Abstract
The species–area relationship (SAR) is one of the most general patterns in ecology. Recently, SARs have been employed as tools for comparing the ecology and biogeography of native and exotic species across spatial scales and exploring the influence of invasive species on native biodiversity. Here, we assess published studies to determine if SARs differ between native and exotic assemblages. We conducted a literature search to find studies that estimated the exponent (z) of the power-law SAR for native and exotic species across the same set of locales. We also compiled intercepts (c) of SARs where available. We used linear mixed models to test if z and c differed between native and exotic SARs and if this relationship differed across taxa. Our literature search produced 36 native-exotic pairs from 23 studies with which to compare the exponent of the power-law SAR. Further, SAR intercepts were available for 21 native-exotic pairs. Overall, exotic SAR exponents (z) did not differ from those of natives. However, this pattern did not hold across all taxonomic groups. Plant assemblages, which are best represented in our data (61% of total), mirrored the overall pattern showing no differences in exponents between native and exotic SARs. On the other hand, SAR exponents were greater for both native bird and animal assemblages. The intercepts (c) of native SARs were significantly greater than those of exotics for all taxa combined and for each individual taxonomic grouping. Our results suggest processes driving the increase in species richness with area are similar for native and exotic plant species, but not for animals. Expanding studies that compare SARs of native and exotic species to more taxonomic groups and different types of SARs (e.g., nested, contiguous, non-contiguous) will facilitate a better understanding of how native and exotic species richness scale with area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The species–area relationship (SAR) is one of the most general patterns in ecology (Rosenzweig 1995; Lomolino 2000). The change in species richness with area is commonly modeled by a power law function (Arrhenius 1921), S = cAz, and is log–log transformed for statistical utility, log(S) = log(c) + z × log(A) (Connor and McCoy 1979; Dengler 2009). The exponent of the power-law SAR, z, models the rate at which species richness increases with area in log–log space (Connor and McCoy 1979). The exponent of the power-law SAR has been studied to elucidate general rules for how species richness scales with area (Preston 1962; Connor and McCoy 1979; Lomolino 2000) and to compare and contrast SARs across taxonomic groups (e.g., Ricklefs and Lovette 1999), island and mainland systems, and locales differing in minimum area, maximum area, and area range (Rosenzweig 1995; Lomolino 2000; Drakare et al. 2006). The intercept of the power-law SAR, c, interpreted as species richness per unit area, has received considerably less attention (Gould 1979; Triantis et al. 2012).
More recently, SARs have been employed as tools for comparing the ecology and biogeography of native and exotic species across spatial scales (Sax and Gaines 2006). Whether or not exotic species are fundamentally different from natives is a central question in ecology (Daehler 2003; Ordonez et al. 2010; Lemoine et al. 2016). SARs have the potential to elucidate differences between native and exotic species because they are influenced by both environmental and species characteristics and integrate several ecological processes such as habitat filtering, dispersal limitation, and species interactions (Rosenzweig 1995; He and Legendre 2002; Rosindell and Cornell 2009). Thus, comparing the z values of SARs can provide insights into how native and exotic species richness covary in response to biotic and abiotic conditions (Sax and Gaines 2006; Stark et al. 2006), which in turn can provide insights about whether they are fundamentally different or not.
Purported differences between native and exotic species (Daehler 2003; Ordonez et al. 2010; Lemoine et al. 2016) and observed patterns of native and exotic species diversity (Stohlgren et al. 1999; Fridley et al. 2007; Dyer et al. 2016; Carpio et al. 2017) can inform predictions of how z values of SARs should differ between native and exotic assemblages. For example, one of the traits that is most associated with variation in z values is dispersal ability (Rosenzweig 1995). Taxa or species groups that have high dispersal ability are predicted to have lower z values. Dispersal ability is thought to be an important characteristic of exotic species (O’Connor et al. 1986; Sakai et al. 2001; Daehler 2003; but see Flores-Moreno et al. 2013). If exotics are better dispersers than native species, then we expect z values from exotic SARs to be lower than those of natives. On the other hand, the fact that native species have had a longer time period than exotics to disperse across the landscape (e.g., archipelago, country, continent) is a potential mechanism by which native SARs can have lower z value than exotic SARs.
Another trait that has been ascribed to exotic and invasive species is adaptability (Daehler 2003; Blackburn et al. 2009). Phenotypic plasticity (Daehler 2003; Funk 2008; Davidson et al. 2011) and traits related to coping with novel environments (e.g., greater diet breadth, habitat breadth, Blackburn et al. 2009) are posited to facilitate the establishment and spread of exotic species. If exotics are more adaptable than natives, then they are likely to be found in more habitat types across their introduced range. As a result of this broader habitat niche, when sampling at larger scales, you are less likely to sample a “new” exotic species than a “new” native species. In terms of the SAR, this mechanism equates to larger z values for native assemblages.
While traits such as dispersal ability or those related to adaptability can drive differences in SARs between native and exotic species, broad-scale native-exotic richness relationships (NERRs) can also inform our predictions for SARs. At large spatial scales, there is strong evidence that NERRs are positive, with exotic species richness increasing with native species richness (Stohlgren et al. 1999, 2003; Herben et al. 2004; Fridley et al. 2007; Dyer et al. 2016; Carpio et al. 2017). This suggests that native and exotic species richness are responding to the same factors at larger spatial scales. Positive NERRs provide a potential mechanism for the null expectation that there is no difference between z values for native and exotic SARs or more generally put, there is no difference between native and exotic species.
Stochastic processes also play a key role in the invasion process (Blackburn et al. 2015). For example, propagule pressure can overwhelm deterministic processes (Holle and Simberloff 2005; Lockwood et al. 2005) resulting in idiosyncratic patterns of exotic establishment at the local scale due to the location of introductions, number of introduction events, and the number of individuals introduced. As a result, local sites may have different exotic species while sharing native species that have had more time to disperse and establish across the landscape. Consequently, as area increases, exotic species richness can increase at a greater rate than native species richness (i.e., have larger SAR z values) because more locally established exotic species (i.e., species with small distributions) occur in larger areas, whereas relatively fewer new native species are added with increases in area because they simply have larger ranges (i.e., natives are more likely to have been previously sampled at a smaller spatial scale). Indirect evidence for this mechanism is that exotic plants have been shown to increase beta-diversity at smaller scales (McKinney 2004; Martin and Wilsey 2015) and non-natives that have had a shorter residence time increase beta-diversity when compared to non-natives that have had a longer residence time (Lososová et al. 2012).
A comparison of SARs may not be able to pinpoint drivers of differences in spatial diversity of native and exotic species as several mechanisms, including the ones mentioned above, can lead to the same patterns. Studies of native and exotic SARs are, however, a step forward in elucidating factors that structure exotics across spatial scales and can generate hypotheses regarding mechanisms. Furthermore, there is recent evidence that SARs serve as proxies for the responses of species to environmental and anthropocentric variables (Tittensor et al. 2007; Li et al. 2018). Here, to test whether native and exotic species respond similarly to changes in area, we compared the exponent of the power-law SAR, z, across 36 pairs of native-exotic SARs compiled from 23 publications. We also tested differences in the intercept, c, of native and exotic SARs across 21 pairs from 14 studies that reported them. We expect that c values for native SARs will be greater than those of exotic SARs. We further explored the role of taxonomic group, minimum and maximum area, area range across study sites, and island vs. mainland location (henceforth denoted as island-mainland) to better understand what drives differences in native and exotic SARs.
Methods
Data
We conducted a systematic literature search using the PRISMA guidelines (Supporting Information, Appendix A; Moher et al. 2009). We searched the terms “species–area” AND “invasive” OR “exotic” OR “non-native” in Google Scholar and the ISI Web of Science database to identify papers that studied SARs for exotic species. We then evaluated each study to determine if: (1) the study was about species area relationships; (2) the authors modeled SARs for both native and exotic species at the same locales, (3) the authors provided the exponent of the SARs (z) and a measure of variance around z from a univariate model (\({ \log }\left( S \right) = log\left( c \right) + z \times { \log }\left( A \right)\)) or provided the data that allowed for its calculation (Supporting Information, Appendix A). For papers that provided estimates of z without a measure of variance, we used the R package “digitize” (Poisot 2011) to extract data from SAR plots and calculated standard errors, when possible. Additionally, for the papers that met these criteria, we explored their references and the papers that cited them and evaluated these additional papers according to the same criteria. The screening process resulted in 36 native-exotic pairs coming from 23 different papers (Table 1). Of these 36 native-exotic pairs, 21 reported SAR intercepts (c). All intercepts were log10 transformed (i.e., intercepts that were log transformed in the original study were back transformed and then log10 transformed) to allow comparisons of the intercepts across studies. We further extracted data on the taxonomic group, the minimum, maximum, and range of area across the study, and whether the locales in the study are islands or mainland locations. We also recorded all available information for each species–area model (e.g., p values, R2; Supporting Information, Appendix B).
Analysis
To test whether native and exotic species have different SAR parameters, we used Linear Mixed models (LMM) with z or c as the response variable and provenance (native or exotic) as the fixed term along with native-exotic pair nested in study as random terms. Each model had a Gaussian error distribution and identity link function. Models were weighted by the inverse of parameter (z or c) standard errors. We assessed residuals to insure model assumptions were met. We fitted models with the R package lme4 (Bates et al. 2015). We conducted individual LMMs for plants, birds, animals (including birds), and all taxa together.
To test whether the differences between SAR parameters of native and exotic species varied among taxonomic groups, we constructed a LMM with provenance, taxa (plant or animal), an interaction between provenance and taxa, island-mainland, minimum area, and area range as fixed terms. The response variable(s), random terms, distribution, link function, and weights are the same as the above LMMs. Minimum area and area range were scaled to have mean of zero and standard deviation of one before fitting the model. The interaction between provenance and taxa accounts for the possibility that SAR slopes of native and exotic species may change across taxa. We did not include maximum area in this model because it was highly correlated with area range (r = 0.99). We also did not include interaction between provenance and island-mainland in the model for the following reasons. First, island-mainland has a high correlation with taxa (Spearman ρ = − 0.50, p < 0.001) because of the uneven spread of plant and animal taxa across islands and mainland (most plant studies were on the mainland while most animal studies were on islands, see details in the Results), including provenance * island-mainland would make it hard to study provenance * taxa given the collinearity between island-mainland and taxa. Second, we did not find any evidence for an interaction between provenance and island-mainland when testing z or c values for the animal or plant data sets separately (Supporting Information, Appendix C and D). We conducted backward stepwise selection on the fixed effects in the full model using the “step” function in the R package lmerTest (Kuznetsova et al. 2017). We used the R package lmerTest (Kuznetsova et al. 2017) to calculate p values for fixed terms in all LMMs with the Kenward-Roger approximation.
Results
Comparison of exponents (z) between native and exotic SARs
Our Web of Science search yielded 106 studies and we evaluated the first 200 studies from the Google Scholar search results. After removing duplicates, our literature search yielded 281 studies. We then screened these 281 studies and added eight additional papers that were either referenced or cited in the papers that met our criteria (Appendix A). Our systematic search yielded 36 native-exotic pairs from 23 studies to compare the exponents of the species–area relationship (Table 1). Of these data pairs, 22 were composed of plant data, five were bird data, four were reptiles, four were invertebrates, and one was composed of amphibians. Due to the small sample size, we included all non-plant taxa in the group “animals” for analyses. Minimum locale area ranged from 3.9 × 10−6 to 1 × 105 km2, maximum locale area ranged from 1.6 × 10−4 to 5 × 106 km2, and the range of area across locales within a study was from 1.6 × 10−4 to 5 × 106 km2. Of the 36 data pairs, 16 were from mainland studies, 20 were from studies where islands were the locales (Supporting Information, Appendix B). The majority of our plant data came from mainland systems (14/22 data pairs) and majority of our animal data came from island systems (12/14 data pairs). All but two of our native-exotic pairs were type IV SARs defined by Scheiner (2003). The other two studies (Hulme 2008; Tarasi and Peet 2017) in our literature search reported multiple SARs built from nested quadrats (e.g., type I, sensu Scheiner 2003) within the same system. Hulme (2008) reported five SARs for plants in England, UK; Tarasi and Peet (2017) reported 4501 SARs for plants in North and South Carolina, USA. For each of these studies, we took the mean z and c estimates and standard errors across all SARs.
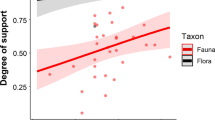
Overall, exotic (observed mean = 0.233, SD = 0.166) and native SARs (observed mean = 0.248, SD = 0.149) did not differ in their z values (Table 2). Fifteen of the comparisons showed that exotic z values were greater than native values, 19 comparisons showed natives with larger SAR exponents, and 2 comparisons had the same native and exotic z values (Fig. 1a). Breaking this result down by taxonomic group, exotic plants (observed mean = 0.293, SD = 0.159) did not have significantly different z values than natives (observed mean = 0.301, SD = 0.144; Table 2), with 10 exotic SARs having greater z values than that of natives, 11 z values were greater for natives, and in one case z was the same between native and exotic SARs (Fig. 1b). Native birds (observed mean = 0.186, SD = 0.129) had significantly greater SAR exponents than exotic birds (observed mean = 0.131, SD = 0.097; Table 2) and showed higher z values for native SARs in four out of five cases (Fig. 1c). The z values of native animals (including birds; observed mean = 0.165, SD = 0.118) were significantly greater than those of exotics (observed mean = 0.139, SD = 0.133; Table 2), with 8 out of 14 native SARs showing higher z values (Fig. 1d). The final LMM model resulting from stepwise selection included the variables provenance, taxa and island-mainland (Table 3). There was no significant effect of provenance on z values while plants and assemblages on islands had significantly greater SAR exponents (Table 3).
Comparison of intercepts (c) between native and exotic SARs
Of our 36 native-exotic pairs that reported z values, 21 reported intercepts (c). Of these data pairs, 13 were composed of plant data, two were bird data, four were reptiles, one was invertebrates, and one was composed of amphibians. Overall, intercepts (c) from native SARs (observed mean = 1.416, SD = 0.825) were greater than those of exotic SARs (observed mean = 0.776, SD = 0.825; Table 4). Eighteen of the comparisons showed that native c values were greater than those of exotics while 3 comparisons showed exotics with larger SAR intercepts (Fig. 2a). Native SAR intercepts we greater than exotic ones for all taxonomic groups. For plants, native SARs (observed mean = 1.746, SD = 0.608) had significantly greater c values than exotics (observed mean = 1.039, SD = 0.933; Table 4), with 11 (84.6%) native SARs having greater c values than exotic SARs (Fig. 2b). The SAR intercepts were greater for the two native bird assemblages (Fig. 2c). The c values of native animals (including birds; observed mean = 0.88, SD = 0.634) were significantly greater than those of exotics (observed mean = 0.35, SD = 0.341; Table 4), with 7 out of 8 native SARs showing higher c values (Fig. 2d). The final LMM model resulting from stepwise selection included the variables provenance, and taxa (Table 5). Exotic assemblages had significantly lower SAR intercepts and plant assemblages had significantly higher intercepts (Table 5).
Discussion
Comparison of exponents (z) between native and exotic SARs
We show that across all data sets exotic and native SARs do not differ in their z values. However, this pattern does not hold across all taxonomic groups. Plant assemblages, which are best represented in our data (61% of total), mirrored the overall pattern showing no difference in z values between native and exotic SARs. On the other hand, SAR exponents were greater for native assemblages for both birds and animals.
Our results for plants suggest that exotic species richness responds similarly to factors that drive native species richness as area increases. This result is in line with expectations based on the positive native-exotic richness relationships (NERRs), which has been observed across taxa (Fridley et al. 2007; Dyer et al. 2016; Carpio et al. 2017). The link between NERRs and SARs is as follows: native and exotic richness are both driven by the same factors (e.g., soil fertility and pH, climate, and habitat heterogeneity; Stohlgren et al. 1999; Davies et al. 2005; Gilbert and Lechowicz 2005; Dyer et al. 2016) and as area increases, both native and invasive richness respond in the same way to changes in these factors resulting in similar SAR z values. For example, if spatial heterogeneity drives NERRs (Davies et al. 2005) and spatial heterogeneity increases with area at a rapid rate in a given system, we would expect that z values for both native and exotic SARs would be relatively large. Conversely, if spatial heterogeneity increases with area at a relatively low rate, we would expect matching low values for the exponents of native and exotic SARs.
One interpretation of the plant SAR result is that provenance is not important in determining how plant species richness responds to increasing area. This interpretation suggests that there are no fundamental differences between native and non-native plant species. While some studies have shown little to no differences between native and exotic species (e.g., Palacio-López and Gianoli 2011; Leffler et al. 2014), others have found significant differences (Daehler 2003; Van Kleunen et al. 2010; Bernard-Verdier and Hulme 2015). Either traits that differ between natives and exotics are not important for SARs or different processes operating on different traits are generating similar SARs for native and exotics species. One way that exotic species can differ from natives yet yield similar SARs relates to the scaling of habitat disturbance with area. Habitat disturbance in terms of roads (Gelbard and Belnap 2003), human population (McKinney 2002), and urbanization (McKinney 2006) are all known drivers of exotic species establishment and richness. If the type and number of disturbances increase with area at the same rate as undisturbed habitat heterogeneity, then native and invasive species might be responding to different aspects of habitat diversity as area increases. However, this scenario assumes that habitat diversity drives SARs (Williams 1964).
The result that native bird and animal assemblages have greater z values than exotics suggests that the relationships between native and exotic SAR exponents differ between plants and animals. However, the discrepancy in sample size and taxonomic resolution between plants and animals indicates that we should interpret these results with some caution. The small sample size for birds (n = 5) and the fact that three of the studies were on oceanic islands in which several islands overlapped across data sets limits the inference we can draw. One possible approach to further exploring SARs for native and exotic birds is pairing native species lists for given locales with exotic species list based on distribution data from the global avian invasions atlas (GAVIA, Dyer et al. 2016). While there was a larger sample size for animals, the grouping of several taxa with very different life histories makes the interpretation of these results challenging. However, if native SARs indeed do have greater z values for animals, this suggests that different processes are governing the responses of native and exotic SARs across taxa. One observation from our data and the results of Triantis et al. (2012) is that plants (not considering provenance) have larger z values than animals. This observation may be a starting point for exploring any potential differences in the relationships between native and exotic SAR exponents between plants and animals.
The other variables in our final linear mixed model showed that SAR z values were higher on islands than mainlands and higher for plants compared to animals (Table 3). Several studies have suggested that the SAR exponent should be steeper on islands because dispersal is lower between islands than locales on the mainland due to the fact that oceans serve as a major barrier to dispersal. However, a meta-analysis of over 500 SARs showed no significant differences between island and mainland z values (Drakare et al. 2006). SAR z values for plants have also been observed to be greater than those of vertebrates and invertebrates (Triantis et al. 2012). Another notable result is that the two studies that used nested SARs (Hulme 2008; Tarasi and Peet 2017, both for plants), which reported multiple SARs that we simply took the average of, have significantly higher z values for exotic SARs.
Comparison of intercepts (c) between native and exotic SARs
The intercept (c) of the SAR can yield insight into the role of invasive species when comparing invaded and uninvaded SARs from different sites within the same system (Powell et al. 2013; Stohlgren and Rejmánek 2014). However, when comparing SAR intercepts of exotic vs. native assemblages for the same sites, the intercept basically quantifies differences in species richness (especially when slopes are similar, as in our study). Thus, we predicted that it would be uncommon for exotic species to have equal or greater c values. Our prediction was confirmed as intercepts of native SARs were greater than those of exotics for all taxa combined and for each individual taxonomic group.
The intercept (c) and slope (z) of SARs can also be utilized to better understand how invasive species alter native biodiversity (Powell et al. 2013; Rejmánek and Stohlgren 2015). Specifically, such studies focus on how the abundance of a particular invasive (Powell et al. 2013) or total percent cover of invasives (Stohlgren and Rejmánek 2014; Chase et al. 2015) alter c and z of the SAR. While this question differs from the one we ask here, these two lines of questioning are clearly linked and may inform one another to provide a better understanding of SARs in an increasingly invaded world. For example, while we clearly do not consider the abundance or percent cover of invaders in our study, information on the presence of an aggressive (abundant) invader could provide another covariate to further test differences in the responses of native and exotic SARs. For instance, similar responses of native and exotic SAR exponents (or intercepts) to the presence or absence of an aggressive invader would be evidence that exotics and natives are ecologically similar. On the other hand, a decrease in z values for exotic SARs in the presence of an abundant invader and an increase in native z values could indicate that the given invader may be facilitating the spread and establishment of a suite of exotic species. Thus, identifying abundant invaders or measuring percent cover of invasives during studies comparing native and exotic SARs could provide additional insight into the ecology of native and exotic species and how they respond to area.
Conclusions
Despite the fact that SARs have been studied for almost 100 years (Arrhenius 1921), a mechanistic understanding of what drives them has been elusive (Lomolino 2001; Turner and Tjørve 2005). Here, we conducted the first synthesis of studies comparing native and exotic SARs. We found that SARs for exotic species have z values similar to native SARs for plants while native z values are larger for animals and birds. SAR intercepts are larger for native assemblages across taxonomic groups. However, as with most SAR studies, we are still lacking a mechanism. The challenge in linking SARs to processes that generate them is in part due to the fact that they likely result from several interacting processes that vary across scale (Turner and Tjørve 2005). Expanding studies that compare native and exotic SARs to more taxonomic groups, different types of SARs, and explicitly testing how disturbance scales with area relative to native habitat diversity will lead to a better understanding of the similarities and differences in how native and exotic species richness scale with area.
References
Ackerman JD, Tremblay RL, Rojas-Sandoval J, Hernández-Figueroa E (2017) Biotic resistance in the tropics: patterns of seed plant invasions within an island. Biol Invasions 19:315–328
Arrhenius O (1921) Species and area. J Ecol 9:95–99
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bennett JR, Dunwiddie PW, Giblin DE, Arcese P (2012) Native versus exotic community patterns across three scales: roles of competition, environment and incomplete invasion. Perspect Plant Ecol Evol Syst 14:381–392
Bernard-Verdier M, Hulme PE (2015) Alien and native plant species play different roles in plant community structure. J Ecol 103:143–152
Blackburn TM, Cassey P, Lockwood J et al (2008) The island biogeography of exotic bird species. Glob Ecol Biogeogr 17:246–251
Blackburn TM, Cassey P, Lockwood JL (2009) The role of species traits in the establishment success of exotic birds. Glob Change Biol 15:2852–2860
Blackburn TM, Lockwood JL, Cassey P (2015) The influence of numbers on invasion success. Mol Ecol 24:1942–1953
Blackburn TM, Delean S, Pyšek P, Cassey P (2016) On the island biogeography of aliens: a global analysis of the richness of plant and bird species on oceanic islands. Glob Ecol Biogeogr 25:859–868
Burns KC (2015) A theory of island biogeography for exotic species. Am Nat 186:441–451
Carpio A, Barasona J, Guerrero-Casado J et al (2017) An assessment of conflict areas between alien and native species richness of terrestrial vertebrates on a macro-ecological scale in a mediterranean hotspot. Anim Conserv 20:433–443
Chase JM, Powell KI, Knight TM (2015) ‘Bigger data’on scale-dependent effects of invasive species on biodiversity cannot overcome confounded analyses: a comment on stohlgren & rejmánek (2014). Biol Let 11:20150103
Chiarucci A, Fattorini S, Foggi B et al (2017) Plant recording across two centuries reveals dramatic changes in species diversity of a mediterranean archipelago. Sci Rep 7:5415
Chown S, Gremmen N, Gaston K (1998) Ecological biogeography of southern ocean islands: species–area relationships, human impacts, and conservation. Am Nat 152:562–575
Connor EF, McCoy ED (1979) The statistics and biology of the species–area relationship. Am Nat 113:791–833
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431
Davies KF, Chesson P, Harrison S et al (2005) Spatial heterogeneity explains the scale dependence of the native–exotic diversity relationship. Ecology 86:1602–1610
Dengler J (2009) Which function describes the species–area relationship best? A review and empirical evaluation. J Biogeogr 36:728–744
Denslow MW, Palmer MW, Murrell ZE (2010) Patterns of native and exotic vascular plant richness along an elevational gradient from sea level to the summit of the appalachian mountains, usa. J Torrey Bot Soc 137:67–80
Drakare S, Lennon JJ, Hillebrand H (2006) The imprint of the geographical, evolutionary and ecological context on species–area relationships. Ecol Lett 9:215–227
Dyer EE, Franks V, Cassey P et al (2016) A global analysis of the determinants of alien geographical range size in birds. Glob Ecol Biogeogr 25:1346–1355
Flaspohler DJ, Giardina CP, Asner GP et al (2010) Long-term effects of fragmentation and fragment properties on bird species richness in hawaiian forests. Biol Cons 143:280–288
Flores-Moreno H, Thomson FJ, Warton DI, Moles AT (2013) Are introduced species better dispersers than native species? A global comparative study of seed dispersal distance. PLoS ONE 8:e68541
Fridley J, Stachowicz J, Naeem S et al (2007) The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17
Funk JL (2008) Differences in plasticity between invasive and native plants from a low resource environment. J Ecol 96:1162–1173
Gao D, Perry G (2016) Species–area relationships and additive partitioning of diversity of native and nonnative herpetofauna of the west indies. Ecol Evol 6:7742–7762
Gelbard JL, Belnap J (2003) Roads as conduits for exotic plant invasions in a semiarid landscape. Conserv Biol 17:420–432
Gilbert B, Lechowicz MJ (2005) Invasibility and abiotic gradients: the positive correlation between native and exotic plant diversity. Ecology 86:1848–1855
Gould SJ (1979) An allometric interpretation of species–area curves: the meaning of the coefficient. Am Nat 114:335–343
Gram WK, Borer ET, Cottingham KL et al (2004) Distribution of plants in a california serpentine grassland: Are rocky hummocks spatial refuges for native species? Plant Ecol 172:159–171
He F, Legendre P (2002) Species diversity patterns derived from species–area models. Ecology 83:1185–1198
Herben T, Mandák B, Bímová K, Münzbergová Z (2004) Invasibility and species richness of a community: a neutral model and a survey of published data. Ecology 85:3223–3233
Holle BV, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212–3218
Houlahan JE, Keddy PA, Makkay K, Findlay CS (2006) The effects of adjacent land use on wetland species richness and community composition. Wetlands 26:79–96
Hulme PE (2008) Contrasting alien and native plant species–area relationships: the importance of spatial grain and extent. Glob Ecol Biogeogr 17:641–647
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Leffler AJ, James JJ, Monaco TA, Sheley RL (2014) A new perspective on trait differences between native and invasive exotic plants. Ecology 95:298–305
Lemoine NP, Burkepile DE, Parker JD (2016) Quantifying differences between native and introduced species. Trends Ecol Evol 31:372–381
Li D, Monahan WB, Baiser B (2018) Species richness and phylogenetic diversity of native and non-native species respond differently to area and environmental factors. Divers Distrib 24:853–864
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Lomolino MV (2000) Ecology’s most general, yet protean pattern: the species–area relationship. J Biogeogr 27:17–26
Lomolino MV (2001) The species–area relationship: new challenges for an old pattern. Prog Phys Geogr 25:1–21
Long JD, Trussell GC, Elliman T (2009) Linking invasions and biogeography: isolation differentially affects exotic and native plant diversity. Ecology 90:863–868
Lososová Z, Chytry M, Tichy L et al (2012) Biotic homogenization of central european urban floras depends on residence time of alien species and habitat types. Biol Cons 145:179–184
Malkinson D, Kopel D, Wittenberg L (2018) From rural-urban gradients to patch–matrix frameworks: plant diversity patterns in urban landscapes. Landsc Urban Plan 169:260–268
Martin LM, Wilsey BJ (2015) Differences in beta diversity between exotic and native grasslands vary with scale along a latitudinal gradient. Ecology 96:1042–1051
McKinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 52:883–890
McKinney ML (2004) Do exotics homogenize or differentiate communities? Roles of sampling and exotic species richness. Biol Invasions 6:495–504
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Cons 127:247–260
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med 6:e1000097
Nichols WF, Nichols VC (2008) The land use history, flora, and natural communities of the isles of shoals, rye, new hampshire and kittery, maine. Rhodora 110:245–295
O’Connor R, Usher M, Gibbs A, Brown K (1986) Biological characteristics of invaders among bird species in britain. Philos Trans R Soc Lond B Biol Sci 314:583–598
Ordonez A, Wright IJ, Olff H (2010) Functional differences between native and alien species: a global-scale comparison. Funct Ecol 24:1353–1361
Palacio-López K, Gianoli E (2011) Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis. Oikos 120:1393–1401
Poisot T (2011) The digitize package: extracting numerical data from scatterplots. R J 3:25–26
Powell KI, Chase JM, Knight TM (2013) Invasive plants have scale-dependent effects on diversity by altering species–area relationships. Science 339:316–318
Preston FW (1962) The canonical distribution of commonness and rarity: part i. Ecology 43:185–215
Pyšek P (1998) Alien and native species in central european urban floras: a quantitative comparison. J Biogeogr 25:155–163
Rejmánek M, Stohlgren TJ (2015) Scale-dependent impacts of invasive species: a reply to chase. Biol Lett 11:20150402
Ricklefs RE, Lovette IJ (1999) The roles of island area per se and habitat diversity in the species–area relationships of four lesser antillean faunal groups. J Anim Ecol 68:1142–1160
Rosenzweig ML (1995) Species diversity in space and time. Cambridge University Press, Cambridge
Rosindell J, Cornell SJ (2009) Species–area curves, neutral models, and long-distance dispersal. Ecology 90:1743–1750
Roura-Pascual N, Sanders NJ, Hui C (2016) The distribution and diversity of insular ants: do exotic species play by different rules? Glob Ecol Biogeogr 25:642–654
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Sax DF, Gaines SD (2006) The biogeography of naturalized species and the species–area relationship: reciprocal insights to biogeography ans invasion biology. In: Conceptual ecology and invasion biology: Reciprocal approaches to nature. Springer, pp 449–480
Scheiner SM (2003) Six types of species–area curves. Glob Ecol Biogeogr 12:441–447
Stark SC, Bunker DE, Carson WP (2006) A null model of exotic plant diversity tested with exotic and native species–area relationships. Ecol Lett 9:136–141
Stohlgren TJ, Rejmánek M (2014) No universal scale-dependent impacts of invasive species on native plant species richness. Biol Let 10:20130939
Stohlgren TJ, Binkley D, Chong GW et al (1999) Exotic plant species invade hot spots of native plant diversity. Ecol Monogr 69:25–46
Stohlgren TJ, Barnett DT, Kartesz JT (2003) The rich get richer: patterns of plant invasions in the united states. Front Ecol Environ 1:11–14
Tarasi DD, Peet RK (2017) The native-exotic species richness relationship varies with spatial grain of measurement and environmental conditions. Ecology 98:3086–3095
Tittensor DP, Micheli F, Nyström M, Worm B (2007) Human impacts on the species–area relationship in reef fish assemblages. Ecol Lett 10:760–772
Triantis KA, Guilhaumon F, Whittaker RJ (2012) The island species–area relationship: biology and statistics. J Biogeogr 39:215–231
Turner WR, Tjørve E (2005) Scale-dependence in species–area relationships. Ecography 28:721–730
Van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245
Whittaker RJ, Rigal F, Borges PA et al (2014) Functional biogeography of oceanic islands and the scaling of functional diversity in the azores. Proc Natl Acad Sci 111:13709–13714
Williams C (1964) Patterns in the balance of nature: and related problems in quantitative ecology. Academic Press, Cambridege
Acknowledgements
Thanks to Edward F. Connor, Sara Kuebbing, and two anonymous reviewers for their feedback on earlier versions of this manuscript. This project was supported by NSF Grant ABI#1458034 to BB.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baiser, B., Li, D. Comparing species–area relationships of native and exotic species. Biol Invasions 20, 3647–3658 (2018). https://doi.org/10.1007/s10530-018-1802-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1802-4