Abstract
Environmental conditions found along an invasion front can mediate spread dynamics of an invasive species. Conditions that affect survival and individual growth rates of founder propagules and the first young-of-year generation can have a profound effect on establishment dynamics. Responses of younger, more sensitive individuals to these novel conditions, in part, determine establishment success. The southern invasion front of zebra mussels in North America expanded into Texas in 2009. To determine mechanisms that could affect establishment of zebra mussel populations at low latitudes in North America, an in situ study was conducted in Lake Texoma, on the Texas-Oklahoma border. Survival and growth were measured of young-of-year juveniles at multiple sites within the reservoir. Age-specific mortality was significantly positively correlated with temperature at all sites and no mortality occurred when temperatures were <26 °C. Shell-length growth rates were the highest ever reported for lentic environments. A decrease in shell-length growth rates was associated with an increase in soft tissue mass, possibly resulting from flexible energy allocation from shell development to gametogenesis after maturation. Overall, survivorship and growth were related to water temperatures and chlorophyll-a concentrations. Warm waters found at lower latitudes along the invasion front could facilitate establishment of populations by increasing growth rates and decreasing time to maturity. However, due to extreme climate events (e.g. drought and flooding) and a strong temperature-mortality relationship, mortality likely will also be high beyond the southern invasion front, resulting in highly dynamic boom and bust cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A salient characteristic of many invasive species is the ability to adapt to novel environmental conditions during geographic spread (Thompson 1998; Lee 2002; Lockwood et al. 2007; Prentis et al. 2008). Establishment success along the invasion front is mediated, in part, by the adaptability of populations to novel environmental conditions (Sakai et al. 2001). Rapid adaptive change of invasive populations can accelerate the rate of invasions and invasive spread (Thompson 1998; Phillips et al. 2006). For example, increased growth rates can reduce generation times and age at maturity thereby facilitating internal propagule loading (i.e. intra-environmental release of offspring by founders), which can have a profound effect on establishment of populations (Lockwood et al. 2007). Similarly, the meta-analysis by Colautti et al. (2006) found that generation time was inversely related to the invasiveness of a species. Selection pressures at the invasion front are particularly important in this regard, as they can affect both the establishment (e.g. environmental tolerances and biotic interactions) and transport (e.g. dispersal capability) phases of the invasion process (Lee 2002).
In aquatic ecosystems, water temperature is one of the primary determinants of species range limits because of its effects on individuals and other water quality parameters (Strayer 1991; Hellmann et al. 2008). On the other hand, broad temperature tolerance, or eurythermality, can facilitate invasive spread across thermal gradients (Zerebecki and Sorte 2011). Survival of invasive propagules in waters that are near or at upper thermal tolerance levels could result in thermally-adapted populations, as reported for zebra mussels Dreissena polymorpha at lower latitudes in North America (Elderkin and Klerks 2005; Morse 2009; Boeckman and Bidwell 2014). After initial detection of zebra mussels in the Laurentian Great Lakes in the late 1980s, spread was expected to be limited by temperature in the southern United States (Strayer 1991; McMahon 1996) based on geographic distributions and thermal tolerances of zebra mussels in Europe (Allen et al. 1999). However, zebra mussel populations found in rivers and reservoirs at lower latitudes in the United States could have adapted to warmer temperature regimes compared to populations found at higher latitudes (Thorp et al. 1998; Elderkin and Klerks 2005; Morse 2009).
Establishment of zebra mussel populations in multiple Texas reservoirs exemplifies the moving invasion front of D. polymorpha farther into the south and southwest United States and into conditions originally predicted to exceed the species’ thermal tolerance. There remains uncertainty regarding the future rate and extent of geographic spread of zebra mussels, especially in warm surface waters of the southern United States (McMahon 1996; Bossenbroek et al. 2007; Cohen 2008; Strayer 2009; Boeckman and Bidwell 2014). Based on data from Europe (Karatayev et al. 1998) and North America (Garton et al. 2014), temperature, calcium, dissolved oxygen, pH, and salinity concentrations influence the distribution of zebra mussels. These limiting environmental factors have been used to predict the potential geographic range (Strayer 1991; Cohen and Weinstein 2001; Cohen 2008; Whittier et al. 2008) and survival, growth, or occurrence of zebra mussels in Europe (Ramcharan et al. 1992; Naddafi et al. 2010) and North America (Hincks and Mackie 1997; Garton et al. 2014). However, understanding effects of environmental conditions found at the current invasion front on zebra mussel survival and growth could be more useful for predicting continuing directional spread.
The objectives of this study were to quantify young-of-year (YOY) zebra mussel survival and growth along natural gradients in a reservoir at the southern invasion front in North America. Recently-settled zebra mussels from the established population in Lake Texoma (Texas, USA) were transplanted to, and incubated in, each of five zones of the reservoir that contain distinct physicochemical characteristics (Atkinson et al. 1999). Water-quality parameters and zebra mussel survival, shell length, and body mass were quantified during a 69-day growing season. Growth rates determined by the current study were compared to those reported from invaded regions in Europe and North America that span the latitudinal range of current zebra mussel geographic distribution. We tested the hypotheses that (1) in general, the zebra mussel population in Lake Texoma exhibits adaptation to local conditions (i.e. growth rates greater than those reported from populations at higher latitudes), and that (2) within this population, survivorship and growth are affected by temperature and an interacting suite of environmental factors.
Methods
Study area
The southern invasion front of zebra mussels expanded into Texas in 2009, when adult zebra mussels were detected in Lake Texoma and Sister Grove Creek (Texas Parks and Wildlife Department 2009a, b). An established zebra mussel population was detected in a neighboring reservoir, Ray Roberts Lake, in July 2012, and by 2013, all three life stages (larval [i.e. veliger], juvenile, and adult) were detected in a third Texas reservoir (Lake Lewisville; Texas Parks and Wildlife Department 2012, 2013a). Additionally, downstream transport of propagules (especially veligers) from reservoirs into the mainstem of the Trinity River and downstream to Lake Livingston has been observed (C. J. Churchill, pers. observ.). Since 2013, established zebra mussel populations have been found in five additional Texas reservoirs (Belton, Bridgeport, Dean Gilbert, Eagle Mountain, and Stillhouse Hollow; Texas Parks and Wildlife Department 2017).
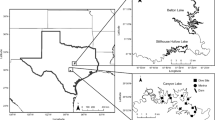
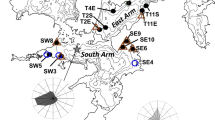
This research was conducted in Lake Texoma, a monomictic, 3.1 km3 reservoir formed by an impoundment of the Red and Washita Rivers in the southern Great Plains ecoregion of North America (Fig. 1). The reservoir was partitioned into five zones based on physicochemical properties (Atkinson et al. 1999): Red River (RRZ), Red River Transition (RRTZ), Main Lake (MLZ), Washita River Transition (WRTZ), and Washita River (WRZ) (Table 1). Differences in zebra mussel survival and growth have been observed within water bodies (MacIsaac 1994; Garton and Johnson 2000), therefore the five zones in Lake Texoma were used to test for effects of these natural environmental gradients on survivorship and growth rates in the Lake Texoma population. Study sites were located near marinas to allow land-based access and consistent depth (3 m) of submerged study equipment and enclosures (see below). Environmental and biological data were collected during 10 visits to each study site in 2012 (July 19 and 27, August 7, 17, 23, and 30, and September 6, 13, 20, and 26).
Environmental data
Discrete water-quality parameters including water temperature, dissolved oxygen concentration (DO), pH, and specific conductance were measured by using a YSI 600 XLM datasonde (YSI Incorporated, Yellow Springs, OH, USA). Continuous water temperature data were recorded at 15-min intervals by submersible data loggers (Onset Computer Corporation, Bourne, MA, USA) that were placed inside experimental enclosures. Continuous water temperature data were used to calculate mean daily temperature (MDT) for each site. Replicate water samples (n = 3) for determination of calcium, salinity, and chlorophyll-a were collected using a Kemmerer sampler (1200-030; Wildlife Supply Company, Saginaw, MI, USA) during visits to each study site. Samples were transferred into opaque 500-ml bottles and stored on ice until analyzed. Calcium concentrations were determined by using an EDTA titrimetric method (American Public Health Association 2005). Salinity was measured by using a benchtop multi-meter (H280G; Hach Company, Loveland, CO, USA). Chlorophyll-a concentrations were determined spectrophotometrically (American Public Health Association 2005).
Artificial substrates/enclosures
Young-of-year zebra mussels were harvested from the main lake zone of the reservoir using stacked-tile artificial substrates (Churchill and Baldys 2012). Using a single site for harvesting study organisms allowed the use of mussels from a single cohort. The main lake zone was selected because it is known to contain reproducing zebra mussel beds (Churchill 2013). Substrates were made of eight 10 × 10-cm high-density fiberboard “hardboard” tiles that were separated by small, plastic spacers. Artificial substrates were deployed in June, prior to settlement of the primary spring cohort. Substrates were retrieved in early July, shortly after initial settlement. This method reduced variability in post-settlement growth, which can depend on settlement date (Wacker and von Elert 2002). Substrates were disassembled and tiles containing recently-settled mussels were randomly selected for reassembly into newly-configured artificial substrates composed of four hardboard tiles each. This method did not require removal of mussels from their original substrate, which could negatively affect survival and growth (Karatayev et al. 2006). Because of a population crash that preceded the study and the desire to track biological measures of individual mussels throughout the experiment, 35 mussels were used at each site at the beginning of the experiment (n = 175). Substrates were deployed at a depth of 3 m at each of the five study sites. Substrates were deployed inside flexible mesh bags with 1.9-mm diagonal openings (Yu and Culver 1999, Garton and Johnson 2000, Boeckman and Bidwell 2014). Although 1.9-mm diagonal openings are large enough to allow veliger entry into the enclosures, no additional settlement was observed inside any of the enclosures during the experimental period. Mesh bags were closed at the top by using zip-ties. Mesh enclosures were opened and cleaned during weekly visits to each study site to prevent fouling of mesh material. During cleaning of mesh enclosures, tiles with attached study organisms were submerged in reservoir water to prevent desiccation. Artificial substrates were deployed at each study site on July 19 and retrieved on September 26 (69-day exposure).
Zebra mussel survival and growth
Survival, shell length, soft tissue mass (i.e. ash-free dry weight), and growth were determined for each individual mussel during each visit to study sites. Mussels were considered alive if they were observed siphoning while submerged. Mussels were considered dead if gaping and their valves were not reclosed upon physical stimulation of internal organs. Dead mussels were removed from enclosures and were not used during statistical analyses for growth data. Mortality was the number of dead organisms observed during each visit. Age-specific mortality (AsM) was calculated as the number of mussels that died at each site between visits divided by the number of surviving mussels counted during the previous visit. AsM, therefore, represents the percentage of mussels that died between visits.
Shell length (SL) of each mussel was measured during each visit to study sites. SL was determined by measuring from umbo to the most distant point on the posterior margin, taken parallel to the ventral surface. Mussels with SLs <8.0 mm were measured to the nearest 0.01 mm by taking pictures using a calibrated handheld microscope (Dino-Lite AM-413T; AnMo Electronics Corporation, New Taipei City, Taiwan) with built-in 1.3 MP digital camera. Mussels with SLs >8.0 mm were measured to the nearest 0.05 mm using vernier calipers. Mussels were not removed from the tiles and were emersed for no more than 3 min while shell lengths were recorded.
Body mass was quantified as ash-free dry weight (AFDW) (Bij de Vaate 1991). Because of the destructive nature of AFDW determinations and the availability of fewer organisms due to a population crash (Churchill 2013), AFDW was not directly determined for study organisms during 2012. Instead, the relationships between SL and AFDW determined for each site during a pilot study (see below) were used to estimate AFDW for individual study organisms during 2012. Total SL and AFDW growth was calculated by subtracting initial values from final values for each individual. Daily SL and AFDW growth rates (SLDG and AWDG, respectively) were determined by dividing individual growth by the number of elapsed days since the most recent measurement.
Individual mussels were tracked throughout the study using digital photographs that were taken during each visit. Artificial substrates were retrieved from each study site prior to secondary spawning of the population, which typically occurs in autumn (Churchill 2013). Intact substrates were covered with paper towels wetted by reservoir water, placed in closed plastic bags, and stored in a cooler containing ice. Substrates were transported to a laboratory within 5 h of removal from the reservoir.
A pilot study was conducted during 2011. Initial SLs were measured and artificial substrates containing juvenile mussels were deployed at each study site on August 26. Environmental data were collected during three visits to each study site (September 8, 22, and 28). After substrate retrieval, 20 mussels from each of the five study sites were randomly selected for SL measurements and AFDW determinations. Selected mussels were dried at 100 °C for 24 h, then ashed at 500 °C for 3 h. Dry weight (DW) and ash weight (AW) were measured to the nearest 0.1 mg using a digital balance. AFDW was calculated as AFDW = DW − AW, and represents a measure of soft body tissue only (without shell). Quadratic regressions were used to determine relationships between SL and AFDW at each site (r2 = 0.93–0.98, p < 0.0001 for all sites). Other methods used during the pilot study were the same as those described above for the full experiment in 2012. Juvenile zebra mussel growth differed significantly between the five study sites during the 33-day pilot study (F = 4.63, p = 0.0017), indicating that our experimental design was suitable to detect population responses.
Statistical analyses
Analyses were conducted on both pooled data (all five sites) and data from each site resulting in six data groupings for each analysis. Pooled data were used during comparisons to published studies. Pearson correlation coefficients were calculated for each pair of environmental variables (Naddafi et al. 2010). Collinear variables (p < 0.05) were not used in subsequent analyses. Specific conductance was significantly correlated with calcium concentration for all six data groupings. Calcium was retained for analyses due to its importance in shell development (Cohen and Weinstein 2001; Whittier et al. 2008). Regressions on raw data were used to determine if relationships between survival or growth and environmental predictor variables were significant. One-way ANOVAs were used to compare mean total SL growth or mean total AFDW growth for individuals among sites. Significant ANOVAs for total growth data were followed by a Tukey multiple comparison test with Kramer adjustment for unequal sample sizes.
Several variables were significantly correlated with time, therefore, ANCOVAs were conducted by using either SLDG or AWDG as the dependent variable and time as the covariate for each site. Regression slopes for the five sites were not significantly different for either dependent variable. Therefore, to account for the effect of time on biological variables (SLDG and AWDG) and environmental factors (MDT, DO, pH, calcium, salinity, and chlorophyll-a) either quadratic (biological variables) or linear (environmental variables) regressions were conducted using each of the aforementioned variables as the dependent variable and time as the independent variable. Regression residuals represent values of dependent variables after the effect of time has been removed, and residuals from each regression analysis were used in subsequent analyses. Residuals of AsM [log (x + 1) transformed], SLDG, and AWDG were regressed against environmental variables and candidate models were compared using corrected Akaike Information Criterion scores (AICc) (Naddafi et al. 2010). Differences in AICc scores (ΔAICc) were used to identify best-fit models considering ΔAICc < 2 indicates a model that has substantial support (Burnham and Anderson 2002). Weighted AICc scores (wAICc) were calculated to determine the best model(s) out of the subset of candidate models with ΔAICc < 2. Evidence ratios (wAICci/wAICcj) were calculated to determine to what extent the best-supported model was better than other candidate models. Variable importance was determined using model averaging. Model inference sampling method was unrestricted with replacement and was run 1000 times. Variables selected in at least 20% of the samples (n ≥ 200) were used during refit analysis. Refit analysis was run 1000 times. During each iteration, a best-fit model was generated and selected variables were recorded. Variable selection percentage describes how often variables were included in best-fit models during 1000 iterations and represents a quantitative measure of variable importance.
Statistical analyses were conducted using SAS 9.3 or SigmaPlot 12. Data were analyzed for parametric assumptions including normality and homoscedasticity. Results were considered significant at p < 0.05.
Results
Environmental variables
Water temperature decreased substantially during the study period. Temperature and chlorophyll-a concentrations were generally higher in the two river zones than in the MLZ (Table 2) but were not significantly different between the study sites. Dissolved oxygen extremes were observed in the river transition zones (minimum at RRTZ, maximum at WRTZ). Calcium and salinity gradients were observed in the reservoir, decreasing from the RRZ to the WRZ.
Survival
Generally, AsM was higher for earlier age classes (Fig. 2). Mean AsM > 13% was observed when temperatures were >29.4 °C. After temperatures decreased to <26 °C, AsM was zero at all sites. A significant curvilinear relationship existed between AsM and water temperature for pooled data (r2 = 0.41, p < 0.0001; Fig. 3). Multiple regressions on pooled data yielded three candidate models with ΔAICc < 2 (Table 3). All three models with ΔAICc < 2 included water temperature and pH. The model with water temperature, pH, and calcium concentration provided the best fit to pooled data (adjusted R2 = 0.54, F = 17.78, p < 0.0001). Multiple regressions on data from RRZ, MLZ, WRTZ, and WRZ yielded models containing temperature with ΔAICc < 2. At sites with lower temperatures (RRTZ and MLZ), chlorophyll-a was included in best-fit models. Water temperature was selected in 100% of best-fit models for pooled data (Table 4). Using data from each site, temperature, pH, or DO was the most important predictor and was selected in at least 77% of best-fit models. In the MLZ, DO was included in the best-fit model (adjusted R2 = 0.95) and was selected in 98% of best-fit models. AsM was significantly inversely related to SL (r2 = 0.44, p < 0.0001) and SLDG (r2 = 0.22, p = 0.0011; Fig. 4). Highest mortality (more than four individuals between visits) was observed when SLs were <5.1 mm. AsM was also significantly inversely related to AFDW (r2 = 0.40, p < 0.0001) and AWDG (r2 = 0.34, p = 0.0002; Fig. 4).
Age-specific mortality of zebra mussels from five sites in Lake Texoma. See Table 1 for study site description
Curvilinear regression of age-specific mortality of zebra mussels and mean daily water temperature from five sites in Lake Texoma. Regression line and 95% confidence bands are shown for pooled data. See Table 1 for study site description
Curvilinear regressions of age-specific mortality and shell length (a), daily shell-length growth (b), ash-free dry weight (c), and daily ash-free dry-weight growth (d) of zebra mussels from five sites in Lake Texoma. Note different axes. Regression line and 95% confidence bands are shown for pooled data. See Table 1 for study site description
Shell-length growth
Mean total SL growth was significantly different between sites (F = 17.42, p < 0.0001), with lowest growth in MLZ (8.49 ± 0.96 mm) and highest in river zones where water temperature and chlorophyll-a concentrations were higher (11.14 ± 0.90 mm in WRZ and 10.83 ± 1.34 mm in RRZ; Fig. 5). Mean SLDG at all sites ranged from 21.46 ± 13.37 to 301.70 ± 80.07 µm × day−1 (Table 5). At each site, SLDG maximum was observed between days 29 and 49, when SLs were 6-8 mm. Mean SLDG was unimodally related to water temperature (r2 = 0.33, p = 0.0002; Fig. 6). At each site, SLDG maximum was observed after reservoir turnover occurred and water temperatures decreased to 27.4–28.1 °C. Although SLDG was not significantly correlated with pH, SLDG maxima were observed when pH was 8.1–8.5 at each site. Using data from each site, chlorophyll-a, DO, or calcium was the most important predictor of SLDG and each was selected in more than 70% of best-fit models (Table 6). Chlorophyll-a was one of the two most important predictor variables at each site except RRZ, where it was most concentrated (Table 2) and, therefore, less likely a limiting factor. At RRZ, DO was included in 74% of best fit models. SLDG was significantly related to time at each site (r2 = 0.67–0.84, p = 0.0039–0.0365; Fig. 7). For pooled data, SLDG was significantly related to SL (r2 = 0.66, p < 0.0001; Fig. 8).
Cumulative shell-length growth (a) and ash-free dry-weight growth (b) of zebra mussels from five sites in Lake Texoma (mean from each time step ± standard error). Note different y-axes. See Table 1 for study site description
Regression of daily shell-length growth of zebra mussels and mean daily water temperature from five sites in Lake Texoma. Regression line and 95% confidence bands are shown for pooled data. See Table 1 for study site description
Regressions of mean daily shell-length growth (a, c, e, g, i) and mean daily ash-free dry-weight growth (b, d, f, h, j) of zebra mussels and day from five sites in Lake Texoma. Note different y-axes. Regression line and 95% confidence bands are shown. See Table 1 for study site description
Regressions of daily shell-length growth and shell length (a) and daily ash-free dry-weight growth and ash-free dry weight (b) of zebra mussels from five sites in Lake Texoma. Note different axes. Regression line and 95% confidence bands are shown for pooled data. See Table 1 for study site description
Soft tissue growth
Mean total AFDW growth was significantly different between sites (F = 10.45, p < 0.0001), with highest growth in river zones (24.51 ± 9.29 mg at RRZ and 23.68 ± 5.60 mg WRZ) and lowest in MLZ (11.61 ± 5.48 mg; Fig. 5). Mean AWDG was positive throughout the experiment (no degrowth was observed) and ranged from 0.01 to 0.79 mg × day−1. AWDG maximum at each site was observed between days 56 and 63, which was 2-4 weeks after maximum SLDGs were observed (Table 7). AWDG maxima were observed when AFDW values were 15–22 mg, except in the MLZ. Maximum AWDG observed for any individual was 1.70 mg × day−1 (WRZ, days 63–69). At each site, AWDG maxima occurred as mussels achieved sexual maturity (C. J. Churchill, unpublished data). Chlorophyll-a was selected in more than 75% of best-fit models for pooled AWDG data (Table 8). Using data from each site, chlorophyll-a, calcium, or DO was the most important predictor of AWDG and each was selected in at least 45% of best-fit models. AWDG was significantly related to time at each site (r2 = 0.86–0.94, all p < 0.001; Fig. 7). For pooled data, AWDG was significantly related to AFDW (r2 = 0.85, p < 0.0001; Fig. 8).
Discussion
Mortality of zebra mussels is related to spatio-temporal dynamics of environmental factors including temperature extremes (Matthews and McMahon 1999; Morse 2009) and limited algal food availability (Jantz and Neumann 1998; Garton et al. 2014). Temperatures associated with mortality of zebra mussels in Lake Texoma were similar to those reported in previous studies. Upper acute thermal tolerances of zebra mussels in North America are between 30 and 32 °C (Iwanyzki and McCauley 1993; Matthews and McMahon 1999; Garton et al. 2014) and can vary latitudinally (Morse 2009). Dense mussel populations are often associated with water temperatures that are 14–28 °C (Wu et al. 2010). Mortality increases with temperature if it exceeds 28 °C (MacIsaac 1994; Karatayev et al. 2011). During the current study, AsM was generally high until temperatures decreased to approximately 28 °C (after reservoir turnover) and no study organism died after temperatures decreased to <26 °C. Therefore, individuals that survive high summer temperatures can persist into autumn/winter. In the south and southwest United States, where water temperatures during summer often exceed reported upper thermal tolerances of zebra mussels, populations will experience higher mortality due to thermal stress. Such selection pressures could result in thermally-adapted populations with enhanced upper thermal tolerances (Elderkin and Klerks 2005; Boeckman and Bidwell 2014). Several studies suggest that zebra mussel populations are adapting to warmer temperatures found in lakes and reservoirs of the southern United States (McMahon 1996; Morse 2009). In areas where temperature is not a limiting factor, limited food availability can increase mortality of zebra mussels (Dorgelo 1993). In Lake Texoma, chlorophyll-a (a surrogate for algal density) was an important factor affecting mortality in the main lake body, where the mean concentration was less than the minimum for long-term survival (Jantz and Neumann 1998) and temperatures were the lowest of all study sites. No other site had higher mortality after temperatures decreased during reservoir turnover (after day 42).
Shell-length growth of zebra mussels from the current study was higher than reported values for lentic populations at higher latitudes: the Netherlands (Bij de Vaate 1991), Lake Erie (MacIsaac 1994), and Lake St. Clair (Bitterman 1992; Mackie 1993). Mean total SL growth during the current study was 9.6 mm for pooled data, whereas maximum SL growth over a single growing season was 5.5 mm in Lake Wawasee (Garton and Johnson 2000) and 8.5 mm in Lake Ijsselmeer (Bij de Vaate 1991). Maximum SL growth of YOY mussels in Lake Erie was 11.2 mm (Chase and Bailey 1999a) but this occurred over a five-month period, which is more than twice the duration of the current 69-day study. Daily shell-length growth rates were significantly related to temperature based on studies from lentic environments that span the latitudinal range of zebra mussel distributions in Europe and North America (r2 = 0.73, p < 0.0001; Fig. 9). Jantz and Neumann (1992), MacIsaac (1994), Garton and Johnson (2000), and Karatayev et al. (2006) found that SL growth of zebra mussels increases with temperature up to 30 °C. Growth is halted at 30 °C and mortality is high when temperatures exceed 32 °C (Karatayev et al. 2006; Boeckman and Bidwell 2014; Garton et al. 2014). In lotic environments, positive associations have been observed between SL growth and temperature (Smit et al. 1992) up to 28 °C (Allen et al. 1999). It is therefore expected that SLDG observed during the current study would increase with temperatures that are lower than the upper thermal tolerance level. In Lake Texoma, SLDG maxima were observed when temperatures decreased to 27–28 °C and before study organisms reached maturity (C. J. Churchill, unpublished data). It has also been reported that low levels of algal density (measured as chlorophyll-a concentration) and dissolved oxygen can retard growth of zebra mussels (Stanczykowska 1977; Jantz and Neumann 1998). Although sufficient chlorophyll-a levels (>7.4 µg/L) were observed at most sites, low chlorophyll levels in less productive areas (e.g. main lake areas, MLZ in the current study) could have reduced shell-length growth. In regions of lakes and reservoirs that contain sufficient levels of chlorophyll-a, other limiting factors can reduce growth rates. For example, at RRZ, dissolved oxygen was an important predictor variable for growth likely because chlorophyll-a, calcium, and pH were not limiting at this site.
Linear regression of daily shell-length growth of zebra mussels and latitude reported from lentic environments in Europe and North America. Regression line and 95% confidence bands are shown. See Table 1 for study site description. References: 1 Chase and Bailey (1999a), 2 MacIsaac (1994), 3 Bij de Vaate (1991); 4 Dorgelo (1993); 5 Mackie (1993); 6 current study; 7 Boeckman and Bidwell (2014)
Growth rates of zebra mussels in the current study are the highest reported for lentic populations (Fig. 9) and are comparable to the highest rates reported for populations found in lotic environments (Smit et al. 1992; Jantz and Neumann 1998; Allen et al. 1999; French et al. 2006). These results support the hypothesis that the zebra mussel population in Lake Texoma could be adapting to local conditions. However, more research is needed to determine if this population has experienced an adaptive response to local conditions as opposed to a physiological response.
Daily shell-length growth rates of zebra mussels are size-dependent with smaller individuals having higher rates (Bij de Vaate 1991; MacIsaac 1994; Jantz and Neumann 1998; Chase and Bailey 1999a; Yu and Culver 1999; Garton and Johnson 2000). In the current study, size-dependent SLDG was observed at all sites. However, SLDG increased between day 0 and 42 for pooled data. SLDG maxima were observed when SLs were 6-8 mm resulting in a unimodal relationship between SLDG and SL. The pattern of increasing, then decreasing SLDG has rarely been reported and is contrary to results from Sprung (1995a). Only one other study reported a similar unimodal pattern (Bitterman 1992), which was also conducted on YOY mussels and with relatively frequent measurements. It is likely that more frequent measures of SL and the use of recently-settled individuals would discern a similar unimodal SLDG-SL relationship in other populations. Subsequent decline of SLDG of organisms longer than 8 mm in SL could be attributed to flexible energy allocation resulting in a diversion of energy from shell development to soft tissue growth and reproduction (Sprung 1995b; Stoeckmann and Garton 2001).
Although AFDW values for the current study were calculated from an earlier pilot study, overall AFDW and AWDG values were similar, yet slightly higher, than reported in previous studies. This agrees with SL data from the current study, which were also higher than reported in previous studies. Generally, AFDW of zebra mussels is size-dependent. Maximum mean AFDW for zebra mussels with 15-mm SL was 14.5 mg in Europe (Mazurian Lakes, Poland, Stanczykowska 1977) and 19.6 mg in North America (Lake St. Clair, Mackie 1991). However, during the current study, maximum mean AFDW for organisms with 14.5-mm SL was 25.2 mg (RRZ). Walz (1978) found that AWDG of 1 + year zebra mussels is size-dependent with exponential AFDW growth occurring for only a short period. Gross growth efficiency (growth per unit of available food) also decreases with size (Sprung 1995b). Similar to Bitterman (1992), size-dependent AFDW growth was observed at all sites with a unimodal relationship peaking at 15-mg AFDW. Maximum AWDG occurred after primary shell development. Flexible energy allocation in zebra mussels is associated with a life history trade-off (Stoeckmann and Garton 2001). Given that allocation of energy to reproduction in adult zebra mussels can be size-specific (Stoeckmann and Garton 2001), it is possible that energy was diverted from shell development to soft tissue growth and gametogenesis (Mackie 1993; Jantz and Neumann 1998; Chase and Bailey 1999b). In addition, smaller zebra mussels can be more thermally tolerant than larger individuals, which can be partly attributed to lower metabolic demand of smaller individuals (Allen et al. 1999). Stoeckmann and Garton (1997) and Allen et al. (1999) found that juvenile zebra mussels can increase shell length and soft tissue mass under the same thermally-stressful, sub-lethal conditions that cause degrowth of adults via tissue remobilization. Karatayev et al. (2006) reported higher mortality and growth for smaller individuals resulting in a survivorship/growth trade-off. This life history trade-off has been described in general by Stearns (1992) and specifically for zebra mussels by Dorgelo (1993), Mackie (1993), and Karatayev et al. (2011). The latter studies found that reduced survivorship was associated with increased growth and temperatures for zebra mussel populations in Lakes Erie and St. Clair. During the current study, AsM was highest for smaller individuals. However, AsM and water temperatures had decreased before SLDG maxima were observed (i.e. decoupling of mortality and growth). It is likely that the survivorship/growth tradeoff would be detected over a longer temporal scale or when considering the entire population (i.e. juveniles and adults). These results support the hypothesis that zebra mussel survivorship and growth are strongly affected by temperature.
Food quantity and quality also affect growth of zebra mussels (Dorgelo 1993; Jantz and Neumann 1998; Stoeckmann and Garton 2001). Strayer and Malcom (2006) found that body condition of zebra mussels is positively correlated with phytoplankton biomass. In general, algal food availability (chlorophyll-a) has a unimodal relationship with AFDW growth (Bitterman 1992). Eutrophic environments are often associated with high growth rates of zebra mussels while hypereutrophic conditions can slow growth or cause mortality (Dorgelo 1993). Oligotrophic environments could affect zebra mussel population density and size structure by reducing growth rates (Dorgelo 1993; Nalepa et al. 1995; Naddafi et al. 2010). Tissue degrowth is more likely to be observed in larger, 1 + year organisms and can be site-specific (Walz 1978; Stoeckmann and Garton 2001). Within-lake variation in soft tissue mass has been observed in Europe (Sprung 1995a) and North America (Bitterman 1992). During the current study, AFDW growth was positive for all organisms and maximum AWDG values were observed in river zones, which had the highest levels of chlorophyll-a (mean of 9.5 µg × L−1). However, in systems with high food quantity/quality coupled with high turbidity or total suspended solids, growth of zebra mussels is reduced due to negative effects on filtration rates (Schneider et al. 1998; Thorp et al. 1998).
Mackie (1993) described two distinct zebra mussel population types based on life history traits (e.g. survival and longevity) and annual SL growth data for populations in Europe and North America. Slow-growing populations, typical of most invaded regions in Europe, are composed of individuals that grow <1.0 cm × year−1, attain maximum SL > 40 mm, and live for longer than three years. In contrast, individuals with growth rates of 1.5–2.0 cm × year−1, maximum SL < 35 mm, and life spans of less than three years comprise fast-growing populations. Most zebra mussel populations in North America would be classified as fast-growing although life-span studies have not been conducted on populations found along the current southern invasion front in the central United States. Mean SL of study organisms at the end of the current experiment was 13.3 mm and maximum SL was 17.7 mm (WRTZ). Considering these SLs were achieved during a 69-day experimental period, it is likely that zebra mussels found at lower latitudes could grow more than 2.0 cm × year−1, especially during their first year. Because larger individuals can have greater overwinter survivorship (Karatayev et al. 2011), it is possible that high growth rates can increase overwinter survivorship of YOY individuals in populations found in warm-water regions (i.e. fast-growing populations that spawn in April or May).
Environmental conditions that alter survivorship or individual growth rates have a profound effect on establishment, persistence, and structure of invasive populations (Lockwood et al. 2007). For example, heat-induced zebra mussel population crashes shift demographics toward YOY or juvenile dominance (Stoeckmann and Garton 2001; Boeckman and Bidwell 2014). These crashes can occur after chronic exposure to sub-lethal temperatures (White et al. 2015) or after extreme, high-temperature droughts, which have been observed in Kansas (Severson 2010), Oklahoma (Boeckman and Bidwell 2014), and Texas (Churchill 2013). Population crashes in these three temperate regions were observed shortly after periods of extreme drought that reduced water levels and desiccated a substantial number of adults in littoral zones. After a population crash, YOY individuals contribute proportionately more to the reproductive population size. Zebra mussel populations that are found in regions that experience intermittent, extreme droughts depend on fast-growing YOY individuals to persist or recover from a crash. In Texas, other extreme climate events also affect zebra mussel population stability. For example, flooding increases reservoir levels, which makes additional substrate available in inundated littoral areas. If flooding occurs during spawning events, mussels could settle in the higher-elevation littoral areas, and be subsequently desiccated when flood waters recede. After a crash, population size recovery would occur if conditions facilitate the development and recruitment of a strong, fast-growing cohort (Strayer and Malcom 2006). At lower latitudes, where YOY zebra mussels grow rapidly, recovery to pre-crash population size could occur quickly. Population recovery would be facilitated if YOY mussels attain maturity during their first year (Jantz and Neumann 1998).
In conclusion, it is likely that warm waters found along the southern zebra mussel invasion front in North America facilitate establishment of populations by enhancing growth rates. Increased growth rates, especially of more sensitive, young-of-year individuals, can increase establishment success (i.e. invasiveness). Therefore, increased invasiveness with continued spread into warmer, lower latitudes is possible if this invasive species shows latitudinal changes in thermal tolerances that produce thermally-tolerant populations composed of fast-growing individuals. Increased growth rates will influence age at maturity, reproductive output, and other reproduction dynamics. However, due to extreme climate events and a strong temperature-mortality relationship, seasonal mortality likely will be high along the southern invasion front, resulting in highly dynamic boom and bust cycles. These dynamics reduce the likelihood of large, sustained zebra mussel populations in low-latitude regions in North America. More research is needed along the current invasion front to determine if environmental conditions and predicted population dynamics will affect regional and continental rates of spread. Future in situ studies of the effects of interacting environmental factors on survival, growth, time to maturity, and reproduction of young-of-year individuals would provide information regarding establishment dynamics, population stability, propagule loading, and continued spread beyond the current invasion front.
References
Allen YC, Thompson BA, Ramcharan CW (1999) Growth and mortality rates of zebra mussels, Dreissena polymorpha, in the lower Mississippi River. Can J Fish Aquat Sci 56:748–759
American Public Health Association, American Water Works Association and Water Pollution Control Federation (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, New York
Atkinson SF, Dickson KL, Waller WT, Ammann L, Franks J, Clyde T, Gibbs J, Rolbiecki D (1999) A chemical, physical, and biological water quality survey of Lake Texoma: August 1996–September 1997 Final Report. Institute of Applied Sciences, University of North Texas, Denton, TX
Bij de Vaate A (1991) Distribution and aspects of population dynamics of the zebra mussel, Dreissena polymorpha (Pallas, 1771), in the lake IJsselmeer area (The Netherlands). Oecologia 86:40–50
Bitterman AM (1992) Comparative growth and mortality rates of the zebra mussel, Dreissena polymorpha, from two sites in Lake St. Clair. Thesis, Oakland University
Boeckman CJ, Bidwell JR (2014) Density, growth, and reproduction of zebra mussels (Dreissena polymorpha) in two Oklahoma reservoirs. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control. CRC Press, Boca Raton, pp 369–382
Bossenbroek JM, Johnson LE, Peters B, Lodge DM (2007) Forecasting the expansion of zebra mussels in the United States. Conserv Biol 21:800–810
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Chase ME, Bailey RC (1999a) The ecology of the zebra mussel (Dreissena polymorpha) in the lower Great Lakes of North America: I. population dynamics and growth. J Gt Lakes Res 25:107–121
Chase ME, Bailey RC (1999b) The ecology of the zebra mussel (Dreissena polymorpha) in the lower Great Lakes of North America: II. Total production, energy allocation, and reproductive effort. J Gt Lakes Res 25:122–134
Churchill CJ (2013) Spatio-temporal spawning and larval dynamics of zebra mussels (Dreissena polymorpha) in a north Texas reservoir: implications for invasions in the southern United States. Aquat Invasions 8:389–406
Churchill CJ, Baldys S (2012) USGS zebra mussel monitoring program for north Texas. United States Geological Survey Fact Sheet 2012–3077
Cohen AN (2008) Potential distribution of zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena bugensis) in California. San Francisco Estuary Institute
Cohen AN, Weinstein A (2001) Zebra mussel’s calcium threshold and implications for its potential distribution in North America. San Francisco Estuary Institute
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037
Dorgelo J (1993) Growth and population structure of the zebra mussel (Dreissena polymorpha) in Dutch Lakes of differing trophic state. In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts, and control. CRC Press, Boca Raton, pp 79–94
Elderkin CL, Klerks PL (2005) Variation in thermal tolerance among three Mississippi River populations of the zebra mussel, Dreissena polymorpha. J Shellfish Res 24:221–226
French JRP, Nichols SJ, Craig JM, Allen JD, Black MG (2006) In situ growth of juvenile zebra mussels in a regulated stream. J Freshw Ecol 21:25–30
Garton DW, Johnson LE (2000) Variation in growth rates of the zebra mussel, Dreissena polymorpha, within Lake Wawasee. Freshw Biol 45:443–451
Garton DW, McMahon R, Stoeckmann AM (2014) Limiting environmental factors and competitive interactions between zebra and quagga mussels in North America. In: Nalepa TF, Schloesser DW (eds) Quagga and zebra mussels: biology, impacts, and control. CRC Press, Boca Raton, pp 383–402
Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS (2008) Five potential consequences of climate change for invasive species. Conserv Biol 22:534–543
Hincks SS, Mackie GL (1997) Effects of pH, calcium, alkalinity, hardness, and chlorophyll on the survival, growth, and reproductive success of zebra mussel (Dreissena polymorpha) in Ontraio lakes. Can J Fish Aquat Sci 54:2049–2057
Iwanyzki S, McCauley RW (1993) Upper lethal temperatures of adult zebra mussels (Dreissena polymorpha). In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts, and control. CRC Press, Boca Raton, pp 667–673
Jantz B, Neumann D (1992) Shell growth and population dynamics of Dreissena polymorpha in the Rhine River. In: Neumann D, Jenner HA (eds) The zebra mussel Dreissena polymorpha: Ecology, biological monitoring, and first applications in the water quality management. Gustav Fisher, Stuttgart, pp 49–66
Jantz B, Neumann D (1998) Growth and reproductive cycle of the zebra mussel in the Rhine River as studied in a river bypass. Oecologia 114:213–225
Karatayev AY, Burlakova LE, Padilla DK (1998) Physical factors that limit the distribution and abundance of Dreissena polymorpha (Pall.). J Shellfish Res 17:1219–1235
Karatayev AY, Burlakova LE, Padilla DK (2006) Growth rate and longevity of Dreissena polymorpha (Pallas): a review and recommendations for future study. J Shellfish Res 25:23–32
Karatayev AY, Mastitsky SE, Padilla DK, Burlakova LE, Hajduk MM (2011) Differences in growth and survivorship of zebra and quagga mussels: size matters. Hydrobiologia 668:183–194
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Lockwood JL, Hoopes MF, Marchetti MP (2007) Invasion ecology. Blackwell, London
MacIsaac HJ (1994) Comparative growth and survival of Dreissena polymorpha and Dreissena bugensis, exotic molluscs introduced to the Great Lakes. J Gt Lakes Res 20:783–790
Mackie GL (1991) Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 219:251–268
Mackie GL (1993) Biology of the zebra mussel (Dreissena polymorpha) and observations of mussel colonization on unionid bivalves in Lake St. Clair of the Great Lakes. In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts, and control. CRC Press, Boca Raton, pp 153–166
Matthews MA, McMahon RF (1999) Effects of temperature and temperature acclimation on survival of zebra mussels (Dreissena polymorpha) and Asian clams (Corbicula fluminea) under extreme hypoxia. J Molluscan Stud 65:317–325
McMahon RF (1996) The physiological ecology of the zebra mussel, Dreissena polymorpha, in North America and Europe. Am Zool 36:339–363
Morse JT (2009) Thermal tolerance, physiological condition, and population genetics of dreissenid mussels (Dreissena polymorpha and D. rostriformis bugensis) relative to their invasion of waters in the Western United States. Dissertation, University of Texas at Arlington
Naddafi R, Pettersson K, Eklov P (2010) Predation and physical environment structure the density and population size structure of zebra mussels. J N Am Benthol Soc 29:444–453
Nalepa TF, Wojcik JA, Fanslow DL, Lang GA (1995) Initial colonization of the zebra mussel (Dreissena polymorpha) in Saginaw Bay, Lake Huron: population recruitment, density, and size structure. J Gt Lakes Res 21:417–434
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439:803
Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ (2008) Adaptive evolution in invasive species. Trends Plant Sci 13:288–294
Ramcharan CW, Padilla DK, Dodson SI (1992) Models to predict potential occurrence and density of the Zebra Mussel, Dreissena polymorpha. Can J Fish Aquat Sci 49:2611–2620
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen J, Ellstrand NC, McCauley DE, O’Neil P, Parket IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332
Schneider DW, Madon SP, Stoeckel JA, Sparks RE (1998) Seston quality controls zebra mussel (Dreissena polymorpha) energetics in turbid rivers. Oecologia 117:331–341
Severson AM (2010) Effects of zebra mussel (Dreissena polymorpha) invasion on the aquatic community of a Great Plains reservoir. Thesis, Kansas State University
Smit HA, Bij de Vaate A, Fioole A (1992) Shell growth of the zebra mussel (Dreissena polymorpha (Pallas)) in relation to selected physicochemical parameters in the Lower Rhine and some associated lakes. Arch Hydrobiol 124:257–280
Sprung M (1995a) Physiological energetics of the zebra mussel Dreissena polymorpha in lakes I: growth and reproductive effort. Hydrobiologia 304:117–132
Sprung M (1995b) Physiological energetics of the zebra mussel Dreissena polymorpha in lakes II: food uptake and gross growth efficiency. Hydrobiologia 304:133–146
Stanczykowska A (1977) Ecology of Dreissena polymorpha (Pall.) in lakes. Pol Arch Hydrobiol 24:461–530
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stoeckmann AM, Garton DW (1997) A seasonal energy budget for zebra mussels (Dreissena polymorpha) in western Lake. Can J Fish Aquat Sci 54:2743–2751
Stoeckmann AM, Garton DW (2001) Flexible energy allocation in zebra mussels (Dreissena polymorpha) in response to different environmental conditions. J N Am Benthol Soc 20:486–500
Strayer DL (1991) The projected distribution of the zebra mussel, Dreissena polymorpha, in North America. Can J Fish Aquat Sci 48:1389–1395
Strayer DL (2009) Twenty years of zebra mussels: lessons from the mollusk that made headlines. Front Ecol Environ 7:135–141
Strayer DL, Malcom HM (2006) Long-term demography of a zebra mussel (Dreissena polymorpha) population. Freshw Biol 51:117–130
Texas Parks and Wildlife Department (2009a) Lone zebra mussel found in Lake Texoma. http://www.tpwd.state.tx.us/newsmedia/releases/index.phtml?req=20090421a. Accessed 3 Sept 2013
Texas Parks and Wildlife Department (2009b) Zebra mussels spreading in Texas. http://www.tpwd.state.tx.us/newsmedia/releases/index.phtml?req=20090817a. Accessed 3 Sept 2013
Texas Parks and Wildlife Department (2012) Zebra mussels found in Lake Ray Roberts. http://www.tpwd.state.tx.us/newsmedia/releases/index.phtml?req=20120718a Accessed 3 Sept 2013
Texas Parks and Wildlife Department (2013a) Zebra mussels documented in Lewisville Lake. http://www.tpwd.state.tx.us/newsmedia/releases/?req=20130620b. Accessed 3 Sept 2013
Texas Parks and Wildlife Department (2017) The zebra mussel threat. http://tpwd.texas.gov/huntwild/wild/species/exotic/zebramusselmap.phtml. Accessed 22 Apr 2017
Thompson JN (1998) Rapid evolution as an ecological process. Trends Ecol Evol 13:329–332
Thorp JH, Alexander JE, Bukaveckas BL, Cobbs GA, Bresko KL (1998) Responses of Ohio River and Lake Erie dreissenid mollusks to changes in temperature and turbidity. Can J Fish Aquat Sci 55:220–229
Wacker A, von Elert E (2002) Strong influences of larval diet history on subsequent post-settlement growth in the freshwater mollusc Dreissena polymorpha. Proc R Soc Lond B 269:2113–2119
Walz N (1978) The energy balance of the freshwater mussel Dreissena polymorpha Pallas in laboratory experiments and in Lake Constance IV: growth in Lake Constance. Arch Hydrobiol Suppl 55:142–156
White JD, Hamilton SK, Sarnelle O (2015) Heat-induced mass mortality of invasive zebra mussels (Dreissena polymorpha) at sublethal water temperatures. Can J Fish Aquat Sci 72:1221–1229
Whittier TR, Ringold PL, Herlihy AT, Pierson SM (2008) A calcium-based invasion risk assessment for zebra and quagga mussels (Dreissena spp). Front Ecol Environ 6:180–184
Wu Y, Bartell SM, Orr J, Ragland J, Anderson D (2010) A risk-based decision model and risk assessment of invasive mussels. Ecol Complex 7:243–255
Yu N, Culver DA (1999) In situ survival and growth of zebra mussels (Dreissena polymorpha) under chronic hypoxia in a stratified lake. Hydrobiologia 392:205–215
Zerebecki RA, Sorte CJ (2011) Temperature tolerance and stress proteins as mechanisms of invasive species success. PLoS ONE 6(4):e14806
Acknowledgements
The authors wish to thank the following marina personnel for allowing access to docks and boat slips; Mark at Lake Texoma Marina, Scott Hayward, Tim Hayes, and Mark Callahan at Highport Resort, James Harber at Eisenhower Yacht Club, Bill Glascock at Alberta Creek Resort and Marina, and Jerry and Serena Current at Newberry Creek Resort. The authors would also like to thank three anonymous reviewers who provided useful suggestions that improved this manuscript. This work was partially supported by National Science Foundation grant CBET-1039172 (DJH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Churchill, C.J., Hoeinghaus, D.J. & La Point, T.W. Environmental conditions increase growth rates and mortality of zebra mussels (Dreissena polymorpha) along the southern invasion front in North America. Biol Invasions 19, 2355–2373 (2017). https://doi.org/10.1007/s10530-017-1447-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1447-8