Abstract
The widespread introduction of Largemouth Bass (Micropterus salmoides) and Florida Bass (M. floridanus) to establish sport fisheries represents a significant conservation concern given their role as apex predators and their ability to alter community diversity and species abundance. In regions like southern Africa, which has both high levels of aquatic endemism and imperilment, limiting the spread of invasive predators is a primary goal of current alien species legislation. Here, we applied two panels of species-diagnostic SNPs for a total of 60 markers to map the distribution of Largemouth Bass, Florida Bass, and their hybrids in 13 southern African water bodies. Using Bayesian clustering algorithms we documented the introgression of Florida Bass alleles across a broad geographic range, from the Cape Floristic region of South Africa to Mozambique. Several populations previously considered pure Largemouth Bass based on mitochondrial DNA sequences were found to consist exclusively of hybrids. Samples collected from Lake Chicamba, which was initially established with pure Largemouth Bass, are now almost exclusively comprised of Florida Bass alleles (89.3 % Florida Bass). The estimated hybrid class of sampled fish (e.g., F1 hybrid, pure Largemouth Bass, pure Florida Bass) showed that with few exceptions, populations were dominated by a single hybrid class. The present work provides enhanced resolution of the distribution and dynamics of Florida Bass, Largemouth Bass, and their hybrids in southern Africa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species introductions are a leading threat to biodiversity worldwide (e.g., Strayer 2010; Vilà et al. 2011) and freshwater ecosystems are particularly susceptible (García-Berthou 2007). In regions of the globe such as southern Africa the long-term isolation of aquatic habitats has resulted in both high levels of endemism and imperilment (Linder et al. 2010; Weyl et al. 2014). Southern Africa was previously identified as a hotspot of aquatic invasions with the ratio of non-native species richness to total species richness being greater than 25% (Leprieur et al. 2008). Given the heightened risks of species imperilment and extinction in freshwater systems of southern Africa, knowledge on the spread and dynamics of invasive species represents an important source of information for management and conservation.
The Largemouth Bass (Micropterus salmoides) is a freshwater fish species native to eastern North America that has been established worldwide for the purpose of recreational fisheries (Robbins and MacCrimmon 1974; Jackson 2002; Hargrove et al. 2015; Taylor et al. 2015). Largemouth Bass is an apex predator in many systems, capable of altering the abundance and distribution of aquatic biota via top-down control (Drenner et al. 2002; Cambray 2003; Warren 2009; Weyl et al. 2010). Because of their widespread introductions and documented negative impacts on native fish communities, they have been characterized as one of the world’s worst invaders (Lowe et al. 2000). Largemouth Bass were first introduced into South Africa in 1928 (Harrison 1936), and since that time their distribution has expanded via intentional translocations to encompass much of southern Africa (DeMoor and Bruton 1988; Ellender and Weyl 2014; Hargrove et al. 2015). Previous genetic work has shown that populations have persisted in isolation in excess of 90 years, despite initial introductions that consisted of small numbers of individuals harboring low levels of neutral genetic diversity (Hargrove et al. 2017).
The Florida Bass (Micropterus floridanus) was first described as a subspecies of Largemouth Bass by Bailey and Hubbs (1949), with a key characteristic being the ability to reach larger maximum size. Shortly after they were described, Florida Bass were purposefully introduced outside their native range to enhance the growth characteristics of existing Largemouth Bass populations (e.g. Sasaki 1961). Record-breaking catches following the introduction of Florida Bass have been documented in Arkansas (Lamothe and Johnson 2013), California (Chew 1974), Louisiana (Hughes and Wood 1995), Oklahoma (Horton and Gilliland 1993), Texas (Forshage and Fries 1995), and South Africa (Weyl et al. 2017). In addition to reaching larger maximum sizes relative to Largemouth Bass, Florida Bass are capable of spawning over a longer time period (Rogers et al. 2006), have a greater tolerance for high temperatures (Philipp and Whitt 1991), and exhibit greater longevity in warm climates (Neal and Noble 2002). The first introductions of Florida Bass into South Africa occurred in 1980 (DeMoor and Bruton 1988), and private fish transfers have increased their prevalence countrywide (Weyl et al. 2017). From the perspective of species invasions, the introduction of Florida Bass represents a significant concern, as their ability to reach larger maximize sizes and persist longer may equate to greater biological impacts on native communities (Weyl et al. 2017).
Because Florida Bass and Largemouth Bass readily hybridize and are morphologically difficult to distinguish (Warren 2009), molecular genetic markers are necessary to identify the taxonomic status of individuals in regions where the two co-occur. Previous genetic work has shown that Florida Bass have been introduced throughout South Africa, with Florida Bass mitochondrial haplotypes (mtDNA) recovered in 13 of 20 sampled populations (Weyl et al. 2017). Conclusions drawn from these results were limited by the use of a uniparentally inherited mtDNA marker which precludes the ability to identify hybrids. Additionally, asymmetrical hybridization may prevent the detection of all present species when using only mtDNA (e.g., Avise et al. 1997). The inclusion of nuclear markers could solve this problem by allowing for the detection of species, and their hybrids, and providing better estimates of introgression. As a result, the use of a species-diagnostic marker such as Single Nucleotide Polymorphisms (SNPs) may serve to increase both the resolution and accuracy of species detection and quantification of introgression between species (Della Croce et al. 2016).
Given the potential for negative impacts associated with Florida Bass introductions in an area which has high numbers of threatened and endangered freshwater taxa, this study sought to refine the estimated distribution of Florida Bass in southern Africa. The goals of this study were to use a suite of species-diagnostic SNPs (n = 60) to identify the presence of Florida Bass in southern Africa and to characterize levels of introgression as well as genealogical class of sampled fish within populations.
Methods
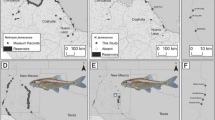
Fish (n = 94) were sampled using hook and line tackle from ten impoundments in South Africa and a single impoundment each in Botswana, Mozambique, and Tanzania (Fig. 1). A 1 × 1 cm2 portion of pectoral fin was clipped from each fish captured and placed into 95% non-denatured ethanol for preservation. DNA was isolated from each fin-clip using a 96-well plate extraction protocol (Ivanova et al. 2006), quantified using a ND-1000 spectrophotometer (Nanodrop, Wilmington, DE), and diluted to a concentration of 20 ng/μL. Both positive and negative controls were included in DNA extractions. Each sample was then assessed following the procedures of Li et al. (2015). Two SNP panels totaling 60 species-diagnostic markers (Li et al. 2015; Zhao et al. 2018) were genotyped using an Agena MassARRAY (Agena Bioscience, San Diego, CA) and called using MassARRAY TYPER 4.0 software.
Sampling locations of Largemouth Bass (Micropterus salmoides) collected for analysis of hybridization with Florida Bass (M. floridanus) in southern Africa. Tanzania was not displayed on this map but GPS coordinates are available in Table 1
Relationships among multilocus genotypes, within and among populations, were explored using Principal Component Analysis (PCA) as implemented in the ade4 and adegenet packages (Thioulouse et al. 1997; Jombart 2008) in R v 3.3 (R Development Core Team 2016). Analyses were performed on a correlation matrix of scaled allele frequencies with genetic variation reduced to two principle components allowing individuals to be clustered along axes based on their allele distributions. To determine the appropriate number of principal components to retain we examined the scree plot, and axes preceding a sharp change in the slopes of adjacent segments were selected. The graphical output displayed the absolute variance (i.e., eigenvalues) explained by each of the principal components with the X and Y labels representing the percentage of total variance explained by the first and second components, respectively.
To estimate the proportion of each individual’s genome descended from Largemouth Bass and Florida Bass, the model-based Bayesian assignment method implemented in structure v 2.3 was used (Pritchard et al. 2000). Analyses were performed under the admixture model with correlated allele frequencies and the number of clusters (k) set to two. Burn-in consisted of 1 × 105 iterations followed by a run length of 2.5 × 105 steps. structure runs were conducted without location priors (locprior) as introductions of Florida Bass were informal (i.e., illegal) and as a result their distribution was expected to be haphazard. Individuals with ancestry coefficients (q) of 1 were designated as Largemouth Bass, q = 0 were designated as Florida Bass, and intermediate values were identified as hybrids. A total of five structure runs were performed and a graph of average q value per individual was generated using the R package pophelper (Francis 2017).
Distinct genealogical and hybrid classes were estimated for each individual based on predictable within and between locus allelic configurations using newhybrids (v 1.1; Anderson and Thompson 2002). Similar to the assignment of individuals to genetic clusters in structure, the assignment of individuals to predefined hybrid classes was performed using the Markov chain Monte Carlo (MCMC) method to estimate the posterior probability of each solution. Thus, in addition to understanding patterns in ancestry (structure), results generated in NewHybrids provided information about the nature of hybridization occurring in populations. For example, if pure Florida Bass have been recently planted into a system with Largemouth Bass we would expect pure Florida Bass and F1 hybrids to be detected. Alternatively, if pure Florida Bass were introduced more distantly in the past or if non-pure strain Florida Bass were used in translocations, we would expect to see predominantly later stage backcrosses (e.g., Largemouth Bass × F1 hybrid) but not Florida Bass or F1 hybrids. newhybrids classified fish into one of six genealogical classes based on patterns of allelic inheritance (Largemouth Bass, Florida Bass, F1 hybrids, F2 hybrids, Largemouth Bass × F1 backcrosses, and Florida Bass × F1 backcrosses).
Results
A total of 94 individuals from 13 different populations were screened with 60 species-diagnostic SNPs (Table 1). The extent of hybridization varied widely among populations with Largemouth Bass alleles ranging from 9.9 to 96.1% (Table 1). Six populations (Binfield, Groendal, Groenvlei, Mankazana, Settlers, and Tanzania) exhibited > 90% Largemouth Bass alleles while four (Theewaterskloof, Letsibogo, Chicamba, and Goedertrouw) exhibited < 40% Largemouth Bass alleles, with the remaining populations exhibiting intermediate values (Table 1). The composition of alleles of fish sampled in Chicamba and Goedertrouw were highly skewed towards Florida Bass, with 10.7% and 9.9% of their alleles being derived from Largemouth Bass, respectively.
The first two PCA axes explained a total of 71.2% of the observed variance in the samples (Fig. 2). Bass from Binfield, Groendal, Groenvlei, Mankazana, Settlers, and Tanzania were tightly clustered and overlapping, indicative of low levels of genetic variation among samples from these populations. Goedertrouw and Chicamba populations formed clusters proximate to a reference Florida Bass individual, indicating similarity in allelic composition. Individuals from Impofu, Wriggleswade, and Theewaterskloof were less tightly bound relative to other populations indicating varying degrees of hybridization and introgression.
Principal Component Analysis of species diagnostic SNP markers amplified for Largemouth Bass (Micropterus salmoides) sampled from southern Africa to examine patterns of hybridization with Florida Bass (M. floridanus). Profiles of pure Florida Bass and Largemouth Bass reference samples (singular individual) are shown on the right and upper left portions of the plot, respectively
Partitioning multilocus genotypes among two clusters using the Bayesian inference approach in structure revealed varying degrees of admixture among populations (Fig. 3). The average q value corresponding to Largemouth Bass across all individuals was 0.68 (SD ± 0.31) and ranged from a low of 0.10 to a high of 0.96. Mean values of q (higher = greater incidence of Largemouth Bass ancestry) were highest for Groendal (0.96 ± 0.002) followed by Settlers (0.95 ± 0.005), Groenvlei (0.95 ± 0.003), Mankazana (0.94 ± 0.002), Binfield (0.94 ± 0.001), and Tanzania (0.93 ± < 0.001). The remaining populations displayed lower average q values and higher variation; Impofu (0.68 ± 0.03), Kowie Weir (0.56 ± 0.03), Wriggleswade (0.51 ± 0.05), Theewaterskloof (0.39 ± 0.05), Letsibogo (0.30 ± 0.01), Chicamba (0.11 ± 0.01), and Goedertrouw (0.10 ± 0.01).
Coefficient of ancestry generated for Largemouth Bass (Micropterus salmoides) and Florida Bass (M. floridanus) collected across southern Africa. Bars denote individuals, dashed white lines separate populations, and colors correspond to the portion of the genome that is derived from Largemouth Bass (red) and Florida Bass (blue). Population abbreviations are as follows; BF Binfield, CH Chicamba, GW Goedertrouw, GD Groendal, GV Groenvlei, KW Kowie Weir, LE Letsibogo, MK Mankazana, IM Impofu, ST Settlers, TZ Tanzania, TH Theewaterskloof, WR Wriggleswade
The assignment of individuals to hybrid classes using newhybrids indicated substantial variation among populations but little variation within populations (Fig. 4). newhybrids identified all samples from Binfield, Groendal, Groenvlei, Mankazana, Settlers, and Tanzania as Largemouth Bass with high levels of probability (> 95%) despite the low-level presence of Florida Bass alleles in these samples. All individuals from Chicamba and Goedertrouw were assigned as Florida Bass, whereas bass from Kowie Weir were classified as second generation hybrids (F2s). All samples from Letsibogo were identified as Florida Bass × F1 backcrosses, and three populations (Impofu, Theewaterskloof, and Wriggleswade) consisted of individuals whose probability of ancestry was split into multiple genealogical classes (e.g., 40% F1, 40% F2, 20% Largemouth Bass backcross).
Probability of Largemouth Bass (Micropterus salmoides) and Florida Bass (M. floridanus) samples collected across southern Africa belonging to potential hybrid classes. Bars denote individuals, dotted white lines denote populations, and the height of colors correspond to the probability of belonging to a particular class. F1 F1 hybrid, F2 F2 hybrid, FLB_Bx Florida Bass × F1 hybrid, FLB Florida Bass, LMB Largemouth Bass, LMB_Bx Largemouth Bass × F1 hybrid, BF Binfield, CH Chicamba, GW Goedertrouw, GD Groendal, GV Groenvlei, KW Kowie Weir, LE Letsibogo, MK Mankazana, IM Impofu, ST Settlers, TZ Tanzania, TH Theewaterskloof, WR Wriggleswade
Discussion
Using 60 species-diagnostic SNPs, our study generated a detailed description of the distribution of Largemouth Bass, Florida Bass, and their hybrids across southern Africa. We revealed the presence of substantial variation in levels of hybridization among water bodies, and identified populations consisting almost exclusively of Florida Bass and Largemouth Bass alleles. Despite the fact that hybridization varied substantially between populations, the genetic composition of individuals within the same population was highly similar, suggesting populations may be in Hardy–Weinberg equilibrium as a result of introductions occurring more distantly in the past. The same pattern was reflected in the distribution of hybrid classes; populations were found to consist generally of a single hybrid class. Combined, the current study highlights the utility of applying high-resolution markers for inferring the distribution and extent of hybridization among co-distributed invasive fish populations.
In general, Florida Bass influence was greatest at lower latitudes but there were exceptions. The three populations exhibiting the highest frequency of Florida Bass alleles were located north of latitude 30°S (Goedertrouw, Chicamba, and Letsibogo), with upwards of 90% of their alleles being diagnostic for Florida Bass. The observed frequency of Florida Bass alleles could be due to selection as a result of superior performance of Florida Bass in warmer climates (Neal and Noble 2002), or simply a reflection of species introductions in different regions. Observations from Lake Chicamba suggest the former since it was originally established with Largemouth Bass (Weyl and Hecht 1999) but now exhibits almost exclusively Florida Bass alleles. The shift in genetic composition may be temperature driven as thermal selection for Florida Bass alleles has been demonstrated in artificial pond settings (Fields et al. 1987; Philipp et al. 2002) and in lakes receiving thermal effluent from power plants (Childers 1979; Allen et al. 2009). Increased size and greater longevity of Florida Bass have been recorded in subtropical environments (Neal and Noble 2002; Peterson et al. 2017) and these facets may promote their survival and reproduction. Additionally, the spawning season of Florida Bass is more protracted relative to Largemouth Bass (Isely et al. 1987; Rogers et al. 2006), and in summer rainfall regions such as Mozambique, the ability to spawn over a greater time period may decrease the probability of spawning failure due to extreme rain events (Weyl and Hecht 1999). Future efforts should focus on sampling in such a manner that the role of temperature could be explicitly tested as a factor that may promote directional hybridization.
Results from our genetic analyses suggested that variation in Florida Bass influence across reservoirs was strongly influenced by introduction history. For example, populations classified as Largemouth Bass (Fig. 4) were likely free from secondary introductions based on their tight overlapping placement in our PCA (Fig. 2) and minimal variation in estimates of genetic admixture (Fig. 3). This pattern was supported by coalescent modeling performed on microsatellite data for four of the same populations (Binfield, Groenvlei, Mankazana, and Settlers) which identified the most probable introduction scenario involved a single ancestral source (Hargrove et al. 2017). The low levels of Florida Bass alleles observed in these populations was unexpected, but may have been an artifact of the original founders being hybrids or typing errors (see below). In contrast to populations classified as Largemouth Bass, water bodies characterized as containing exclusively Florida Bass were most likely later generation backcross hybrids. Support for this argument includes the broader distribution of individuals along Principal Component axes (Fig. 2) and larger variance in genetic admixture levels (Fig. 3). That Chicamba consisted almost exclusively of Florida Bass alleles, yet was originally founded with Largemouth Bass (Weyl and Hecht 1999) lends further support to a potential selective gradient by latitude influencing allele composition. Unfortunately, our ability to relate allele frequencies with stocking history was limited because records of fish introductions in southern Africa are commonly incomplete or occur as a result of illegal transfers (McCafferty et al. 2012; Ellender and Weyl 2014; Hargrove et al. 2017). However, several populations in southern South Africa exhibited moderate Florida Bass influence (Impofu, Theewaterskloof, Kowie Weir, and Wriggleswade), and with the exception of Kowie Weir, these systems represent popular angling venues (i.e., host regular bass angling competitions; Hargrove et al. 2015). Proximity to population centers and elevated angler use have been previously identified as factors that may promote the introduction of invasive species (Johnson et al. 2008; Sharma et al. 2009), and this may in part explain the observed Florida Bass alleles. Ultimately, the study of admixture in the success of introduced populations is relatively nascent (e.g., Rius and Darling 2014), and in this scenario, sampling allele frequencies through time and characterizing the performance of different phenotypes may help to explain the observed patterns of hybridization.
Results from the current study generally confirmed the trends observed in previous genetic assessments of South African bass populations (Table 2), with two notable exceptions (Hargrove et al. 2017; Weyl et al. 2017). Ten populations examined here were also evaluated by Weyl et al. (2017), and both studies detected Florida Bass influence in Goedertrouw, Impofu, Theewaterskloof, and Wriggleswade populations. Because uniparentally inherited markers (mtDNA sequence data) were used for species identification in Weyl et al. (2017), a direct comparison of the extent of introgression between studies was not possible. One notable deviation between previous studies and the current one involves the detection of Florida Bass alleles in the Kowie Weir population. Hargrove et al. (2017) identified higher levels of microsatellite diversity in this population relative to others in the same region, and results from coalescent based models identified this population as likely derived from a secondary introduction. Hargrove et al. (2017) postulated that introgression with another species of the genus Micropterus may have explained the observed elevated genetic diversity. Spotted Bass (M. punctulatus) have been recorded in the Kowie River and adjacent river systems (James et al. 2008, OLF Weyl unpublished data), but the hypothesis that elevated genetic diversity was driven by intraspecific hybridization was ultimately rejected as all mtDNA sequences from this population matched those of Largemouth Bass. Using SNP markers we identified that interspecific hybridization between Largemouth Bass and Florida Bass has occurred in this system most likely as a result of Florida Bass from privately stocked ponds making their way into the Kowie River during flood events. Such a prospect is reasonable given that the Kowie Weir population consists of a pooled section of the Kowie River situated in the lower reaches of a 580 km2 river catchment (Watling and Watling 1983; Wasserman et al. 2011). Future work is necessary to identify the role of connectivity and gene flow among water bodies in the Kowie Weir system for sake of explaining Florida Bass influence.
A second deviation between the current study and previous analyses is the prevalence of low-levels of Florida Bass alleles in every population sampled. This finding was largely unexpected, and there are several potential explanations. Hargrove et al. (2017) identified that five populations of bass in South Africa contained a mitochondrial haplotype that was identical to fish sampled in Maryland, USA, and argued this may have been a potential source population. However, no records of bass in Maryland exist prior to 1874 (Truitt et al. 1929; Powell 1967), which strongly suggests that bass in Maryland were themselves introduced. It is possible that bass introduced into Maryland were Florida Bass × Largemouth Bass hybrids, however, patterns of allelic inheritance in our SNP data suggest otherwise. Specifically, in the five populations which contained low levels of Florida Bass influence (≤ 5%) all Florida bass alleles were restricted to 5 of the 60 SNPs. This nonrandom pattern suggests that something other than hybridization may be driving the observed genotypes. Potential explanations include selection operating on these specific loci (Gompert and Alex Buerkle 2010), typing errors associated with the MassARRAY platform (Johansen et al. 2013), or that SNPs within duplicated genes are being amplified (Li et al. 2015). Whether or not the observed patterns are an artifact of methodology or differences in introgression among loci is currently unknown.
A potential criticism of the current study involves the small sample sizes collected from the focal water bodies. The probability of detecting introgression within a population is commonly modeled as a function of the number of individuals sampled and number of markers amplified per individual (Rasmussen et al. 2010; Amish et al. 2012). Effectively, small numbers of individuals and few markers would result in a high likelihood that introgression would not be detected (Della Croce et al. 2016). Additionally, the likelihood of detecting introgression is thought to be positively influenced by the time since introgression first occurred (Della Croce et al. 2017). Several facets of our methods provide strong evidence that estimates of introgression generated here are likely to be accurate. First, using equation 1 of Della Croce et al. (2016) to estimate the probability of failing to detect introgression assuming a sample size of eight fish, 60 markers, and a population admixture rate of 0.05, is 4.1 × 10−22. In other words, even in instance where admixture is low, the large number of markers used here ensured a high likelihood of detecting introgression. Secondly, as was noted earlier, variation in admixture among individuals of the same population was generally low.
In conclusion, our study represents the first detailed accounts of hybridization among Largemouth Bass and Florida Bass introduced outside of North America. Future studies should focus on relating the biotic and abiotic characteristics of individual water to explore which factors may promote or inhibit hybridization among black basses. Additional efforts should also be directed towards identifying potential differences in performance (e.g., growth and longevity) among populations that vary in their genetic composition. Combined, such efforts will help to create a better understanding of how genetic and environmental factors interact to affect the dynamics of invasive fish populations.
References
Allen R, Cato C, Dennis C et al (2009) Condition relative to phenotype for bass populations in southern Arkansas lakes. J Ark Acad Sci 63:20–27
Amish SJ, Hohenlohe PA, Painter S et al (2012) RAD sequencing yields a high success rate for Westslope cutthroat and Rainbow trout species-diagnostic SNP assays. Mol Ecol Resour 12:653–660
Anderson EC, Thompson EA (2002) A model-based method for identifying species hybrids using multilocus genetic data. Genetics 160:1217–1229
Avise J, Pierce P, Van Den Avyle M et al (1997) Cytonuclear introgressive swamping and species turnover of bass after an introduction. J Hered 88:14–20
Bailey RM, Hubbs CL (1949) The black basses (Micropterus) of Florida, with description of a new species. Occasional Papers of the Museum of Zoology University of Michigan 516
Cambray JA (2003) Impact on indigenous species biodiversity caused by the globalisation of alien recreational freshwater fisheries. Hydrobiologia 500:217–230
Chew RL (1974) Early life history of the Florida largemouth bass. Game and Fresh Water Fish Commission, Fishery Bulletin 7, Tallahassee, Florida
Childers WF (1979) The effect of a heated effluent on the frequency of two alleles at the Mdh-B locus in largemouth bass, Micropterus salmoides. In: Philipp DP, Childers WF, Whitt WF (eds) Evaluation of a cooling lake fishery, vol III. Electric Power Research Institute, Palo Alto, p 45
Della Croce P, Poole GC, Luikart G (2016) Detecting and quantifying introgression in hybridized populations: simplifying assumptions yield overconfidence and uncertainty. Mol Ecol Resour 16:1287–1302
Della Croce P, Poole GC, Payn RA et al (2017) Early detection of nonnative alleles in fish populations: when sample size actually matters. Fisheries 42:44–56
DeMoor I, Bruton M (1988) Atlas of alien and translocated indigenous aquatic animals in southern Africa. CSIR, National Scientific Programmes Unit, p 317
Drenner RW, Baca RM, Gilroy JS et al (2002) Community responses to piscivorous largemouth bass: a biomanipulation experiment. Lake Reserv Manag 18:44–51
Ellender BR, Weyl OLF (2014) A review of current knowledge, risk and ecological impacts associated with non-native freshwater fish introductions in South Africa. Aquat Invasions 9:117–132
Fields R, Lowe SS, Kaminski C et al (1987) Critical and chronic thermal maxima of northern and Florida largemouth bass and their reciprocal F1 and F2 hybrids. Trans Am Fish Soc 116:856–863
Forshage A, Fries L (1995) Evaluation of the Florida largemouth bass in Texas, 1972–1993. In: Schramm H Jr, Piper RG (eds) Uses and effects of cultured fishes in aquatic ecosystems. American Fisheries Society, Symposium, pp 484–491
Francis RM (2017) pophelper: an R package and web app to analyse and visualize population structure. Mol Ecol Resour 17:27–32
García-Berthou E (2007) The characteristics of invasive fishes: what has been learned so far? J Fish Biol 71:33–55
Gompert Z, Alex Buerkle C (2010) introgress: a software package for mapping components of isolation in hybrids. Mol Ecol Resour 10(2):378–384
Hargrove JS, Weyl OLF, Allen MS et al (2015) Using tournament angler data to rapidly assess the invasion status of alien sport fishes (Micropterus spp.) in southern Africa. PLoS ONE 10:e0130056
Hargrove JS, Weyl O, Austin JD (2017) Reconstructing the introduction history of an invasive fish predator in South Africa. Biol Invasions 19:2261–2276
Harrison A (1936) Black bass in the Cape Province: second report on the progress of American largmeouth black bass (Micropterus salmoides; Lacepede). South African Division of Fisheries Investigational Report, p 119
Horton RA, Gilliland ER (1993) Monitoring trophy largemouth bass in Oklahoma using a taxidermist network. In: Proceedings of the annual conference Southeastern Association of Fish and Wildlife Agencies vol 47, pp 679–685
Hughes JS, Wood MG (1995) Development of a trophy largemouth bass fishery in Louisiana. In: Proceedings of annual conference of the Southeastern Association of Fish and Wildlife Agencies, pp 58–68
Isely JJ, Noble RL, Koppelman JB et al (1987) Spawning period and first-year growth of northern, Florida, and intergrade stocks of largemouth bass. Trans Am Fish Soc 116:757–762
Ivanova NV, Dewaard JR, Hebert PDN (2006) An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol Ecol Notes 6:998–1002
Jackson D (2002) Ecological effects of Micropterus introductions: the dark side of black bass. In: Philipp D, Ridgway M (eds) Black bass: ecology, conservation, and management. American Fisheries Society Symposium, Bethesda, MD, pp 221–232
James NC, Whitfield AK, Cowley PD (2008) Long-term stability of the fish assemblages in a warm-temperate South African estuary. Estuar Coast Shelf Sci 76:723–738
Johansen P, Andersen JD, Borsting C et al (2013) Evaluation of the iPLEX® Sample ID Plus Panel designed for the Sequenom MassARRAY® system. A SNP typing assay developed for human identification and sample tracking based on the SNPforID panel. Forensic Sci Int Genet 7:482–487
Johnson PT, Olden JD, Vander Zanden MJ (2008) Dam invaders: impoundments facilitate biological invasions into freshwaters. Front Ecol Environ 6(7):357–363
Jombart T (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics (Oxford, England) 24:1403–1405
Lamothe K, Johnson RL (2013) Microsatellite analysis of trophy largemouth bass from Arkansas reservoirs. J Ark Acad Sci 67:71–80
Leprieur F, Beauchard O, Blanchet S et al (2008) Fish invasions in the world’s river systems: when natural processes are blurred by human activities. PLoS Biol 6:e28
Li C, Gowan S, Anil A et al (2015) Discovery and validation of gene-linked diagnostic SNP markers for assessing hybridization between Largemouth bass (Micropterus salmoides) and Florida bass (M. floridanus). Mol Ecol Resour 15:395–404
Linder HP, Johnson SD, Kuhlmann M et al (2010) Biotic diversity in the Southern African winter-rainfall region. Curr Opin Environ Sustain 2:109–116
Lowe S, Browne M, Boudjelas S et al (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Invasive Species Specialist Group Auckland, New Zealand
McCafferty J, Ellender B, Weyl O et al (2012) The use of water resources for inland fisheries in South Africa. Water SA 38:327–344
Neal JW, Noble RL (2002) Growth, survival, and site fidelity of Florida and intergrade largemouth bass stocked in a tropical reservoir. N Am J Fish Manag 22:528–536
Peterson NR, Lutz-Carrillo D, Neal JW (2017) Genetic composition of largemouth bass in Puerto Rico reservoirs. N Am J Fish Manag 37:1199–1207
Philipp DP, Whitt GS (1991) Survival and growth of northern, Florida, and reciprocal F1-hybrid largemouth bass in central Illinois. Trans Am Fish Soc 120:58–64
Philipp DP, Claussen JE, Kassler TW et al (2002) Mixing stocks of largemouth bass reduces fitness through outbreeding depression. In: Philipp DP, Ridgway MS (eds) Black bass: ecology, conservation, and management. American Fisheries Society, Bethesda, pp 349–364
Powell AM (1967) Historical information of Maryland Commission of Fisheries: with some notes on game. Maryland Department of Natural Resources, Annapolis
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasmussen JB, Robinson MD, Heath DD (2010) Ecological consequences of hybridization between native westslope cutthroat (Oncorhynchus clarkii lewisi) and introduced rainbow (Oncorhynchus mykiss) trout: effects on life history and habitat use. Can J Fish Aquat Sci 67:357–370
Rius M, Darling JA (2014) How important is intraspecific genetic admixture to the success of colonising populations? Trends Ecol Evol 29:233–242
Robbins WH, MacCrimmon HR (1974) The blackbass in America and overseas. Biomanagement and Research Enterprises, Sault Sainte Marie, Ontario
Rogers MW, Allen MS, Porak WF (2006) Separating genetic and environmental influences on temporal spawning distributions of largemouth bass (Micropterus salmoides). Can J Fish Aquat Sci 63:2391–2399
Sasaki S (1961) Introduction of Florida largemouth bass into San Diego County. Inland Fisheries Administrative Report. California Department of Fish and Game, Sacramento, California, p 6
Sharma S, Herborg LM, Therriault TW (2009) Predicting introduction, establishment and potential impacts of smallmouth bass. Divers Distrib 15:831–840
Strayer DL (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174
Taylor GC, Weyl OLF, Cowley PD et al (2015) Dispersal and population-level mortality of Micropterus salmoides associated with catch and release tournament angling in a South African reservoir. Fish Res 162:37–42
Thioulouse J, Chessel D, Doledec S et al (1997) ADE-4: a multivariate analysis and graphical display software. Stat Comput 7:75–83
Truitt RV, Bean BA, Fowler HW (1929) The fishes of Maryland. State of Maryland Conservation Department, Annapolis
Vilà M, Espinar JL, Hejda M et al (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Warren M Jr (2009) Centrarchid identification and natural history. In: Cooke S, Philipp D (eds) Centrarchid fishes diversity, biology and conservation. Wiley, West Sussex, pp 375–533
Wasserman R, Strydom N, Weyl OLF (2011) Diet of largemouth bass, Micropterus salmoides (Centrarchidae), an invasive alien in the lower reaches of an Eastern Cape river, South Africa. Afr Zool 46:378–386
Watling R, Watling H (1983) Metal surveys in South African estuaries VII Bushmans, Kariega, Kowie and Great Fish Rivers. Water SA 9:66–70
Weyl OL, Hecht T (1999) A successful population of largemouth bass, Micropterus salmoides, in a subtropical lake in Mozambique. Environ Biol Fishes 54:53–66
Weyl PS, DeMoor F, Hill M et al (2010) The effect of largemouth bass Micropterus salmoides on aquatic macro-invertebrate communities in the Wit River, Eastern Cape, South Africa. Afr J Aquat Sci 35:273–281
Weyl OLF, Finlayson B, Impson ND et al (2014) Threatened endemic fishes in South Africa’s Cape Floristic region: a new beginning for the Rondegat River. Fisheries 39:270–279
Weyl OLF, Schirrmann MK, Hargrove JS et al (2017) Invasion status of Florida bass Micropterus floridanus (Lesueur, 1822) in South Africa. Afr J Aquat Sci 42:359–365
Zhao H, Li C, Hargrove JS, Bowen BR et al (2018) SNP marker panels for parentage assignment and traceability in the Florida bass (Micropterus floridanus). Aquaculture 485:30–38
Acknowledgements
We thank Neil Deacon for his help with planning and execution of field sampling. Financial assistance for JSH was provided by the Institute of Food and Agricultural Sciences, University of Florida (UF), the UF Center for African Studies, the Jeanne and Hunt Davis Graduate Research Award, and the UF International Center Research Abroad for Graduate Students Program. We acknowledge use of infrastructure and equipment provided by the SAIAB Research and Molecular Genetics Platforms and the funding channeled through the NRF-SAIAB Institutional Support system. This study was partially funded by the National Research Foundation—South African Research Chairs Initiative of the Department of Science and Technology (Inland Fisheries and Freshwater Ecology, Grant No. 110507).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hargrove, J.S., Weyl, O.L.F., Zhao, H. et al. Using species-diagnostic SNPs to detail the distribution and dynamics of hybridized black bass populations in southern Africa. Biol Invasions 21, 1499–1509 (2019). https://doi.org/10.1007/s10530-018-01912-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-01912-8