformed species richness (spp/log[m2]; mean ± SE) and cover index of native and non-native plants at freshwater springs across biomes in Alberta, Canada. Letters indicate statistical significance between biomes (uppercase = richness, lowercase = cover index). Note the difference in y-axis scales
Environmental factors
Pearson correlation analyses demonstrated autocorrelation among several environmental factors and vegetation variables (Table 1). Site elevation was positively related to springs discharge (Q, flow rate), and negatively related to the human impact (HI) score. Human impact was, in turn, positively related to latitude. This reflected the general decline in elevation moving eastward away from the Rocky Mountains, where springs at higher elevations and in that wetter montane biome had increased groundwater discharge.
Vegetation characteristics were related to several environmental factors, but not discharge (Table 1). Native species richness was related to elevation, with higher-elevation springs displaying increased native plant richness, accounting for approximately 10% of the observed variation (Fig. 6; note the plotting from high to low elevation, which generally corresponded to the west-to-east longitudinal pattern from montane to prairie biomes). However, we found no association between native species cover index values and elevation (Table 1; Fig. 6). In contrast, non-native plant richness was highest at intermediate elevations (1100–1700 msl) and a quadratic function provided a better fit than a linear function (Fig. 6; r2 = 0.17 vs. 0.10). The non-native plant cover index was negatively correlated longitude and slightly less strongly with elevation (Table 1; Fig. 7), as opposed to the absence of those patterns among native species (Fig. 6). The relationship between non-native cover index and longitude was amplified by anomalously high values at two eastern (prairie biome) springs, which were situated in the heavily grazed and elevated Cypress Hills (Figs. 1 and 7), which interrupt the pattern of progressive decline in elevation from the Rocky Mountains in the west to the prairies in the east.
Native species richness was apparently weakly negatively related to HI score (r2 = 0.05), suggesting that slightly fewer native plant species occurred around heavily disturbed springs (Fig. 8). Confirming this influence on native vegetation, the native cover index was more strongly negatively correlated with human impacts (Fig. 8). Non-native richness and cover index both demonstrated positive correlations with HI score (Fig. 8). Thus, we found that cumulative anthropogenic impacts negatively affected native plant species, but increased the diversity and cover of non-native species.
Native plant cover was positively correlated with native richness (Table 1). But while neither non-native richness nor cover were correlated with native richness, both variables were negatively correlated with native vegetation cover (r2 = 0.17 and 0.13, respectively). Within the full matrix of environmental factors and vegetation variables, the strongest correlation was between non-native richness and cover, which were positively correlated with one-third correspondence (r2 = 0.33; Table 1).
For combinations of environmental factors, the best fit multiple linear regression models were: non-native species richness with elevation and HI score (adjusted r2 = 0.13); native species richness with elevation and latitude (adjusted r2 = 0.12); non-native cover index with longitude and HI score (adjusted r2 = 0.31); and native cover index with HI score (adjusted r2 = 0.14; Table 2).
Plant occurrences
Carex sedges were the most commonly detected native taxa, with 101 observations of as many as 32 species (Table 3). The second most common taxa were Juncus rushes, which were observed 67 times, with 11 species identified. The most common non-native taxa were Cirsium thistles, with 40 occurrences and at least two species identified. The numbers of species within the non-native genera were lower compared to the native genera, with three being the greatest number of species identified among the Trifolium clovers and the Rumex sorrels (or docks). Among both native and non-native taxa, most plants detected were classified as facultative relative to their wetland indicator status (USDA 2017). Three facultative wetland genera were among the most commonly observed taxa and all of which were native, including Carex, Juncus, and Salix (willows). Non-native plants fell within facultative, facultative upland, or upland categories of the wetland indicator status.
Life history traits
With respect to life history traits, the most commonly observed taxa were either forbs or graminoids, and most exhibited perennial growth (Table 4). Most flower during spring, with similar proportions among natives and non-native species, and dominant pollination strategies included zoophily (animals) and anemophily (wind). Two non-native taxa, Taraxacum (dandelions) and Medicago (burclovers) reproduced through autogamous fertilization, and the majority of taxa produced achenes. Zoochory (animal-assisted seed dispersal) was the most common with two-thirds of taxa employing this mechanism, split almost evenly among natives and non-native species. More than half of the taxa were capable of vegetative reproduction through rhizomous or stoloniferous growth, or by clonal suckering.
Each of the 22 most commonly detected taxa exhibited at least one trait conferring disturbance tolerance, including resistance to drought, inundation, herbivory, association with primary successional habitats, or vigorous growth in disturbed soils. Because livestock, and primarily cattle grazing is a common form of disturbance in southern Alberta (Alberta Environment and Parks 2014) and has been associated with the increased occurrence of non-native plants, we specifically documented herbivory tolerance and defences among plants to assess traits associated with disturbance from grazing. Slightly less than half of the most common taxa were native species that exhibited tolerance to grazing, and one quarter of the common taxa displayed structural or phytochemical defences against herbivory. These included structural defences of spines and silica, or phytochemicals including thiaminase, coumarins, and saponins (Moore 1975; Turkington et al. 1978; Cody and Wagner 1980; Small 1996).
Multivariate ordination
In both non-metric multidimensional scaling (NMDS) ordinations, elevation and spring flow provided the strongest associations with the distribution of the most common taxa (Fig. 9). Among presence/absence and cover index ordinations, HI score and longitude were associated with axis 1, and latitude, discharge and elevation were associated with axis 2. In both ordinations, non-native taxa were shifted with the HI score vector. Also in both ordinations, non-native taxa were somewhat clustered while the native taxa appeared more dispersed, reflecting the larger species pool and increased diversity.
Non-metric multidimensional scaling of the richness (top) and cover index (bottom) of the most commonly observed plant genera native (white circle, n = 63) and non-native (filled circle, n = 11) at 55 springs in Alberta, Canada. Large circles represent mean (± SE) position of native and non-native taxa in ordination space. Arrows represent vectors for environmental factors (Elv = elevation, Long = longitude, Lat = latitude, HI = human impact score, Q = spring discharge). Numbers and letters correspond to native and non-native taxa in Table 3, respectively
Discussion
This study investigated the extent of invasion of Alberta freshwater springs ecosystems by non-native plant species, with two objectives: (1) to identify physical factors that increase ecosystem invasibility, or vulnerability to invasion; and (2) to identify life history traits that increased invasiveness, or invasion success of non-native plants. Our results reveal pervasive influences of human disturbances in the distribution of non-native plants at springs with respect to both site invasibility and species invasiveness. Plant invasions often are largely facilitated by human actions and disturbances (reviewed in Mack et al. 2000), and this was reflected in our results as non-native plant richness and success (as cover index) were positively related to the HI score (Fig. 8). This pattern has been recognized in a range of other ecosystems (Catford et al. 2009; Pyšek et al. 2010; Inderjit et al. 2017).
The parkland biome, the zone between the flatter, drier prairies where crop production predominates and the foothills with greater topographic relief, is used extensively for livestock production, and this activity directly and indirectly increases disturbance on the landscape (Alberta Environment and Parks 2014). Cattle physically disturb springs ecosystems through trampling and pugging, reducing the abundance of mature native plant species and increasing available ground for colonization opportunities for native and non-native propagules (Kimball and Schiffman 2003). Additionally, livestock readily disperse propagules, especially seeds, where they graze (Chuong et al. 2016). Our analysis of the life history traits of common non-native plants revealed that zoochory was a common method of seed dispersal, increasing the ability of those taxa to invade rangeland springs (Table 4). While this often increases establishment potential, the efficacy of this trait can be environment-dependent (Pyšek and Richardson 2007). In contrast to springs ecosystems, some native and non-native species along riparian corridors rely upon hydrochory as a method dispersal whereby seeds or propagules are transported by flowing water (Nilsson et al. 2010; Rood et al. 2010). Because springs represent groundwater emergence with no upstream and often no downstream connectivity, hydrochory would not represent a viable mechanism of plant dispersal for springs colonizers.
In southern Alberta, abundant livestock and native animals make zoochory an efficient mode of seed dispersal for non-native plant species. Following dispersal and initial establishment, cattle and native ungulates avoid grazing on unpalatable non-native taxa, an influence that, combined with the reduction in cover and loss of native species, can facilitate rapid invasion into grazed areas by non-native plant species (DiTomaso 2000). Our life history trait analysis supports this interaction, since the majority of common plants with herbivory defences were non-native (Table 4). Similarly, at springs in the American Mojave Desert, Fleishman et al. (2006) reported that grazing intensity was positively related to non-native plant occurrence. In their study, non-native species richness and cover were greatest at moderate to high levels of grazing intensity, which is consistent with our findings. Herbivory tolerance also was a characteristic of the most common native plant species in our study (Table 4), likely as an evolutionary consequence of selective grazing pressure by native vertebrate and invertebrate herbivores (Stevens and Meretsky 2008; Springer et al. 2015). Native springs plants sustain selective pressures for increased disturbance tolerance from native wildlife, such as deer, elk (Cervus canadensis), and plains bison (Bison bison ssp. bison), which can heavily graze and trample springs habitats. With respect to grazing intensity, pressures from contemporary native herbivores typically occur at much lower levels than those resulting from non-native grazing vertebrates, particularly cattle. This supports the observed pattern of increased non-native plant invasion at springs subjected to heavy livestock grazing. Prior to 1800, plains bison would have been abundant throughout southern Alberta, particularly in the parkland ecoregion. Their grazing patterns would have been as or even more severe than cattle but more temporary because bison herds regularly moved to avoid predators and to seek fresh forage (Morgan 1980).
The largest urban centres in Alberta are located in the parkland biome (e.g., Calgary; Fig. 1), which are major sources of non-native propagules. Thus, springs in closer proximity to these urban centres are more likely to sustain increased propagule occurrence and the establishment of non-native plant populations, especially in the early stages of regional invasion (Alston and Richardson 2006; Catford et al. 2009).
In contrast to the parklands, the high-elevation Rocky Mountains biome is more sparsely populated and less developed, and includes several large national and provincial parks. We found reduced richness and abundance of non-native plants around springs in the Rocky Mountain region (Fig. 5). Parks and protected areas are managed specifically to reduce human impacts to conserve biodiversity, as well as to limit invasion of non-native flora and fauna (Downing and Pettapiece 2006; Alberta Environment and Parks 2014). These management efforts and ecological processes often result in lower rates of non-native species invasions due to reduced anthropogenic disturbances (Foxcroft et al. 2011). Preservation or simulation of natural disturbance regimes (e.g., controlled burning) can reduce non-native plant invasion success in park ecosystems because native species are generally adapted to such disturbances (Alpert et al. 2000; Havill et al. 2015).
Surprisingly, our results indicated that the prairie biome, the eastern-most and lowest elevation region in the province, displayed reduced richness and abundance of non-native plant species as compared to the parkland biome, and approached the scarcity of non-native species detected in the Rocky Mountains biome (Fig. 5). The prairie biome supports extensive agricultural crop production and livestock grazing, along with many important transportation corridors, along which non-native plants often disperse (McKinney 2008), and thus it was expected that this region would support the greatest richness and abundance of non-native species. However, we only sampled three prairie springs, all of which were relatively ecologically intact, limiting our confidence in this conclusion. Further sampling of springs in this region is warranted to explore this result.
In slight contrast to Springer et al. (2015), we did not find a significant positive correlation between native and non-native richness (Table 1). We used a subset of the plants included by Springer et al. (2015), and excluded aquatic plants and taxa that could not be reliably assigned as native or non-native, or those that were not resolved to the genus or species level. The pattern reported in Springer et al. (2015) has been observed where non-native plants have readily invaded hotspots of native plant richness (Stohlgren et al. 1999, 2002), but this pattern is apparently scale-dependent (Fridley et al. 2007). Results from small-scale experiments and islands may support a negative correlation between native and non-native richness (Stohlgren et al. 1999, 2003), although Pauchard and Shea (2006) suggest that spatially-limited ecosystems with high native biodiversity can support rich communities of non-native plants. They propose that the particular characteristics of the non-native species is of greater importance to invasion risk than the richness of non-native species (Pauchard and Shea 2006). Our results display a trend toward positive correlation between native and non-native species richness, likely because these spring ecosystems contained multiple microhabitats, which offer greater habitat diversity and thus greater biodiversity of both native and non-native plants.
As Springer et al. (2015) reported, we identified one quarter of all Alberta plant species at a small number of freshwater springs with a total study area of 3.8 ha, which comprises less than 0.01% of the provincial land area. This finding underscores the remarkable plant species packing and high level of biodiversity of freshwater springs ecosystems. For hydrophytic and wetland specialist plants around springs that face increased stress under climate change, disturbance and habitat loss due to human impacts including groundwater pumping or springs diversion, could result in the loss or extirpation of a potentially large number of native species. Given that springs in arid and mesic regions alike function as refugia for wetland and riparian species, increased human disturbance of springs ecosystems may greatly reduce or threaten regional plant species richness and diversity, particularly of rare wetland taxa (Kløve et al. 2011). In addition, springs vegetation provides food and cover for a host of springs-dependent invertebrate and some vertebrate species, as well as upland taxa, thereby serving as a conservation umbrella for entire springs biotic assemblages (Hendrickson and Minckley 1984; Shepard 1993). The decline in native plant abundance at sites with greater human disturbance is a common theme in prior studies (reviewed in Mack et al. 2000). This trend is of concern for springs in southern Alberta, since reliance on groundwater is anticipated to increase over the coming decades, with greater drought frequency predicted for the region under some climate projections (Forbes et al. 2011; Alberta Environment and Parks 2014). Increased development of groundwater resources would result in greater disruption and habitat loss of springs ecosystems (Kløve et al. 2014). With the combination of native plant extirpation and increased ecological pressure from non-native species, springs ecosystems are likely to be particularly vulnerable to increased invasion with climate change.
In summary, our study demonstrated that both the environmental conditions affecting site invasibility and life history traits influencing species invasiveness affect the vulnerability of springs ecosystems to non-native plant invasion. To best manage springs for long-term biodiversity and sustainable ecosystem services in southern Alberta and elsewhere, conservation efforts should focus on limiting human disturbance, and particularly limiting livestock impacts. Disturbance from livestock can readily be mitigated through actions that limit access to springs sources, such as exclusion fencing. To provide drinking water for cattle, water from the springs can easily, and often passively, be piped to troughs situated away from ecologically sensitive springs sources. However, the integrity of piping comes at the cost of long-term monitoring and maintenance. Our findings highlight springs as ecosystems that sustain high levels of human disturbance, and support high concentrations of plant taxa that are adapted to those environments. We recommend that those interested in improved understanding and stewardship of springs pay special attention to non-native taxa, particularly species with vigorous reproduction and with high tolerance for disturbance. Such non-native plant species represent the greatest potential long-term threats to the ecological sustainability and functionality of these distinctive but highly threatened ecosystems, which deserve increased recognition and protection in Alberta and worldwide.
References
Alberta Conservation Information Management System (2018) List of all vascular plant elements recorded for Alberta in the ACIMS database—March 2018 https://albertaparks.ca/media/6493459/list-of-elements-ab-vascular-plants.xlsx
Alberta Environment and Parks (2014) South Saskatchewan Region Plan 2014–2024: an Alberta land-use framework integrated plan. Government of Alberta, Edmonton, Canada
Alpert P, Bone E, Holzapfel C (2000) Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol 3:52–66. https://doi.org/10.1078/1433-8319-00004
Alston KP, Richardson DM (2006) The roles of habitat features, disturbance, and distance from putative source populations in structuring alien plant invasions at the urban/wildland interface on the Cape Peninsula, South Africa. Biol Conserv 132:183–198. https://doi.org/10.1016/j.biocon.2006.03.023
Borneuf D (1983) Springs of Alberta 82-3. Alberta Research Council Earth Sciences Report, Edmonton, Canada
Bozdogan H (1987) Model selection and Akaike’s Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika 52:345–370. https://doi.org/10.1007/BF02294361
Broennimann O, Mráz P, Petitpierre B, Guisan A, Müller-Schärer H (2014) Contrasting spatio-temporal climatic niche dynamics during the eastern and western invasions of spotted knapweed in North America. J Biogeogr 41:1126–1136. https://doi.org/10.1111/jbi.12274
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40. https://doi.org/10.1111/j.1472-4642.2008.00521.x
Chuong J, Huxley J, Spotswood EN, Nichols L, Mariotte P, Suding KN (2016) Cattle as dispersal vectors of invasive and introduced plants in a California annual grassland. Rangel Ecol Manag 69:52–58. https://doi.org/10.1016/j.rama.2015.10.009
Cody WJ, Wagner V (1980) The biology of Canadian weeds. 49. Equisetum arvense L. Can J Plant Sci 61:123–133. https://doi.org/10.4141/cjps81-015
Core Team R (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
DiTomaso JM (2000) Invasive weeds in rangelands: species, impacts, and management. Weed Sci 48:255–265. https://doi.org/10.1614/0043-1745(2000)048%5b0255:IWIRSI%5d2.0.CO;2
Downing DJ, Pettapiece W (2006) Natural regions and subregions of Alberta. Government of Alberta, Edmonton, Canada
Fleishman E, Murphy DD, Sada DW (2006) Effects of environmental heterogeneity and disturbance on the native and non-native flora of desert springs. Biol Invasions 8:1091–1101. https://doi.org/10.1007/s10530-005-7564-9
Forbes KA, Kienzle SW, Coburn CA, Byrne JM, Rasmussen J (2011) Simulating the hydrological response to predicted climate change on a watershed in southern Alberta, Canada. Clim Change 105:555–576. https://doi.org/10.1007/s10584-010-9890-x
Foxcroft LC, Jarošík V, Pyšek P, Richardson DM, Rouget M (2011) Protected-area boundaries as filters of plant invasions. Conserv Biol 25:400–405. https://doi.org/10.1111/j.1523-1739.2010.01617.x
Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, Stohlgren TJ, Tilman D, Von Holle B (2007) The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17. https://doi.org/10.1890/0012-9658(2007)88%5b3:TIPRPA%5d2.0.CO;2
Genovesi P (2005) Eradications of invasive alien species in Europe: a review. Biol Invasions 7:127–133. https://doi.org/10.1007/s10530-004-9642-9
Giraudoux P, Antonietti J, Beale C, Pleydell D, Tregalia M (2018) pgirmess: spatial analysis and data mining for field ecologists. https://CRAN.R-project.org/package=pgirmess
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Grime JP (2006) Plant strategies, vegetation processes, and ecosystem properties. Wiley, Chinchester
Grubb PJ (1977) The maintenance of species richness in plant communities: the importance of the regeneration niche. Biol Rev 52:107–145. https://doi.org/10.1111/j.1469-185X.1977.tbo1347.x
Hasselquist EH, Nilsson C, Hjalten J, Jorgensen D, Lind L, Polvi LE (2015) Time for recover of riparian plants in restored northern Swedish streams: a chronosequence study. Ecol Appl 25:1373–1389
Havill S, Schwinning S, Lyons K (2015) Fire effects on invasive and native warm-season grass species in North American grassland at a time of extreme drought. Appl Veg Sci 18:637–649. https://doi.org/10.1111/avsc.12171
Hendrickson DA, Minckley WL (1984) Ciénegas: vanishing climax communities of the American Southwest. Desert Plants 6:131–174
Inderjit Catford JA, Kalisz S, Simberloff D, Wardle DA (2017) A framework for understanding human-driven vegetation change. Oikos 126:1687–1698. https://doi.org/10.1111/oik.04587
Keeley JE, Lubin D, Fotheringham CJ (2003) Fire and grazing impacts on plant diversity and alien plant invasions in the southern Sierra Nevada. Ecol Appl 13:1355–1374. https://doi.org/10.1890/02-5002
Kimball S, Schiffman PM (2003) Differing effects of cattle grazing on native and alien plants. Conserv Biol 17:1681–1693. https://doi.org/10.1111/j.1523-1739.2003.00205.x
Kløve B, Ala-aho P, Bertrand G et al (2011) Groundwater dependent ecosystems. Part I: hydroecological status and trends. Environ Sci Policy 14:770–781. https://doi.org/10.1016/j.envsci.2011.04.002
Kløve B, Ala-Aho P, Bertrand G et al (2014) Climate change impacts on groundwater and dependent ecosystems. J Hydrol 518:250–266. https://doi.org/10.1016/j.jhydrol.2013.06.037
Kreamer DK, Stevens LE, Ledbetter JD (2015) Groundwater dependent ecosystems—science, challenges, and policy. In: Adelana SM (ed) Groundwater. Nova Science Publishers, Hauppauge, pp 205–230
Kruskal JB (1964) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29:115–129. https://doi.org/10.1007/bf02289694
MacDougall AS, Gilbert B, Levine JM (2009) Plant invasions and niche. J Ecol 97:604–615. https://doi.org/10.1111/j.1365-2745.2009.01514.x
Mack RN, Lonsdale WM (2001) Humans as global plant dispersers: getting more than we bargained for. Bioscience 51:95–102. https://doi.org/10.1641/0006-3568(2001)051%5b0095:HAGPDG%5d2.0.CO;2
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.2307/2641039
McCune B, Mefford M (2011) PC-ORD: multivariate analysis of ecological data, version 6. MjM Software, Gleneden Beach, Lincoln, USA
McKinney ML (2002) Influence of settlement time, human population, park shape and age, visitation and roads on the number of alien plant species in protected areas in the USA. Divers Distrib 8:311–318. https://doi.org/10.1046/j.1472-4642.2002.00153.x
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176. https://doi.org/10.1007/s11252-007-0045-4
Moore RJ (1975) The biology of Canadian weeds.: 13. Cirsium arvense (L.) Scop. Can J Plant Sci 55:1033–1048. https://doi.org/10.4141/cjps75-163
Morgan RG (1980) Bison movement patterns on the Canadian plains: an ecological analysis. Plains Anthropol 25:144–160. https://doi.org/10.1080/2052546.1980.11908993
Nilsson C, Brown RL, Jansson R, Merritt DM (2010) The role of hydrochory in structuring riparian and wetland vegetation. Biol Rev 85:837–858
Paffett K, Stevens LE, Springer AE (2018) Ecological assessment and rehabilitation prioritization for improving springs ecosystem stewardship. In: Dorney J et al (eds) Wetland and stream rapid assessments: development, validation, and application. Elsevier, Cambridge, pp 475–487
Pauchard A, Shea K (2006) Integrating the study of non-native plant invasions across spatial scales. Biol Invasions 8:399–413. https://doi.org/10.1007/s10530-005-6419-8
Pettapiece WW (1986) Physiographic subdivisions of Alberta. Research Branch, Agriculture Canada, Ottawa, Canada
Pino J, Font X, Carbo J, Jové M, Pallares L (2005) Large-scale correlates of alien plant invasion in Catalonia (NE of Spain). Biol Conserv 122:339–350. https://doi.org/10.1016/j.biocon.2004.08.006
Pyšek P, Richardson DM (2007) Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W (ed) Biological invasions. Ecological studies (analysis and synthesis), vol 193. Springer, Berlin, pp 97–125
Pyšek P, Richardson DM (2010) Invasive species, environmental change and management, and health. Annu Rev Environ Resour 35:25–55. https://doi.org/10.1146/annurev-environ-033009-095548
Pyšek P, Jarošík V, Hulme PE et al (2010) Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc Natl Acad Sci USA 107:12157–12162. https://doi.org/10.1073/pnas.1002314107
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Change Biol 18:1725–1737. https://doi.org/10.1111/j.1365-2486.2011.02636.x
Richardson DM, Pyšek P (2006) Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog Phys Geogr 30:409–431. https://doi.org/10.1191/0309133306pp490pr
Richardson DM, Pyšek P (2012) Naturalization of introduced plants: ecological drivers of biogeographical patterns. New Phytol 196:383–396. https://doi.org/10.1111/j.1469-8137.2012.04292.x
Rood SB, Braatne JH, Goater LA (2010) Favorable fragmentation: river reservoirs can impede downstream expansion of riparian weeds. Ecol Appl 20:1664–1677. https://doi.org/10.1890/09-0063.1
Rosbakh S, Paccini E, Nepi M, Poschlod P (2018) An unexplored side of regeneration niche: seed quantity and quality are determined by the effect of temperature on pollen performance. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01036
Samuelson GM, Rood SB (2004) Differing influences of natural and artificial disturbances on riparian cottonwoods from prairie to mountain ecoregions in Alberta, Canada. J Biogeogr 31:435–450. https://doi.org/10.1111/j.0305-0270.2003.01052.x
Samuelson GM, Rood SB (2011) Elevated sensitivity: riparian vegetation in upper mountain zones is especially vulnerable to livestock grazing. Appl Veg Sci 14:596–606. https://doi.org/10.1111/j.1654-109X.2011.01137.x
Sciance MB, Patrick CJ, Weller DE, Williams MN, McCormick MK, Hazelton ELG (2016) Local and regional disturbances associated with the invasion of Chesapeake Bay marshes by the common reed Phragmites australis. Biol Invasions 18:2661–2677. https://doi.org/10.1007/s10530-016-1136-z
Shepard WD (1993) Desert springs—both rare and endangered. Aquat Conserv 3:351–359. https://doi.org/10.1002/aqc.3270030409
Simberloff D (2013) Invasive species: what everyone needs to know. Oxford University Press, New York
Simieon G, Stevens LE (2015) Tamarix (Tamaricaceae), Opsius stactogalus (Cicidellidae), and litter fungi interactions limit riparian plant establishment. Adv Entomol 3:65–81. https://doi.org/10.4236/ae.2015.32008
Small E (1996) Adaptations to herbivory in alfalfa (Medicago sativa). Can J Bot 74:807–822. https://doi.org/10.1139/b96-102
Springer AE, Stevens LE (2009) Spheres of discharge of springs. Hydrogeol J 17:83–93. https://doi.org/10.1007/s10040-008-0341-y
Springer AE, Stevens LE, Ledbetter JD, Schaller EM, Gill KM, Rood SB (2015) Ecohydrology and stewardship of Alberta springs ecosystems. Ecohydrology 8:896–910. https://doi.org/10.1002/eco.1596
Stevens LE, Ayers TJ (2002) The biodiversity and distribution of alien vascular plant and animals in the Grand Canyon region. In: Tellman B (ed) Invasive exotic species in the Sonoran Region. University of Arizona Press, Tucson, pp 241–265
Stevens LE, Meretsky VJ (2008) Aridland springs in North America: ecology and conservation. University of Arizona Press, Tucson
Stevens LE, Springer AE, Ledbetter JD (2016) Springs ecosystem inventory protocols. Springs Stewardship Institute, Museum of Northern Arizona, Flagstaff, Arizona. http://docs.springstewardship.org/PDF/ProtocolsBook.pdf. Accessed 25 Nov 2018
Stewart S (2009) Locations of Alberta springs. GIS Shapefile. http://www.ags.gov.ab.ca/publications/DIG/ZIP/DIG_2009_0002.zip. Accessed 31 July 2013
Stohlgren TJ, Binkley D, Chong GW, Kalkhan MA, Schell LD, Bull KA, Otsuki Y, Newman G, Bashkin M, Son Y (1999) Exotic plant species invade hot spots of native plant diversity. Ecol Monogr 69:25–46. https://doi.org/10.1890/0012-9615(1999)069%5b0025:EPSIHS%5d2.0.CO;2
Stohlgren TJ, Chong GW, Schell LD, Rimar KA, Otsuki Y, Lee M, Kalkhan MA, Villa CA (2002) Assessing vulnerability to invasion by nonnative plant species at multiple spatial scales. Environ Manag 29:566–577. https://doi.org/10.1007/s00267-001-0006-2
Stohlgren TJ, Barnett DT, Kartesz JT (2003) The rich get richer: patterns of plant invasions in the United States. Front Ecol Environ 1:11–14. https://doi.org/10.1890/1540-9295(2003)001%5b0011:TRGRPO%5d2.0.CO;2
The PLANTS Database (2017) National plant data team. http://plants.usda.gov. Accessed 18 Sept 2017
Toop DC, de la Cruz N (2002) Hydrogeology of the Canmore Corridor and Northwestern Kananaskis Country, Alberta. Alberta Environment Hydrogeology Section, Edmonton, Canada
Turkington RA, Cavers PB, Rempel E (1978) The biology of Canadian weeds. 29. Melilotus alba Desr. and M. officinalis (L.) Lam. Can J Plant Sci 58:523–537. https://doi.org/10.4141/cjps78-078
Unmack PJ, Minckley WL (2008) The demise of desert springs. Aridland springs of North America: ecology and conservation. University of Arizona Press, Tucson, pp 11–34
Vilà M, Espinar JL, Hejda M et al (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708. https://doi.org/10.1111/j.1461-0248.2011.01628.x
Acknowledgements
This study was supported by funding from the Imperial Oil Foundation, Alberta Environment and Parks, Alberta Innovates, and the Natural Sciences and Engineering Council of Canada. We extend sincere thanks to Alberta Parks, Parks Canada, and the Nature Conservancy of Canada for access, and insights and historical accounts about their springs. The authors gratefully acknowledge field assistance from Joanne Golden, Samuel Woodman, and others.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nielson, K.G., Gill, K.M., Springer, A.E. et al. Springs ecosystems: vulnerable ecological islands where environmental conditions, life history traits, and human disturbance facilitate non-native plant invasions. Biol Invasions 21, 2963–2981 (2019). https://doi.org/10.1007/s10530-019-02025-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02025-6